
Case report of rare hereditary disease in an icteric woman with cirrhosis
Highlight box
Key findings
• Report here about key findings of the study.
• Cirrhosis concomitant with HE has been reported by us for the first time.
What is known and what is new?
• Report here about what is known: HE has similar clinical manifestation of end-stage cirrhosis with both jaundice and splenomegaly.
• Report here about what does this manuscript adds: HE and cirrhosis can coexist and cause splenomegaly, even possibly worsen progress of disease each other.
What is the implication, and what should change now?
• Report here about implications and actions needed.
• We should pay attention to the differentiation between liver disease and hematologic disease when patient presented jaundice and/or hypersplenism.
Introduction
Cirrhosis is a serious cause of death worldwide. There are liver fibrosis and structurally abnormal nodules among diffuse hepatic process in cirrhosis, which representing the final histological change for a multiple of chronic liver disease. Hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection are major causes of cirrhosis in China. Decompensated cirrhosis has numerous complications, including gastroesophageal varices, non-obstructive jaundice, ascites, hepatic encephalopathy, and even variceal bleed. It occurs mainly as consequence of portal hypertension and hyperdynamic circulation and their hemodynamic and metabolic effects (1).
Hereditary elliptocytosis (HE) is an inherited hematological disorder characterized by elliptically-shaped erythrocytes and hemolytic anemia. It is distributed by numerous ethnic groups, but it is more common in countries and regions of endemic malaria, particularly in the Africa (2,3). In China, HE is a rare disease which individual family cases or sporadic cases have been reported (4,5). Most of them are asymptomatic and it may present symptoms of anemia like fatigue. HE may rarely have jaundice and splenomegaly due to long-standing hemolytic anemia.
Cirrhosis don’t definitely have relation to HE, it has not reported that it concurrently happens in one patient yet. Here, we present a Chinese patient suffering from cirrhosis complicated with persistent jaundice, anemia and splenomegaly, as well as HE diagnosed by peripheral blood smear and the next genomic sequencing. We present this case in accordance with the CARE reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-62/rc).
Case presentation
A 52-year-old women was admitted to department of Infectious diseases on August 7, 2020, because of persistent jaundice and fatigue for more than 4 years. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The patient was diagnosed HBV-related cirrhosis and started the treatment of lamivudine 4 years ago, but she had then changed to entecavir therapy after one year in her past medical history. Before admitted to our hospital, the patient had the treatment of hepatoprotective drugs, but it was ineffective. She had no history of hypertension, diabetes mellitus, coronary heart disease, tuberculosis, residence of pastoral area, surgery or trauma. She had no history of cancer in the relatives. Her younger brother had chronic hepatitis B and treated with entecavir, and her son had also chronic HBV infection.
On physical examination, icteric sclera and skin, severe splenomegaly had been found, but no abdominal tenderness, ascites, heart problem, petechiae and ecchymoses were shown in the patient.
The liver function profiling at admission showed serum levels of total bile acid (T.BA), total bilirubin (T.BIL) and indirect bilirubin (I.BIL) elevated to 145.7 µmol/L (normal range, 0–10 µmol/L), 89.86 µmol/L (normal range, 0–23 µmol/L) and 65.65 µmol/L (normal range, 0–19 µmol/L), respectively. The blood routine tests showed decreased lower leukocytes of 2.17×109/L (normal range, 3.5×109/L to 9.5×109/L) and platelets of 37×109/L (normal range, 125×109/L to 350×109/L), respectively. Mean corpuscular volume and mean corpuscular hemoglobin were 109.3 fL (normal range, 82–100 fL) and 35.8 pg (normal range, 27–34 pg), respectively. The levels of hemoglobin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (γ-GT), immunoglobulin G4 (IgG4), ceruloplasmin, glucose-6-phosphate dehydrogenase, folate and vitamin B12 were normal. Serum auto-antibodies of autoimmune liver diseases, direct and indirect combs tests, serum tumor markers and other hepatitis viral markers of hepatitis A virus (HAV), HCV, hepatitis D virus (HDV), cytomegalovirus (CMV) and Epstein-Barr virus (EBV) were negative. The abdominal ultrasonography indicated an image of cirrhosis, and revealed diffusive enlargement of the spleen (the long axis and thickness were 16.9 and 5.5 cm, respectively). Transient elastography showed liver stiffness of 25.4 kPa. Gastro-endoscopy showed severe esophago-gastric varices and chronic gastritis.
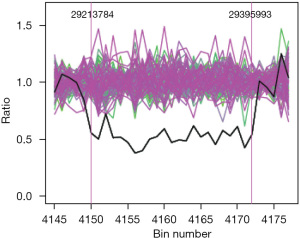
Based on the evidence of biochemical parameters and imaging results, the patient was confirmed the diagnosis with cirrhosis and anti-HBV therapy with entecavir (500 mg, once per day) continued. In addition, the patient had been done platelet transfusion at hospital, but all parameters did not improve. Because of hemolytic icteric in this patient, a peripheral blood smear showed approximately 60% of abnormal shape and appeared oval or elongated erythrocytes, from slightly egg-shaped to rod or pencil forms (elliptocytes) (Figure 1). Then we did the next genomic sequencing, a large fragment about 182.2 Kilo base pairs of heterozygous deletion was detected in p35.3 region of chromosome 1, including 1–15 exons of the erythrocyte membrane protein 4.1 (EPB41) gene (Figure 2).
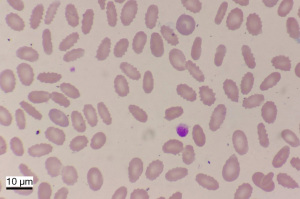
The patient had lower platelets and ecchymosis in abdomen. We gave the suggestion of splenectomy and continued anti-HBV treatment of entecavir for her, but she refused to surgery. The patient lived in her hometown all time and did not come back to our hospital because of coronavirus disease 2019 (COVID-19) epidemic for about 2 years. We did the follow-up by call and the patient had a persistent jaundice and lower platelets as similar as before. Peripheral blood smear showed 70% elliptocytes of red blood cell (RBC) in her younger brother and her brother was surely made the clinical diagnosis of HE, but we have not done the gene profiling of HE in the relatives because they are living in different provinces.
Discussion
HE, inherited as an autosomal dominant trait, is attributed to mutation in any one of genes encoding skeleton proteins of the red cell membrane. It is not common in China and usually misdiagnosed. Mutations of HE located in the a-spectrin, b-spectrin, or protein 4.1 genes (6). Most abnormality was recognized the occurrence within spectrin so far (7), but the mutation caused by gene of 4.1 protein is rarely found. Heterozygous or homozygous mutation in the gene encoding EPB41 on chromosome 1p35 caused HE-1 and account for 5% (8). Over 25% of elliptocytes on peripheral blood smear and a positive family history should be important for diagnosis of HE (6), it might have slight decline of elliptocytes percent after splenectomy, it is helpful to distinguish liver disease from microvascular hemolytic disease. Here we report for the first Chinese patient with both HBV-related cirrhosis and HE, who had jaundice, splenomegaly, and pancytopenia for a long time. Peripheral blood smear showed approximately 70% elliptocytes in RBCs, as well as high-throughput sequencing revealed the EPB41 gene mutation in the patient.
The hematological characteristic of HE has an obvious increase of the elliptocytes in peripheral blood smear, due to the loss of the surface area of red blood cells and then the shape from a double concave disc to oval or even cell fragments (6,9). Abnormal erythrocytes and cell fragments are isolated and swallowed by the spleen due to overload, so that it has anemia and splenomegaly in patient with HE. HE is usually classified into 3 stages (10), there are normal hemoglobin levels and reticulocyte counts, and even no abnormal erythrocytes occurred in smears in asymptomatic patient with HE in stage 1. In stage 2 (hemolytic-compensated patients) the patient shows elevated reticulocyte counts and normal hemoglobin. In stage 3, the patient with hemolytic anemia, as well the increased heteromorphic erythrocytes and cell fragments. We thought this patient was in stage 3 due to persistent mild anemia during hospitalization.
Jaundice, as a common problem in clinical practice, it occurs secondary to intrahepatic and extrahepatic etiology and displays elevated serum bilirubin levels in the unconjugated or conjugated form. In this case, the patient indicated elevated unconjugated bilirubin that already had a hint to clinician. Similar clinical manifestation in both HE and cirrhosis, such as anemia, jaundice, and splenomegaly (11,12), which making diagnosis a challenge, may initially be the reason of no diagnosis of elliptocytosis in this patient. The patient has HBV-related cirrhosis and hypersplenism for a long time. We analyze the laboratory tests when the patient did not have good response to the therapy. Then we found numerous elliptocytes in the blood smear and finally diagnosed HE by gene detection.
HE and cirrhosis can cause splenomegaly and possibly worsen progress of disease each other. It is rarely needed in therapy of HE unless severe cases, RBC transfusions may particularly require during the neonatal period and in intercurrent illness (13,14). Because the spleen is an important organ for the site of erythrocyte sequestration and destruction in the adult, splenectomy has been the cornerstone of therapy for case with severe hemolytic HE to improve anemia, and avoid the formation of bilirubin gallstones. Most patients of HE with improvement in anemia and clinical symptoms after splenectomy operation. However, splenectomy is also associated with increased risk of the complications, such as infection, bleeding, and thrombosis. The mortality rate of splenectomy is about 5–10% (15). Our patient had made the detailed examination when she was admitted to hospital, and we finally diagnosed cirrhosis concomitant with HE. The case had some limitations. Firstly, the patient did not have splenectomy after the understanding for high risk of surgery, and no regular follow-up in clinic. Secondly, we have not done the family tree of HE including genetic background.
Conclusions
We firstly reported a case of cirrhosis concomitant with HE in China. We learnt more from all mentioned above. Firstly, the clinicians are easy to neglect usually hematologic diseases because of the patient with cirrhosis. Secondly, HE can also aggravate hypersplenism. Thirdly, we should understand the reasons when the patient has splenomegaly, low platelets and/or ineffective treatment. Finally, peripheral blood smear should be the best choose for clinicians, and it is cheap and convenient.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-62/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-62/coif). DC is a current employee of Guangzhou KingMed Diagnostics Group Co. Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- European Association for the Study of the liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Glele-Kakai C, Garbarz M, Lecomte MC, et al. Epidemiological studies of spectrin mutations related to hereditary elliptocytosis and spectrin polymorphisms in Benin. Br J Haematol 1996;95:57-66. [Crossref] [PubMed]
- Dhermy D, Schrével J, Lecomte MC. Spectrin-based skeleton in red blood cells and malaria. Curr Opin Hematol 2007;14:198-202. [Crossref] [PubMed]
- Cao M, Huang Z, Zhou H, et al. Clinical and molecular genetic analysis of a Chinese family with hereditary elliptocytosis caused by a novel mutation in the EPB41 gene. J Clin Lab Anal 2021;35:e23781. [Crossref] [PubMed]
- Wang X, Liu A, Huang M, et al. Hereditary elliptocytosis with variable expression and incomplete penetrance in a Chinese family. Br J Haematol 2019;186:e159-62. [Crossref] [PubMed]
- Narla J, Mohandas N. Red cell membrane disorders. Int J Lab Hematol 2017;39:47-52. [Crossref] [PubMed]
- Da Costa L, Galimand J, Fenneteau O, et al. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev 2013;27:167-78. [Crossref] [PubMed]
- McGuire M, Smith BL, Agre P. Distinct variants of erythrocyte protein 4.1 inherited in linkage with elliptocytosis and Rh type in three white families. Blood 1988;72:287-93. [Crossref] [PubMed]
- McGuire M, Agre P. Clinical disorders of the erythrocyte membrane skeleton. Hematol Pathol 1988;2:1-14. [PubMed]
- King MJ, Garçon L, Hoyer JD, et al. ICSH guidelines for the laboratory diagnosis of nonimmune hereditary red cell membrane disorders. Int J Lab Hematol 2015;37:304-25. [Crossref] [PubMed]
- Zaidi AU, Buck S, Gadgeel M, et al. Clinical Diagnosis of Red Cell Membrane Disorders: Comparison of Osmotic Gradient Ektacytometry and Eosin Maleimide (EMA) Fluorescence Test for Red Cell Band 3 (AE1, SLC4A1) Content for Clinical Diagnosis. Front Physiol 2020;11:636. [Crossref] [PubMed]
- Bahr TM, Lozano-Chinga M, Agarwal AM, et al. Dizygotic twins with prolonged jaundice and microcytic, hypochromic, hemolytic anemia with pyropoikilocytosis. Blood Cells Mol Dis 2020;85:102462. [Crossref] [PubMed]
- Mohandas N. Inherited hemolytic anemia: a possessive beginner's guide. Hematology Am Soc Hematol Educ Program 2018;2018:377-81. [Crossref] [PubMed]
- Iolascon A, Andolfo I, Russo R. Advances in understanding the pathogenesis of red cell membrane disorders. Br J Haematol 2019;187:13-24. [Crossref] [PubMed]
- Leukemia and Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guideline on the diagnosis and treatment of primary myelofibrosis. Zhonghua Xue Ye Xue Za Zhi 2019;40:1-7. [PubMed]
Cite this article as: Pi S, Liang X, Chen D, Yang M, Chen Y. Case report of rare hereditary disease in an icteric woman with cirrhosis. Dig Med Res 2023;6:20.


