
Hepatorenal syndrome: a case based practical guide
A 65-year-old man with cirrhosis secondary to chronic alcohol use, decompensated by ascites and prior variceal bleeding presents to the hospital with confusion. His home medications are furosemide 80 mg/day, spironolactone 200 mg/day, nadolol 40 mg/day and a multivitamin. There is no history of recent non-steroidal anti-inflammatory drug use. On examination, heart rate is 110 beats/minute, blood pressure is 85/40 mmHg, and temperature is 37 ℃. Ascites (shifting dullness), asterixis and bilateral lower extremity edema are noted. A large area of erythema and increased warmth is noted in the left lower extremity. Laboratory studies show that serum creatinine level is 2.3 mg/dL, serum sodium is 126 mEq/L, total serum bilirubin is 3.2 mg/dL and international normalization ratio (INR) is 1.8. Creatinine 3 weeks ago was 1.0 mg/dL.
Does this patient have hepatorenal syndrome (HRS)?
This patient has acute kidney injury (AKI) as defined by increase in serum creatinine by ≥0.3 mg/dL (within 48 hours) or percentage increase in 50% from baseline (1,2). AKI is common in hospitalized patients with decompensated cirrhosis and ascites and is associated with severe morbidity and mortality (3). HRS-AKI (formerly known as HRS-type 1) is a type of AKI seen exclusively in patients with cirrhosis and ascites, but it is not the only type of AKI that these patients can have. Up to half the patients can have AKI due hypovolemia. Acute tubular necrosis (ATN) is seen in up to one third of patients. A small group of patients may have AKI due to other intrinsic causes (e.g., bile cast nephropathy, glomerulonephritis, contrast nephropathy) or post-renal obstruction. The rest of the patients, who do not respond to volume expansion and do not have any intrinsic renal or post -renal injury, are diagnosed with HRS-AKI (2,3). This patient does not have HRS-non-AKI (formerly known as HRS-type 2) which is slow progressive form of renal injury seen in patients with diuretic refractory ascites and has no specific precipitating factor. For easier readability, HRS-AKI will be referred to as HRS in the rest of the article.
Management of AKI in cirrhosis should be systematic and swift as early diagnosis and treatment are critical for better outcomes (4). To diagnose HRS (I) other causes of AKI should be ruled out; (II) kidney function should not improve with intravenous volume expansion with albumin (1 g/kg per day for 48 hours) and withdrawal of diuretics (2,5). In addition, simultaneous, prompt investigations should be conducted to determine and resolve precipitating factors (2,5). Figure 1 summarizes the approach to diagnosis and management of AKI in patients with cirrhosis.
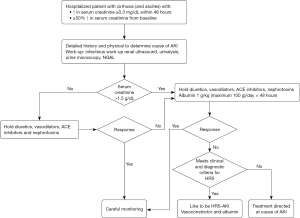
What factors can precipitate AKI in this patient?
Most cases of AKI in decompensated cirrhosis with ascites are pre-renal caused by hypovolemia or HRS. They represent the perturbation of existing hemodynamic abnormalities which is characterized by splanchnic and systemic vasodilation. Vasodilatation leads to decrease in effective arterial volume and activation of vasoconstrictor and anti-natriuretic neurohumoral systems (the renin-angiotensin-aldosterone system and sympathetic nervous system), which in turns leads to sodium and water retention and a hyperdynamic circulation. Progressive vasodilation can cause renal vasoconstriction and decrease in renal blood flow leading to development of HRS. Any factor that decreases effective arterial volume such as intravascular volume depletion (e.g., excessive diuresis or laxative use, gastrointestinal bleeding, large volume paracentesis), excessive vasodilation (use of beta blockers or nitrates, large volume paracentesis) or a systemic inflammatory response (e.g., infection) can precipitate AKI and HRS. A comprehensive infectious work-up including a diagnostic paracentesis is imperative. Elevated INR should not prevent a diagnostic paracentesis. In an American study of patients with infection related acute on chronic liver failure (defined as two or more organ failures in patients with decompensated cirrhosis), the commonest infections were urinary tract infection and spontaneous bacterial peritonitis (6). About 10% of the patients had soft tissues infections (6). This patient has clinical signs of cellulitis which is likely to be the precipitant of AKI.
The patient is admitted to the step-down unit and undergoes infectious work-up with blood cultures, urinalysis, urine culture, chest X-ray and diagnostic paracentesis. Diuretics and beta-blockers held. The edges of the red and warm area on the left shin are marked as there is high suspicion for cellulitis. A diagnostic paracentesis rules out spontaneous bacterial peritonitis. A urine test, obtained by straight catheterization is not suggestive of a urinary tract infection. Urine sediment in bland. Renal ultrasonography is normal. Ceftriaxone and albumin (1 g/kg) are started. At 48 hours, creatinine is 2.4 mg/dL. The patient is awake and oriented and is very uncomfortable due to tense ascites.
What should be the next steps?
The patient now meets diagnostic criteria for HRS as other causes of AKI have been ruled out and as AKI did not resolve with albumin challenge. An expedited transplant referral should be considered (2,5) as HRS without liver transplantation is associated with poor survival (7,8). Renal function, including urine output should be closely monitored. A high index of suspicion should be maintained for additional infections which can worsen liver and renal function and eventually lead to multi-organ failure.
Vasoconstrictors and albumin are the mainstay of therapy and should be continued for 14 days or until creatinine returns to baseline (1,2,5). The most important positive predictor of response to pharmacologic treatment is lower baseline creatinine (9,10) and thus treatment should start as soon as HRS is diagnosed.
The following vasoconstrictors can be used in HRS (Table 1):
- Terlipressin: terlipressin, a long-acting synthetic derivative of vasopressin, is the first line treatment for HRS and has been shown to be effective in reversing HRS (improvement in serum creatinine <1.5 mg/dL) in multiple clinical trials (2,5,11,12). Terlipressin is administered as an intravenous infusion at an initial dose of 2 mg/day that can be increased to 12 mg/day until creatinine decreases (13). Figure 2 shows an algorithm for administration of terlipressin. Though bolus dosing commonly used, there is some evidence to show that administration of terlipressin as an infusion (as compared to bolus dosing) has a more favorable side effect profile and can lead to a more sustained decrease in portal pressure (14).
- Norepinephrine: norepinephrine can be used instead of terlipressin and may be equally effective (15). As it requires close monitoring, patients are transferred to the intensive care unit (ICU). Norepinephrine is started at 0.5 mg/h and titrated to a maximum of 3 mg/h, with a goal of increasing mean arterial pressure by 10 mmHg or urine output of >200 mL/4 h (2).
- Octreotide and midodrine: octreotide and midodrine are often used in the non-ICU setting, where terlipressin is not available, as they do not require intensive monitoring like norepinephrine (16). Octreotide is administered subcutaneously at 100–200 µg every 8 hours. Midodrine is given orally at a starting dose of 7.5 g three times a day and titrated up to a dose of 12.5 mg to achieve a 15 mmHg increase in mean arterial blood pressure (2). This regimen is less effective than terlipressin (17) and in clinical practice is noted to have poor response rates (18).
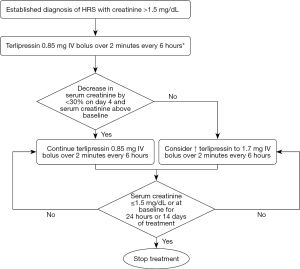
Table 1
| Drugs | Dosage and route | Goal | Side effects |
|---|---|---|---|
| Octreotide and midodrine | Octreotide: 100–200 µg every 8 hours subcutaneously; midodrine: 7.5–15 mg three times a day orally | Increase in MAP by 15 mmHg | Mild and rare, transient decrease in heart rate and cardiac output, hypoglycemia |
| Noradrenaline | 0.5 mg/hour titrated to 3 mg/hour (infusion) | Increase in MAP by 10 mmHg or increase in urine output by 200 mg in 4 hours | Bradycardia, cardiac arrhythmia, cardiomyopathy, peripheral vascular insufficiency, anxiety, transient headache, dyspnea |
| Terlipressin | 2 mg/day titrated to 12 mg/day (infusion) | Improvement in serum creatinine to <1.5 mg/dL | Hyponatremia, abdominal ischemia, circulatory overload, myocardial ischemia, peripheral ischemia, skin necrosis, arterial hypertension, arrythmias and persistent diarrhea |
HRS-AKI, hepatorenal syndrome-acute kidney injury; MAP, mean arterial pressure.
Albumin is an important adjunct to vasoconstrictor therapy and should be continued at 40–50 g/day (2). It expands volume, diminishes endothelial dysfunction, and improves cardiac inotropic effect by binding to vasodilators like nitrous oxide and other deleterious cytokines (19-21).
In patients with tense ascites, intra-abdominal hypertension can adversely affect renal function (22,23). Reduction of intra-abdominal pressure with paracentesis and albumin substitution has been shown to improve renal function by improving renal blood flow (23). Measurement of inferior vena cava diameter and inferior vena cava collapsibility index by point-of-care ultrasound can used to determine if the patient is fluid depleted or overloaded and thus guide paracentesis (24). As this patient is very uncomfortable, 5–6 L of ascitic fluid can be removed to provide comfort and potentially reduce intra-abdominal pressure.
Early involvement of palliative care is recommended to set treatment goals and expectations (25).
The patient remains in the step-down unit and is started on midodrine and octreotide. Albumin is continued at the dosage of 50 g/day. Initially, there is a slight decline in creatinine. But, by day 5 of therapy, creatinine has risen to 3.2 mg/dL and urine output has fallen. Mean arterial pressures are low. The patient has also developed asterixis and has a new oxygen requirement at 2 L/min by nasal cannula. The patient is transferred to the ICU and started on noradrenaline. Transplant evaluation is ongoing. Infectious work-up is repeated. A chest X-ray shows signs of pulmonary interstitial edema. Examination of urine sediment reveals 3–4 granular casts and an occasional renal tubular epithelial cell per high powered field. Urine sodium is 20 mEq/L and fractional excretion of sodium (FeNa) is 0.5%.
Does this patient have ATN?
Traditionally, AKI in cirrhosis was thought to be “structural” (such as ATN) or “functional” (such as hypovolemia or HRS). However, in reality, patients with decompensated cirrhosis and ascites can exhibit features of both structural and functional renal injury and these conditions can overlap (1). FeNa cannot be used to differentiate HRS from other causes of AKI in decompensated cirrhosis. FeNa is low (<1%) in HRS but can also be low in other etiologies of AKI in decompensated cirrhosis (26). Moreover, in this patient, FeNa cannot be used, as diuretic use would have affected sodium excretion, and hence FeNa.
HRS can be difficult to diagnose. It is a diagnosis of exclusion as diagnostic algorithms are designed to rule out other causes of AKI. Diagnosis of HRS by these algorithms has not been validated against a gold standard such as kidney biopsy. Biomarkers such as neutrophil gelatinase associated lipocalin (NGAL) have a good performance to differentiate ATN from other causes of AKI, but may not be easily available (27,28). In addition, it is now recognized that “structural” or parenchymal renal injury is also a component of HRS and is caused by systemic inflammation, oxidative stress and bile salt related tubular damage (1). Thus, response to vasoconstrictors in pure HRS may be variable.
The diagnosis of ATN has important implications for this patient. Dialysis should be actively considered as the next treatment option. This patient has also received overzealous albumin infusions, as evidenced by pulmonary edema. This is very common and can have deleterious effects in these critically ill patients (29). In the landmark multicenter, placebo controlled, double blind, CONFIRM trial, designed to confirm the efficacy and safety of intravenous terlipressin in the treatment of adult subjects with HRS, more patients in the terlipressin arm had respiratory failure, most likely due to overzealous use of albumin (18,30). Furthermore, the ATTIRE randomized controlled trial, which studied the utility of protocolized albumin infusion in hospitalized cirrhotic patients with normal creatinine in preventing a composite end point of infection, AKI, or death, showed that patients in the albumin arm were more likely to develop pulmonary edema (31). To prevent volume overload, point-of-care ultrasound can be used to guide fluid resuscitation, as described above (24). At the minimum, we recommend, albumin in divided doses over the day, with careful monitoring of respiratory status.
On day 6, the patient is in the ICU on a norepinephrine drip with minimal effect on serum creatinine and urine output. He is somnolent and has increased O2 requirement, now up to 4 L/min by nasal cannula. INR is 3.0. No infectious source has been found over the last 24 hours. The transplant team determines that the patient is not a good candidate for liver transplant, due to various reasons including comorbidities and lack of good social support.
Should this patient be started on dialysis?
The use of dialysis as a treatment for HRS is controversial as there is paucity of data to guide selection of patients and the timing of dialysis initiation. The decision to start (or not start) dialysis requires multidisciplinary consensus from hepatologists, nephrologists, critical care specialists and the patient and their family. Dialysis is often considered to be a bridge to liver transplant. But the 30-day transplant free survival in listed patients is 25%, indicating the severity of underlying disease (32). In patients not listed for liver transplant, short term mortality rate is high (up to 85% by some estimates), with little chance of renal recovery and high risk of complications (32-34). In this patient, who has acute on chronic liver failure with multi-organ failure and is not listed for liver transplantation, the chances of survival and renal recovery are very low.
After discussion with the family, dialysis was not initiated. The patient was eventually transitioned to comfort measures and died with family members at bedside.
Conclusions
HRS is a devastating complication of decompensated cirrhosis and has high morbidity and mortality. Early diagnosis and initiation of treatment with vasopressors and albumin, is critical to reverse kidney injury before permanent damage sets in. An evidence-based protocol for diagnosis and treatment has been associated with lower mortality, easier recognition, and earlier initiation of treatment. Institutional protocols may allow for earlier diagnosis and treatment initiation (35).
We now know that “structural” renal damage due to systemic inflammation, oxidative stress and cholemic nephropathy can co-exist with “functional” renal failure in HRS. Further research into these pathophysiological insights, to develop novel biomarkers for diagnosis and measuring treatment response and to identify targets for therapy is needed to improve outcomes in HRS.
Acknowledgments
Funding: This study was supported in part by
Footnote
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-45/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Angeli P, Garcia-Tsao G, Nadim MK, et al. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol 2019;71:811-22. [Crossref] [PubMed]
- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;74:1014-48. [Crossref] [PubMed]
- Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48:2064-77. [Crossref] [PubMed]
- Wong F, O'Leary JG, Reddy KR, et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology 2013;145:1280-8.e1. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- O'Leary JG, Reddy KR, Garcia-Tsao G, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018;67:2367-74. [Crossref] [PubMed]
- Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology 2005;41:1282-9. [Crossref] [PubMed]
- Martín-Llahí M, Guevara M, Torre A, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 2011;140:488-96.e4. [Crossref] [PubMed]
- Piano S, Schmidt HH, Ariza X, et al. Association Between Grade of Acute on Chronic Liver Failure and Response to Terlipressin and Albumin in Patients With Hepatorenal Syndrome. Clin Gastroenterol Hepatol 2018;16:1792-800.e3. [Crossref] [PubMed]
- Boyer TD, Sanyal AJ, Garcia-Tsao G, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol 2011;55:315-21. [Crossref] [PubMed]
- EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397-417. [Crossref] [PubMed]
- Allegretti AS, Israelsen M, Krag A, et al. Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst Rev 2017;6:CD005162. [Crossref] [PubMed]
- Clària J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249-64. [Crossref] [PubMed]
- Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology 2016;63:983-92. [Crossref] [PubMed]
- Israelsen M, Krag A, Allegretti AS, et al. Terlipressin versus other vasoactive drugs for hepatorenal syndrome. Cochrane Database Syst Rev 2017;9:CD011532. [Crossref] [PubMed]
- Angeli P, Volpin R, Gerunda G, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology 1999;29:1690-7. [Crossref] [PubMed]
- Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology 2015;62:567-74. [Crossref] [PubMed]
- Belcher JM, Parada XV, Simonetto DA, et al. Terlipressin and the Treatment of Hepatorenal Syndrome: How the CONFIRM Trial Moves the Story Forward. Am J Kidney Dis 2022;79:737-45. [Crossref] [PubMed]
- Krag A, Bendtsen F, Henriksen JH, et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 2010;59:105-10. [Crossref] [PubMed]
- Fernández J, Clària J, Amorós A, et al. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology 2019;157:149-62. [Crossref] [PubMed]
- Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836-46. [Crossref] [PubMed]
- Umgelter A, Reindl W, Wagner KS, et al. Effects of plasma expansion with albumin and paracentesis on haemodynamics and kidney function in critically ill cirrhotic patients with tense ascites and hepatorenal syndrome: a prospective uncontrolled trial. Crit Care 2008;12:R4. [Crossref] [PubMed]
- Umgelter A, Reindl W, Franzen M, et al. Renal resistive index and renal function before and after paracentesis in patients with hepatorenal syndrome and tense ascites. Intensive Care Med 2009;35:152-6. [Crossref] [PubMed]
- Velez JCQ, Petkovich B, Karakala N, et al. Point-of-Care Echocardiography Unveils Misclassification of Acute Kidney Injury as Hepatorenal Syndrome. Am J Nephrol 2019;50:204-11. [Crossref] [PubMed]
- Rogal SS, Hansen L, Patel A, et al. AASLD Practice Guidance: Palliative care and symptom-based management in decompensated cirrhosis. Hepatology 2022;76:819-53. [Crossref] [PubMed]
- Alsaad AA, Wadei HM. Fractional excretion of sodium in hepatorenal syndrome: Clinical and pathological correlation. World J Hepatol 2016;8:1497-501. [Crossref] [PubMed]
- Huelin P, Solà E, Elia C, et al. Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study. Hepatology 2019;70:319-33. [Crossref] [PubMed]
- Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 2014;60:622-32. [Crossref] [PubMed]
- Schrier RW. Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 2010;5:733-9. [Crossref] [PubMed]
- Wong F, Pappas SC, Curry MP, et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med 2021;384:818-28. [Crossref] [PubMed]
- China L, Freemantle N, Forrest E, et al. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N Engl J Med 2021;384:808-17. [Crossref] [PubMed]
- Allegretti AS, Parada XV, Eneanya ND, et al. Prognosis of Patients with Cirrhosis and AKI Who Initiate RRT. Clin J Am Soc Nephrol 2018;13:16-25. [Crossref] [PubMed]
- Zhang Z, Maddukuri G, Jaipaul N, et al. Role of renal replacement therapy in patients with type 1 hepatorenal syndrome receiving combination treatment of vasoconstrictor plus albumin. J Crit Care 2015;30:969-74. [Crossref] [PubMed]
- Staufer K, Roedl K, Kivaranovic D, et al. Renal replacement therapy in critically ill liver cirrhotic patients-outcome and clinical implications. Liver Int 2017;37:843-50. [Crossref] [PubMed]
- Terres AZ, Balbinot RS, Muscope ALF, et al. Evidence-based protocol for diagnosis and treatment of hepatorenal syndrome is independently associated with lower mortality. Gastroenterol Hepatol 2022;45:25-39. [Crossref] [PubMed]
Cite this article as: Hantzidiamantis K, Mohanty A. Hepatorenal syndrome: a case based practical guide. Dig Med Res 2023;6:24.


