Gastric hyperplastic polyps: a narrative review
Introduction
This review will compile most of the current data in bibliography regarding gastric hyperplastic polyps (GHP). By reviewing the epidemiology, pathophysiology, associations, clinical presentation, microscopic and macroscopic appearance and endoscopic management, this article will bridge the basic science behind GHP development with their clinical management. GHP are frequently encountered during endoscopy and even though initially it was hypothesized that they represent 30–93% of all gastric polyps, more recent studies reveal that the exact number is unknown and may be anywhere between 7–88% (1-3). Their histological characteristics include dilated and elongated (i.e., hyperplastic) foveola in an inflamed stoma (4,5). Multiple associations, such as Helicobacter pylori (H. pylori) gastritis, autoimmune and atrophic gastritis, chronic proton pump inhibitors (PPI) use and portal hypertension have been described, and even though hypergastrinemia has been linked to the pathogenesis of GHP, the exact pathogenetic mechanism leading to GHP development has yet to be elucidated (6-8). Clinically, GHP are usually silent, however they may present with non-specific symptomatology such as gastroesophageal reflux disease (GERD) and dyspepsia (4,7). GHP may also result to slow bleeding and as such to iron deficiency anemia (9,10) More recently, a lot of attention has been drawn to GHP which even though traditionally considered benign lesions, apparently, they carry a malignant transformation risk of approximately 0.6–6.6% (11). The risk increases with size and as such recent guidelines suggest that GHP >1 cm should be resected (12). Summarizing, GHP comprise a large proportion of gastric polyps encountered during endoscopy (1-3). It is imperative to recognize conditions such as H. pylori infection that have been associated with GHP (6-8). The symptoms may be subtle, however cases of overt symptomatology such as bleeding and subsequent anemia have been described (9,10). Even though GHP were classically believed to be benign lesions, apparently recently their malignant potential has been clearly identified, leading to recommendations for removal, especially when they reach certain size (12). We present this article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-38/rc).
Methods
A review of literature until December 31, 2021, was conducted in the PubMed, MEDLINE and EMBASE databases. Three independent researchers assessed clinical trials, case reports, case series and reviews regarding the pathogenesis, associations, clinical features, microscopic and macroscopic characteristics, and management of GHP. To find and assess articles, our search in the databases included keywords connected with Boolean operators “OR” and “AND”. Moreover, after reading the selected articles the researchers would assess the references to find similar or related publications. The search strategy used in the PubMed platform is delineated in Table 1. Similar strategy was used in the other databases. Reviewers assessed and discussed all the articles that resulted from the search to determine if they were suitable to be included in the review. However, articles not in English language were automatically excluded. At the same time, an independent researcher contributed microscopy images and analyzed gastric hyperplastic polyp’s histology features.
Table 1
| Step | Search terms (search conducted for dates up to December 31, 2021) |
|---|---|
| 1 | “gastric polyp*” |
| 2 | “gastric hyperplastic polyp*” |
| 3 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “epidemiology” |
| 4 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “incidence” |
| 5 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “etiology” |
| 6 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “risk factors” |
| 7 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “H. pylori” |
| 8 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “gastritis” |
| 9 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “proton pump inhibitors” |
| 10 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “GERD” |
| 11 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “reflux” |
| 12 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “smoking” |
| 13 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “pathophysiology” |
| 14 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “symptoms” |
| 15 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “clinical presentation” |
| 16 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “anemia” |
| 17 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “bleeding” |
| 18 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “pain” |
| 19 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “maligancy” |
| 20 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “maligant” |
| 21 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “cancer” |
| 22 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “malignant potential” |
| 23 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “cancer risk” |
| 24 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “metaplasia” |
| 25 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “dysplasia” |
| 26 | “TP53 protein” (review articles only) |
| 27 | “Ki-67 antigen” (review articles only) |
| 28 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “macroscopic” |
| 29 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “endoscopy” |
| 30 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “microscopy” |
| 31 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “microscopic” |
| 32 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “histology” |
| 33 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “anatomy” |
| 34 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “management” |
| 35 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “resection” |
| 36 | “gastric hyperplastic polyp*” OR “gastric polyp*” AND “surveillance” |
Incidence
GHP, prior to knowledge and treatment of H. pylori in recent decades, were much more common finding during endoscopy. Since eradication of H. pylori, especially in North America, the incidence of GHP has decreased (13). Studies report GHP are found in 1.2–8% of endoscopies and make up anywhere from 7% to 88% of gastric polyps identified (2,3). GHP tended to be found in older populations, with an age predominance of 60–80 years old (14,15). GHP are not found to have any sexual predominance, although few sources estimate females having a 1.4–1.5 times higher risk than males (3,14,16).
Pathogenesis, predisposing conditions and other associations
As GHP have become less frequently found, and fundic gastric polyps (FGP) more common, the suspected etiology of GHP formation has become more focused. The primary etiology of GHP formation has thus far been linked to hypergastrinemia (7). Elevated gastrin levels have been tied to H. pylori infection, long term PPI use, reactive gastritis, chemical gastritis, autoimmune gastritis or background metaplasia (3,7,17). Elevated gastrin levels, either due to an inability to produce acid, such is the case with autoimmune gastritis, or as a secondary response to PPI therapy has well known tropic and pro-carcinogenic effects, resulting in oxyntic cell atrophy which leads to hyperplasia of acid producing cells and eventual polyp formation (6,7). Hongo et al. correlated serum gastrin levels with GHP incidence while on PPIs and found that incidence was significantly higher with serum gastrin >400 pg/mL (18).
H. pylori infection
H. pylori infection is probably the most well described risk factor for GHP development, by increasing serum gastrin levels as discussed previously (6,7). However, persistent H. pylori infection may also lead to increased cytokine production and chronic active gastritis with subsequent epithelial cell proliferation (19). Vieth et al. revealed that patients with GHP had underlying H. pylori infection at a rate of 37% while Di Giulio et al. found an even higher rate, showing evidence of past or active H. pylori infection in up to 79% of patients developing benign epithelial gastric polyps (most of them being GHP) (11,20). Jeong et al. in their prospective study reported an intermediate number for the proportion of H. pylori infection in patients with GHP at 69.2% (6). Another observation supporting H. pylori infection as significant risk factor for GHP development, is the disappearance of GHP after eradication of the infection (21,22). Both Nam et al. and Ouyang et al. found that over 70% of GHP disappeared completely within 7–10 months after eradication of H. pylori infection (23,24). Ouyang et al. reported this improvement was 20 times more responsive than their control group (23).
Autoimmune and atrophic gastritis
Based on current data, an estimated proportion of 10.5% of atrophic gastritis patients may develop GHP. As such, atrophic gastritis, a condition considered to be an independent risk factor for malignancy, was also linked with GHP development. The underlying mechanism is, again, hypothesized to be hypergastrinemia. However, the detection of GHP in areas other than gastric body and fundus may signify the presence of more factors leading to GHP development. The link between enterochromaffin cell proliferation and hypergastrinemia, seen in atrophic gastritis, is considered key in the GHP formation; these neuroendocrine cells may release hormones and peptides that can induce growth and eventually GHP formation (7,20). Autoimmune gastritis, leading to atrophy has also been observed among patients who develop GHP. However, and despite the exact cause of autoimmune gastritis is unknown, this condition leads to complete atrophy of the oxyntic glands, resulting in significant secondary hypergastrinemia when compared to other causes of atrophic gastritis (7). As such it’s not surprising that GHP arise quite often in autoimmune gastritis, while more than 30% of patients with GHP have been found to suffer from autoimmune gastritis (7,11,25).
PPI
PPI leading to hypergastrinemia and as such to increased risk for polyp formation has been heavily investigated. It has been well established that long term PPI use can lead to multiple histopathologic changes and is associated with fundic gland polyp development (26). However, even though there are studies reporting high PPI use in patients developing GHP, or disappearance of GHP after PPI discontinuation others fail to reveal an association between their use and GHP presence (6,7,18).
GERD
Zeng et al. described an increased incidence of gastric polyps in GERD patients with most of them being GHP. Acid reflux is causing direct tissue damage via mucosal exposure to gastric acids, which leads to mucosal inflammation. Allegedly, the inflammation and chronic mucosal injury results to metaplasia and development of gastric polyps. The association between GERD and GHP was further supported by the disappearance of the polyps after antacid treatment (27). Given those observations along with the presence of reflux and dyspepsia symptomatology in patients with GHP multiple questions can be raised for future studies (4,6,28). Are GHP causing GERD symptoms or is GERD an independent risk factor for GHP development by inducing metaplasia and regenerative changes as described earlier? Is there an underlying cofounding factor predisposing to both GERD and GHP development? Further studies could elucidate the theory underlying the pathophysiologic association of GERD and GHP.
Chronic liver disease and portal hypertension
While GERD has been described as possible association with H. pylori, Livovsky et al., found that patients with chronic liver disease develop GHP more often and at higher numbers when compared to GERD patients, revealing a strong relationship between chronic liver disease (or its complications) and GHP development (27,29). This association was also supported by Waldum et al., endorsing hyperplastic polyps in patients with portal hypertension (7).
Smoking
Di Giulio et al. found that patients with atrophic gastritis who used to smoke were at higher risk for developing benign epithelial gastric polyps in general (20).
Clinical features associated with H. pylori presence
GHP usually do not display overt symptomatology, however multiple case reports, and review studies have described an array of possible symptoms. Patients with GHP have been noted to frequently experience reflux disease symptoms, while dyspepsia has also been described (4,6,30). Even though rare, there have been case reports describing gastric outlet obstruction because of GHP presence (31-34). Another, also rare but well described, potential symptom patients with GHP exhibit is chest pain that may be associated with the location of GHP (35,36). Associations with anemia have also been described. GHP are known to be highly vascular lesions of the gastric mucosa, potentially leading to slow bleeding and as such iron deficiency anemia (9,10). Al-Haddad et al. investigating patients with anemia not only revealed 1.4% of anemic patients examined, were found to have GHP, but in a couple of those patients, anemia failed to respond to other endoscopic therapies but removal of underlying GHP, concluding that GHP should be considered as source of bleeding when other sources cannot be identified (37). Markowski et al. suggested GHP may lead to upper gastrointestinal bleeding, but this would be a latent sign. Ultimately that led to similar recommendation for GHP removal in anemic patients when another actively bleeding source cannot be identified (28). More recently, Hu et al. noted a significant correlation with anemia that was four times more likely to be associated with women than men (38).
Given GHP association with atrophic and autoimmune gastritis, the presence of pernicious anemia in patients carrying GHP would not be unexpected. Indeed, Chan et al. investigating patients with pernicious anemia, revealed presence of polyps in 12.8% with most of them being identified as GHP (39).
Potential for malignant transformation
Even though GHP are considered benign neoplasms of the gastric mucosa, they have been linked to malignant potential in multiple studies (4,40-42). The risk for malignant transformation has been evaluated in detail with estimations mainly between 0.6–6.6% while a few studies suggest higher rates, up to 8.6% (11,42-44).
Histological analysis reveals intestinal metaplasia and dysplastic features in a significant proportion of up to 19% of resected GHP, indicating their significant risk for neoplastic progression even though earlier studies used to describe lower incidence/prevalence rates of dysplastic changes (7,19,27,45,46). Presence of true neoplastic foci within GHP has also been observed (45). Orlowska et al. described a prevalence of neoplasm-bearing GHP at around 2.1% while Terada et al., reported malignant transformation in 2.2% of GHP studied (45,46). More recently Nam et al., revealed true neoplastic foci in 2.7% of GHP analyzed, while Zeng et al. also found adenocarcinoma presence in around 2.1% of resected GHP (22,27).
The exact mechanism of malignant transformation has yet to be understood but multiple studies evaluating the underlying histopathology and immunochemistry tried to reveal possible underlying pathogenetic mechanisms of hyperplasia to carcinoma sequence.
TP53 is a well-known gene encoding a tumor suppressor protein able to break cell’s normal cycle and induce apoptosis while it may also act to repair DNA or alternate cell’s metabolism (47). Ki-67 on the other hand is a protein associated with cell proliferation. In resting cells, the protein is absent. However, during cell’s mitosis, Ki-67 antigen is present in all cell cycles. Thus Ki-67, can highlight the growth fraction of a given cell-population by using the Ki-67 labeling index (48). Both entities have been heavily investigated in GHP. TP53 has been reported to have high expression in neoplastic and dysplastic foci of GHP while in other studies high activity in majority of dysplasia-bearing GHP has been reported (19,46). Genome analysis studies have also reported TP53 or other somatic changes in the neoplastic component of neoplasm-bearing GHP but also in dysplasia-bearing GHP (49,50). More mutations including RB1, PIK3CA and copy number alterations have also been described in dysplasia-bearing GHP (50,51). Interestingly KRAS and APC mutations have been detected in hyperplastic areas of GHP while KRAS mutations were also found in dysplastic and neoplastic areas (52,53). Simultaneously, Ki-67 protein has been reported to have higher activity in neoplasm-harboring and dysplasia-harboring GHP (12,19,46). High Ki-67 labeling index was found in all carcinomatous foci of carcinoma-bearing GHP in Terada et al. study. Based on their findings Terada et al. proposed that malignant changes may develop via hyperplasia-dysplasia-carcinoma sequence. Interestingly the same study failed to show relationship between intestinal metaplasia and malignant transformation (46).
Irrespective of the underlying mechanism, multiple risk factors have been associated with neoplasia development in GHP. Older age, number of GHP detected, size, lobulation, pedunculated shape are proven to have positive correlation with dysplasia/neoplasia presence within GHP (12,19,40). H. pylori infection is also associated with increased risk for dysplasia/ neoplasia development within GHP, while eradication of the organism may even prevent malignant transformation (19). More specifically, when size is taken into consideration, Ahn et al. revealed a median size of carcinoma-bearing GHP at 25 mm. A proportion of 3.7% of all >1 cm GHP were found to have dysplastic changes while half of those contained adenocarcinoma, concluding that GHP >1 cm should be considered for resection (12). Other studies agree with that recommendation suggesting that 5–10 mm GHP have lower malignant transformation risk especially when eradication of underlying possible H. pylori infection is achieved (5,19).
Finally, given their association with H. pylori infection, autoimmune gastritis, increased gastrin levels and gastric atrophy, GHP presence has also been identified as marker indicating high risk for gastric carcinoma development even in other stomach locations (4,7,25).
Endoscopic and microscopic presentation and management
Visual and microscopic examination of any abnormal growth in the stomach necessitates endoscopy with biopsy of the polyp and surrounding tissue.
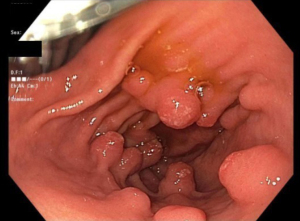
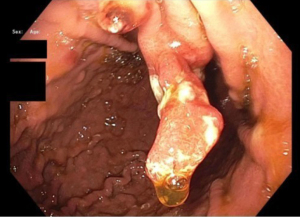
GHP usually present as solitary masses measuring <10 mm and 40–60% are found in the gastric antrum (3,14,16,24). GHP, upon endoscopic examination, appear smooth with a strawberry-like surface that is either broad based/sessile or pedunculated (3,38,54). The distal surface of these masses may contain superficial erosions or ulceration, which can become sites of rare but possible malignancy (54). Upon microscopic examination, tissue samples of GHP show proliferation of superficial foveolar cells with tortuous, cystic pits and varying regions of inflamed stroma (3,16,18) (Figures 1-3).
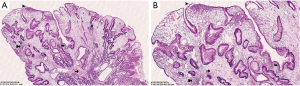
Histology usually reveals typical findings as shown in the images. The above pictures demonstrate typical features of GHP. Gastric foveolae (➥) show elongation, distortion, branching, dilatation, and hyperplasia. The background stroma (➽) is edematous, inflamed, rich in vasculature, and has small, haphazardly distributed smooth muscle bundles (➜). Surface mucosal erosion (➤) is also present (55). GHP are considered to arise from hyperproliferative response to tissue injury. Polypoid foveolar hyperplasia is regarded as a precursor of GHP. It shows elongation but not dilatation of gastric pits and the stroma appears normal or minimally swollen (56). Histologically, GHP should be differentiated from other types of polyps including fundic gland polyps, adenomatous polyps, gastric mucosal prolapse polyps, gastritis cystica polyposa, and hamartomatous inverted polyp (55,57) (Figure 4A,4B).
As discussed previously, resection of these masses is generally considered when size exceeds 10 mm as these polyps tend to have a greater risk of malignancy, especially when sizes reach 20–25 mm (2,3). One study reports a risk of malignancy as high as 58.3% if a polyp grows to >40 mm (2). In the same study the potential for recurrence of GHP after resection was investigated. They found that 51% of GHP regrew after the first resection, 78% regrew after a second resection and 89% regrew after a third resection, suggesting that treatment of the underlying etiology was central to GHP regression (2). Multiple sources recommend a 1-year follow-up esophagogastroduodenoscopy (EGD) to assess for recurrence (23,24,54).
History of GHP knowledge and potential for further studies
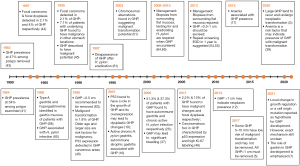
A landmark study investigating the prevalence of GHP was performed in Germany in 1983 and reported that almost 50% of the gastric polyps removed were characterized as GHP, while 1 year later another study reported a prevalence of 34% among the gastric polyps that were examined and associated GHP presence with atrophic gastritis (41,43). Bordi et al. in 1984 first associated GHP development with hypergastrinemia through serum studies (58). A few years later, Daibo et al. reported that 2.1% of GHP contained focal carcinomas and 4% contained dysplastic foci, while Orlowska et al., in a 13-year study, noted similar prevalence of neoplasia-bearing GHP but also that 7.1% of GHP patients were found to have carcinomatous lesions in other gastric locations. In contrast to what was currently believed, these studies elucidated GHP malignant potential (44,45). In 1990, the surrounding gastric mucosa in GHP patients was assessed endoscopically. Type A gastritis and hypergastrinemia were frequently encountered in patients suffering from GHP (59). The following decade produced many more reports attempting to understand GHP, although after 1997 when H. pylori became a large focus in the community, studies into GHP dispersed (60-62). In 1996, Ginsberg et al. linked GHP size with their malignant potential, suggesting that GHP >0.5 cm should be removed (63). The same year another study, also investigating GHP malignant potential, found an incidence of malignant transformation in 1.8% of GHP. The study further investigated possible risk factors for malignant transformation, determining them to be advanced age and size >2 cm. Further histological analysis of GHP noted p53 expression in GHP cancerous areas (40). Murakami et al. also revealed that p53 plays an important role in GHP dysplastic changes and in 2011 another study reported carcinomatous foci expressed p53 as well as high Ki-67 labeling (19,46). In 2001, Abraham et al. again found a strong association between gastritis and GHP development as active chronic H. pylori gastritis and autoimmune gastritis were within the most common mucosal pathologies encountered in GHP patients (16). In 2006, it was reported that 51.3% of patients with GHP suffered from autoimmune gastritis while 37.3% had chronic active H. pylori gastritis, further supporting these associations (25). In 2003, GHP malignant potential was confirmed by identifying chromosomal aberrations in GHP lesions (51). A few years later, Al-Haddad et al. investigated the relationship between GHP and anemia, reporting that GHP may be a significant cause of gastrointestinal bleeding and that excision may be required for resolution of the bleeding (37). Following this, the first comprehensive review on GHP can be found, summarizing the past and present discoveries surrounding GHP (4). Around the same time, more studies were released regarding GHP management. Carmack et al. and Goddard et al., both agreed that when GHP are encountered, mucosal biopsies from other gastric regions are required. It was also mentioned that testing for H. pylori and eradicating any possible infection is critical. Even though, no clear conclusion was reached regarding GHP removal, those studies also suggested that at least biopsies must be taken when GHP are encountered (64,65). Over the coming years many studies attempted to discern the size of GHP that would require excision. In 2013, comprehensive reviews by Islam et al. and Shaib et al., highlighted the need to biopsy surrounding gastric mucosa. Shaib et al. suggested that GHP >1 cm should be excised while Islam et al. lowered the threshold to 0.5 cm and recommended a 1-year repeat surveillance endoscopy (54,55). Ahn et al. later concluded that GHP size larger than 1cm may indicate presence of neoplastic lesions (12). In 2017, another study also suggested that GHP >1 cm must be removed, rather than biopsied, and emphasized the need for mucosa sampling when GHP are encountered (5). More recently, Hu et al. conducted a study comparing GHP that showed neoplastic changes with non-neoplastic GHP. Even though this study revealed again that GHP may undergo malignant transformation, the underlying mechanism was not completely understood (38). The acknowledgment that the exact mechanism of GHP development is unclear was also addressed in a more recent literature review by Waldum et al. (7) (Figure 5).
In conclusion, strong evidence exists regarding GHP prevalence, symptoms, associations, and malignant potential. Even though, recently, a consensus has been reached regarding their endoscopic management, there is still a gray area regarding the management of GHP measuring 0.5–1 cm. Despite multiple studies, to date, have continuously brought to light the importance of managing chronic inflammation, the need for biopsies to assess for chronic gastritis and the potential for GHP malignant transformation, the exact pathophysiologic mechanism leading to GHP development and the underlying process resulting in their malignant transformation has yet to be elucidated and may be a topic for further studies.
Conclusions
Even though GHP are frequently discovered during endoscopy, comprising a significant proportion of polyps encountered, their incidence has significantly decreased the last years after successful eradication of H. pylori infection which along with autoimmune and atrophic gastritis and PPI use are major risk factors leading to hypergastrinemia and GHP development. Patients affected by GHP, won’t frequently exhibit overt symptomatology but when they do, non-specific signs and symptoms such as dyspepsia, reflux disease, chest pain may be observed. It is crucial that GHP are vascular lesions that can exhibit slow blood oozing, leading to iron-deficiency anemia, which often is the only sign in patients affected by GHP. Classically, GHP are considered benign, however growing number of evidence, reveal their malignant potential, directly correlated with their size. Due to that risk, current evidence suggest removal of GHP especially if >1 cm. However, the exact mechanism and genetics of GHP has yet to be understood and more studies are needed to elucidate the underlying pathophysiology, that will lead to better understanding of GHP, and their potential for malignant transformation. Lastly, the current review should be considered with some limitations. Some of the main limitations include that only articles in English language were considered, only articles published until December 2021 were analyzed and there is a lack of mathematical analysis in the creation of this narrative review.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-38/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Borch K, Skarsgård J, Franzén L, et al. Benign gastric polyps: morphological and functional origin. Dig Dis Sci 2003;48:1292-7. [Crossref] [PubMed]
- Forté E, Petit B, Walter T, et al. Risk of neoplastic change in large gastric hyperplastic polyps and recurrence after endoscopic resection. Endoscopy 2020;52:444-53. [Crossref] [PubMed]
- Kővári B, Kim BH, Lauwers GY. The pathology of gastric and duodenal polyps: current concepts. Histopathology 2021;78:106-24. [Crossref] [PubMed]
- Jain R, Chetty R. Gastric hyperplastic polyps: a review. Dig Dis Sci 2009;54:1839-46. [Crossref] [PubMed]
- Castro R, Pimentel-Nunes P, Dinis-Ribeiro M. Evaluation and management of gastric epithelial polyps. Best Pract Res Clin Gastroenterol 2017;31:381-7. [Crossref] [PubMed]
- Jeong CY, Kim N, Lee HS, et al. Risk Factors of Multiple Gastric Polyps according to the Histologic Classification: Prospective Observational Cohort Study. Korean J Gastroenterol 2019;74:17-29. [Crossref] [PubMed]
- Waldum H, Fossmark R. Gastritis, Gastric Polyps and Gastric Cancer. Int J Mol Sci 2021;22:6548. [Crossref] [PubMed]
- João M, Areia M, Alves S, et al. Gastric Hyperplastic Polyps: A Benign Entity? Analysis of Recurrence and Neoplastic Transformation in a Cohort Study. GE Port J Gastroenterol 2021;28:328-35. [Crossref] [PubMed]
- Nelson M, Ganger D, Keswani R, et al. Endoscopic resection is effective for the treatment of bleeding gastric hyperplastic polyps in patients with and without cirrhosis. Endosc Int Open 2016;4:E874-7. [Crossref] [PubMed]
- Nayudu SK, Niazi M, Balar B, et al. A rare complication of hyperplastic gastric polyp. Case Rep Gastrointest Med 2013;2013:631975. [Crossref] [PubMed]
- Vieth M, Stolte M. Elevated risk for gastric adenocarcinoma can be predicted from histomorphology. World J Gastroenterol 2006;12:6109-14. [Crossref] [PubMed]
- Ahn JY, Son DH, Choi KD, et al. Neoplasms arising in large gastric hyperplastic polyps: endoscopic and pathologic features. Gastrointest Endosc 2014;80:1005-13.e2. [Crossref] [PubMed]
- Cao H, Wang B, Zhang Z, et al. Distribution trends of gastric polyps: an endoscopy database analysis of 24 121 northern Chinese patients. J Gastroenterol Hepatol 2012;27:1175-80. [Crossref] [PubMed]
- Gao W, Huang Y, Lu S, et al. The clinicopathological characteristics of gastric polyps and the relationship between fundic gland polyps, Helicobacter pylori infection, and proton pump inhibitors. Ann Palliat Med 2021;10:2108-14. [Crossref] [PubMed]
- Horvath B, Pai RK. Prevalence of Helicobacter pylori in Gastric Hyperplastic Polyps. Int J Surg Pathol 2016;24:704-8. [Crossref] [PubMed]
- Abraham SC, Singh VK, Yardley JH, et al. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol 2001;25:500-7. [Crossref] [PubMed]
- Sonnenberg A, Genta RM. Prevalence of benign gastric polyps in a large pathology database. Dig Liver Dis 2015;47:164-9. [Crossref] [PubMed]
- Hongo M, Fujimoto KGastric Polyps Study Group. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol 2010;45:618-24. [Crossref] [PubMed]
- Murakami K, Mitomi H, Yamashita K, et al. p53, but not c-Ki-ras, mutation and down-regulation of p21WAF1/CIP1 and cyclin D1 are associated with malignant transformation in gastric hyperplastic polyps. Am J Clin Pathol 2001;115:224-34. [Crossref] [PubMed]
- Di Giulio E, Lahner E, Micheletti A, et al. Occurrence and risk factors for benign epithelial gastric polyps in atrophic body gastritis on diagnosis and follow-up. Aliment Pharmacol Ther 2005;21:567-74. [Crossref] [PubMed]
- Ohkusa T, Takashimizu I, Fujiki K, et al. Disappearance of hyperplastic polyps in the stomach after eradication of Helicobacter pylori. A randomized, clinical trial. Ann Intern Med 1998;129:712-5. [Crossref] [PubMed]
- Nam SY, Park BJ, Ryu KH, et al. Effect of Helicobacter pylori infection and its eradication on the fate of gastric polyps. Eur J Gastroenterol Hepatol 2016;28:449-54. [Crossref] [PubMed]
- Ouyang Y, Zhang W, Huang Y, et al. Effect of Helicobacter pylori eradication on hyperplastic gastric polyps: A systematic review and meta-analysis. Helicobacter 2021;26:e12838. [Crossref] [PubMed]
- Nam SY, Lee SW, Jeon SW, et al. Helicobacter pylori Eradication Regressed Gastric Hyperplastic Polyp: A Randomized Controlled Trial. Dig Dis Sci 2020;65:3652-9. [Crossref] [PubMed]
- Dirschmid K, Platz-Baudin C, Stolte M. Why is the hyperplastic polyp a marker for the precancerous condition of the gastric mucosa? Virchows Arch 2006;448:80-4. [Crossref] [PubMed]
- Kim GH. Proton Pump Inhibitor-Related Gastric Mucosal Changes. Gut Liver 2021;15:646-52. [Crossref] [PubMed]
- Zeng SX, Liang YP, Wu XY, et al. Gastroesophageal reflux is associated with an increased risk of gastric cardiac polyps: a case-control study of 140 cases. Ann Palliat Med 2021;10:7173-83. [Crossref] [PubMed]
- Markowski AR, Markowska A, Guzinska-Ustymowicz K. Pathophysiological and clinical aspects of gastric hyperplastic polyps. World J Gastroenterol 2016;22:8883-91. [Crossref] [PubMed]
- Livovsky DM, Pappo O, Skarzhinsky G, et al. Gastric Polyp Growth during Endoscopic Surveillance for Esophageal Varices or Barrett's Esophagus. Isr Med Assoc J 2016;18:267-71. [PubMed]
- Markowski AR, Guzinska-Ustymowicz K. Gastric hyperplastic polyp with focal cancer. Gastroenterol Rep (Oxf) 2016;4:158-61. [Crossref] [PubMed]
- Gencosmanoglu R, Sen-Oran E, Kurtkaya-Yapicier O, et al. Antral hyperplastic polyp causing intermittent gastric outlet obstruction: case report. BMC Gastroenterol 2003;3:16. [Crossref] [PubMed]
- Aydin I, Ozer E, Rakici H, et al. Antral hyperplastic polyp: A rare cause of gastric outlet obstruction. Int J Surg Case Rep 2014;5:287-9. [Crossref] [PubMed]
- Pontone S, Pironi D, Eberspacher C, et al. Endoscopic management of multiple large antral hyperplastic polyps causing gastric outlet obstruction. Ann Ital Chir 2011;82:297-300. [PubMed]
- Parikh M, Kelley B, Rendon G, et al. Intermittent gastric outlet obstruction caused by a prolapsing antral gastric polyp. World J Gastrointest Oncol 2010;2:242-6. [Crossref] [PubMed]
- Erdoğan Z, Silov G, Ozdal A, et al. Enterogastroesophageal reflux detected on 99m-technetium sestamibi cardiac imaging as a cause of chest pain. Indian J Nucl Med 2013;28:45-8. [Crossref] [PubMed]
- Tarar ZI, Tahan V, Yin F, et al. An Unusual Case of Esophageal Hyperplastic Polyp with Scleroderma: A Case Report and Review of the Literature. Cureus 2021;13:e12500. [Crossref] [PubMed]
- Al-Haddad M, Ward EM, Bouras EP, et al. Hyperplastic polyps of the gastric antrum in patients with gastrointestinal blood loss. Dig Dis Sci 2007;52:105-9. [Crossref] [PubMed]
- Hu H, Zhang Q, Chen G, et al. Risk factors and clinical correlates of neoplastic transformation in gastric hyperplastic polyps in Chinese patients. Sci Rep 2020;10:2582. [Crossref] [PubMed]
- Chan JCW, Liu HSY, Kho BCS, et al. Pernicious anemia in Chinese: a study of 181 patients in a Hong Kong hospital. Medicine (Baltimore) 2006;85:129-38. [Crossref] [PubMed]
- Zea-Iriarte WL, Sekine I, Itsuno M, et al. Carcinoma in gastric hyperplastic polyps. A phenotypic study. Dig Dis Sci 1996;41:377-86. [Crossref] [PubMed]
- Laxén F. Gastric carcinoma and pernicious anaemia in long-term endoscopic follow-up of subjects with gastric polyps. Scand J Gastroenterol 1984;19:535-40. [Crossref] [PubMed]
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784-9. [Crossref] [PubMed]
- Seifert E, Gail K, Weismüller J. Gastric polypectomy. Long-term results (survey of 23 centres in Germany). Endoscopy 1983;15:8-11. [Crossref] [PubMed]
- Daibo M, Itabashi M, Hirota T. Malignant transformation of gastric hyperplastic polyps. Am J Gastroenterol 1987;82:1016-25. [PubMed]
- Orlowska J, Jarosz D, Pachlewski J, et al. Malignant transformation of benign epithelial gastric polyps. Am J Gastroenterol 1995;90:2152-9. [PubMed]
- Terada T. Malignant transformation of foveolar hyperplastic polyp of the stomach: a histopathological study. Med Oncol 2011;28:941-4. [Crossref] [PubMed]
- TP53 tumor protein p53 [Homo sapiens (human)] [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited YYYY Mmm DD]. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=7157
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311-22. [Crossref] [PubMed]
- Takayama Y, Ono Y, Mizukami Y, et al. Comparative genome-wide analysis of gastric adenocarcinomas with hyperplastic polyp components. Virchows Arch 2019;475:383-9. [Crossref] [PubMed]
- Salomao M, Luna AM, Sepulveda JL, et al. Mutational analysis by next generation sequencing of gastric type dysplasia occurring in hyperplastic polyps of the stomach: Mutations in gastric hyperplastic polyps. Exp Mol Pathol 2015;99:468-73. [Crossref] [PubMed]
- Weiss MM, Kuipers EJ, Postma C, et al. Genome wide array comparative genomic hybridisation analysis of premalignant lesions of the stomach. Mol Pathol 2003;56:293-8. [Crossref] [PubMed]
- Saab J, Estrella JS, Chen YT, et al. Immunohistochemical and molecular features of gastric hyperplastic polyps. Adv Cytol Pathol 2017;2:25-31.
- Dijkhuizen SM, Entius MM, Clement MJ, et al. Multiple hyperplastic polyps in the stomach: evidence for clonality and neoplastic potential. Gastroenterology 1997;112:561-6. [Crossref] [PubMed]
- Islam RS, Patel NC, Lam-Himlin D, et al. Gastric polyps: a review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol (N Y) 2013;9:640-51. [PubMed]
- Shaib YH, Rugge M, Graham DY, et al. Management of gastric polyps: an endoscopy-based approach. Clin Gastroenterol Hepatol 2013;11:1374-84. [Crossref] [PubMed]
- Gonzalez-Obeso E, Fujita H, Deshpande V, et al. Gastric hyperplastic polyps: a heterogeneous clinicopathologic group including a distinct subset best categorized as mucosal prolapse polyp. Am J Surg Pathol 2011;35:670-7. [Crossref] [PubMed]
- Spechler SJ, Merchant JL, Wang TC, et al. A Summary of the 2016 James W. Freston Conference of the American Gastroenterological Association: Intestinal Metaplasia in the Esophagus and Stomach: Origins, Differences, Similarities and Significance. Gastroenterology 2017;153:e6-e13. [Crossref] [PubMed]
- Bordi C, Bertelè A, Davighi MC, et al. Clinical and pathological associations of argyrophil cell hyperplasias of the gastric mucosa. Appl Pathol 1984;2:282-91. [PubMed]
- Nakano H, Persson B, Slezak P. Study of the gastric mucosal background in patients with gastric polyps. Gastrointest Endosc 1990;36:39-42. [Crossref] [PubMed]
- Veereman Wauters G, Ferrell L, Ostroff JW, et al. Hyperplastic gastric polyps associated with persistent Helicobacter pylori infection and active gastritis. Am J Gastroenterol 1990;85:1395-7. [PubMed]
- Suzuki S, Ohkusa T, Shimoi K, et al. Disappearance of multiple hyperplastic polyps after the eradication of Helicobacter pylori. Gastrointest Endosc 1997;46:566-8. [Crossref] [PubMed]
- Ohkusa T, Miwa H, Hojo M, et al. Endoscopic, histological and serologic findings of gastric hyperplastic polyps after eradication of Helicobacter pylori: comparison between responder and non-responder cases. Digestion 2003;68:57-62. [Crossref] [PubMed]
- Ginsberg GG, Al-Kawas FH, Fleischer DE, et al. Gastric polyps: relationship of size and histology to cancer risk. Am J Gastroenterol 1996;91:714-7. [PubMed]
- Carmack SW, Genta RM, Graham DY, et al. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009;6:331-41. [Crossref] [PubMed]
- Goddard AF, Badreldin R, Pritchard DM, et al. The management of gastric polyps. Gut 2010;59:1270-6. [Crossref] [PubMed]
Cite this article as: Zouridis S, Michael M, Arker SH, Sangha M, Batool A. Gastric hyperplastic polyps: a narrative review. Dig Med Res 2023;6:8.