
Mediastinoscopic esophagectomy for esophageal cancer: will this procedure achieve radical esophagectomy?—a narrative review
Introduction
Esophagectomy is performed using transthoracic and transmediastinal approaches. In cancer surgery, it is necessary to resect the primary lesion and dissect the locoregional lymph nodes. In addition, it is important secure the safety and reduce the invasiveness of the procedure. We herein describe the history and development of the procedures of esophagectomy.
Esophagectomy with dissection of the mediastinal and abdominal lymph nodes or with the addition of cervical lymph node dissection is needed for the radical treatment of thoracic esophageal cancer with invasion of the submucosal layer or deeper layers. For the abovementioned reason, transthoracic and abdominal esophagectomy {the Ivor-Lewis procedure [1946]} (1) and total thoracic esophagectomy with additional neck lymph node dissection {the McKeown procedure [1969]} were developed (2,3). Lymph node dissection of the upper mediastinum was expanded to improve the curability by the 1980s. On the other hand, the incidence of complications, such as recurrent laryngeal nerve palsy (RLNP) and tracheobronchial ischemia increased. As the rationalization of upper mediastinal lymph node dissection has progressed, these complications have decreased (4).
Currently, radical esophagectomy through right thoracotomy has changed to esophagectomy via a thoracoscopic and/or laparoscopic approach, including robot-assisted surgery, which reduces the invasiveness of the procedure by decreasing the destruction of the thoracic and abdominal walls (5-11). As a result, the transthoracic approach has now gained curability, safety and minimal invasiveness, and has become the standard surgical procedure for esophageal cancer.
On the other hand, conventional transhiatal esophagectomy (THE) {blunt dissection [1978]} (12), has been developed for mediastinal esophagectomy. This procedure is also recognized as a type of minimally invasive esophagectomy (MIE) (12-14). However, due to the blind maneuvering in the upper and middle mediastinum, which is necessary in this procedure, as well as the difficulty of systematic lymph node dissection, it is usually only applied in limited cases, such as cases of esophagogastric junction cancer, very early-stage cancers, or some cases of advanced thoracic esophageal cancer for the purpose of palliative resection (13). Thus, these procedures have not been used for radical esophagectomy because the mediastinal lymph node dissection is less radical.
Recently, mediastinoscopic radical esophagectomy under pneumomediastinum has been developed. This has the potential to be the ultimate procedure for esophagectomy, based on its reduced invasiveness and its radicality. Mediastinoscopic esophagectomy under pneumomediastinum was considered as a breakthrough for the THE.
In this review, we describe the development of mediastinoscopic esophagectomy, including the transition of esophagectomy until the present time. In addition, we discuss whether or not mediastinoscopic esophagectomy can become a radical procedure.
Regarding the lymph node names and numbers, we used the Japanese Classification of Esophageal Cancer, 11th Edition (15,16).
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-64).
Methods
The research strategy was based on analyzing and reviewing recent principal research and articles on mediastinoscopic esophagectomy. In addition, the transition of esophagectomy is reviewed. All downloaded papers were collected from PubMed Central, Medline, Google Scholar, Web of Science, and various open access journals.
In addition, we examine whether or not mediastinoscopic esophagectomy can become a radical procedure, from the viewpoint of integrity of mediastinal lymph node dissection and the short-term outcomes in the recent literature. In addition, the indication of this procedure is discussed.
Discussion
Development of esophagectomy
Only 100 years ago, esophagectomy was considered a very difficult procedure and was associated with high mortality. In 1913, Torek successfully performed esophageal resection for thoracic esophageal cancer for the first time in the world at 1913 (17,18). At first, the thoracic esophagus including thoracic esophageal cancer, was resected using left thoracotomy, then cervical esophagostomy and gastrostomy were performed with rubber tube replacement. Subsequently, the esophagostoma and gastrostoma were connected with a rubber tube [1913] (17,18).
Later, Adams and Phemister reported a successful case of esophagectomy for lower thoracic esophageal cancer (19). Garlock successfully performed esophageal resection for three cases in 1938. The performance of anesthesia using intra-tracheal intubation in the United States of America, enabled the performance of transthoracic surgery. Garlock [1944] reported that the success of esophagogastric suturing on the cranial side of the aortic arch using whole stomach after resection of the esophagus for carcinoma of the upper and middle thoracic esophagus using left thoracotomy (20). The patient had an esophageal tumor of approximately 2 inches in length below the superior margin of the aortic arch. The supra-aortic portion of the esophagus was mobilized and the entire organ was drawn upward from behind the aortic arch. The pulling through procedure was completed and the mobilized organ rested over the aortic arch. The esophagus was divided well above the tumor. Esophagogastric suturing was performed at the cranial side of the aortic arch using the whole stomach. In this case, the two upper branches of the left gastric artery were divided.
Later, Sweet [1945] reported transthoracic resection of the esophagus and stomach for carcinoma (21). In this article, he reported 11 cases of esophagectomy and esophagogastric suturing at the cranial side of the aortic arch using whole stomach for esophageal carcinoma of the middle thoracic esophagus.
Unfortunately, the short-term outcomes of esophagectomy for upper and middle thoracic esophageal cancers were very poor. Garlock et al. [1952] reported 91 cases of esophagectomy for upper and middle thoracic esophageal cancers and noted that the operative mortality rate was 38.4% (35/91) (22). Sweet et al. [1952] reported the 107 cases of esophagectomy for thoracic esophageal cancers and noted that the operative mortality rate was 24.3% (26/107) (23). Nakayama [1952] reported 70 cases of esophagectomy for thoracic esophageal cancers and noted that the operative mortality of rate was 14.2% (10/70) (24). McKeown [1969] developed total esophagectomy with a “three-phase operation” (2,3). He performed esophagogastric anastomosis via a right cervical approach instead of a left approach. The article did not report the performance of systematic lymph node dissection. Later, esophagectomy with lymph node dissection was gradually established.
In Japan, Akiyama et al. [1981] reported the significance of extensive lymph node dissection in the posterior mediastinum and abdomen during resection of esophageal carcinomas (4). Okuma et al. (25) reported the performance of transthoracic esophagectomy with three-field dissection (mediastinal, abdominal, and cervical-LN), followed by esophageal reconstruction in 68 cases. They described that metastatic cervical lymph nodes were detected in 20 (29.4%) patients. Thus, three field dissection is needed to improve the prognosis of thoracic esophageal cancer. Below, the classification of esophagectomy and its history are described.
Types of esophagectomy
Esophagectomy is classified as radical esophagectomy or palliative esophagectomy. The characteristics of each method of esophagectomy are shown in Table 1.
Table 1
| Method/approach | Target lesion | Lymphadenectomy | Invasiveness | Radicality | Safety | Specific Risks | ||
|---|---|---|---|---|---|---|---|---|
| Location | Depth | Others | ||||||
| Thoracotomy | ||||||||
| Ivor-Lewis procedure | Mt, Lt, Ae | ALL | N/A | Two-field | ++ | ++ | ++ | Pneumoniae |
| McKeown procedure | ALL | ALL | N/A | Three-field | ++ | ++ | ++ | Pneumoniae |
| Transhiatal | ||||||||
| Blunt esophagectomy | ALL | Superficial | Poor risk | Poor | + | − | − | Bleeding |
| Esophageal stripping | ALL | Superficial | Poor risk | Poor | + | − | − | Bleeding |
| Endodissection | ALL | Superficial | Poor risk | Poor | + | − | − | Bleeding |
| MATHE | ALL | Superficial | Poor risk | Poor-slightly | + | + | − | Bleeding |
| Mediastinoscopic | ALL* | ALL | N/A | Three-field | + | + or ++ | + | RLNP, Bleeding |
*, except for the upper thoracic region in cases with a bulky tumor. Ae, abdominal esophagus; Lt, lower thoracic esophagus; MATHE, mediastinoscope-assisted transhiatal esophagectomy; Mediastinoscopic, mediastinoscopic esophagectomy; Mt, middle thoracic esophagus; N/A, not applicable; RLNP, recurrent laryngeal nerve palsy; Thoracotomy, esophagectomy through a right thoracotomy; Three-field, three-field lymphadenectomy; Transhiatal, transhiatal esophagectomy; Two-field, two-field lymphadenectomy.
Radical esophagectomy (radical lymph node dissection)
Ivor-Lewis method
In Western countries, abdominal esophageal cancer and esophagogastric junctional cancers are well observed and the histological types are frequently diagnosed as “adenocarcinoma”. In these cases, two-field abdominal-thoracic lymph node dissection is performed with reconstruction in the thoracic lesion using the gastric conduit. This is called the Ivor-Lewis method (1).
McKeown method
Esophageal cancers in the middle thoracic or more proximal esophagus are frequently associated with upper mediastinal and cervical lymph node metastasis. Thus, the Ivor-Lewis method is not suitable for these esophageal cancers. To resolve this problem, In 1969, McKeown developed total esophagectomy with additional neck lymph node dissection (2,3). This procedure came to be widely performed. Currently, radical esophagectomy through right thoracotomy has changed to esophagectomy via a thoracoscopic or laparoscopic approach, which reduces the invasiveness of the procedure by decreasing the destruction of the thoracic and abdominal walls. At first, thoracoscopic esophagectomy was performed in the left lateral decubitus position. Later [2006], thoracoscopic esophagectomy in the prone position was developed (26). This procedure is widely used, and it is mainly performed as thoracoscopic esophagectomy. Recently, thoracic surgery has been replaced by robot-assisted surgery. However, in thoracoscopic esophagectomy, including robot-assisted surgery, lung collapse using artificial pneumothorax or pulmonary exclusion is necessary. Thus, there are concerns that pulmonary complications will be induced. Takeuchi et al. reported that MIE was associated with a significantly lower amount of bleeding and lower incidence of prolonged respiratory ventilation (>48 h) in comparison to open esophagectomy (OE) group in a retrospective study of the short-term outcomes of MIE (11). This report indicates that incidence of necrosis of the gastric conduit and reoperation rate within 30 days were significantly higher in the MIE group than in the OE group. Thus, a prospective study (JCOG1409) has been scheduled and is under registration in Japan.
Palliative esophagectomy (palliative lymph node dissection)
Palliative esophagectomy has long been performed. Transhiatal esophagectomy (blunt dissection) is one of the methods by which this is performed.
Blunt esophagectomy (Transhiatal esophagectomy)
In 1981, Orringer and Sloan reported the performance of “blunt esophagectomy” without thoracotomy in 26 patients: four with benign disease and 22 with carcinomas involving various levels of the esophagus (12). The average intraoperative blood loss was 1,350 ml. Complications in these patients included pneumothorax (8 cases), transient hoarseness (5 cases), anastomotic leakage (4 cases) and cerebrovascular accident (1 case). Because this method does not require thoracotomy and the esophagus is dissected using a transhiatal and trans-left cervical approach, so it is considered to be a type of MIE. However, this method is a blind maneuver; thus, the systematic dissection of the upper and middle mediastinal lymph nodes is difficult.
Akiyama reported that the technique of esophagectomy without thoracotomy should not be used for malignant lesions of the thoracic esophagus, except under particular circumstances, such as resection of carcinoma in an extremely early stage or palliative resection of advanced carcinoma of the thoracic esophagus without local invasion (13).
Transhiatal esophagectomy using special devices
Akiyama et al. reported eversion esophageal stripping using a vein stripper (13,27). In this technique, although the esophagus can be removed relatively easily, it is necessary to pay sufficient attention to contamination of the surgical field because the mucosal layer side is outside. This surgery is a good indication for elderly patients, cases of severe lung disorders in which it is desirable to avoid thoracotomy, and early cancer of the thoracic esophagus. In advanced esophageal cancer, it is not indicated because of curability.
In 1993, Bumm et al. reported on non-transthoracic esophagectomy using an “endodissector” (28,29). Endodissection was performed by a left cervical approach. A 10- to 15-cm incision was made at the anterior edge of the musculus sternocleidomastoideus. This instrument was inserted anterior and posterior of the esophagus and dissection was performed. The instrument carried a multifunctional handle that could be rotated 360 degrees and enabled dissection of the esophagus on all sides. Thirty unselected patients, mostly with adenocarcinoma of the esophagus, underwent endodissection. The clinical results of the 30 unselected patients who underwent endodissection, were compared with those of 30 patients who underwent conventional THE and who were selected using a matched pair algorithm. In the endodissection group, adenocarcinoma of the esophagus was most frequent and early tumor stages were predominantly observed. Tumors were mostly located below the tracheal bifurcation. Three significant intraoperative complications were recorded during endodissection (mediastinal bleeding, 1 case; postoperative bleeding, 1 case; and lesion of the right main bronchus, 1 case), all were managed without further morbidity. The mortality rate (30 days) was 6.6% (2 cases) in the endodissection group and 9.9% (3 cases) in the THE group; the difference was not statistically significant. One patient died of septic organ failure caused by interponate necrosis; the other had acute cardiorespiratory failure at 14 days after the operation. There were no deaths associated with endodissection in this study. The frequency of postoperative severe pulmonary complications was 13.3% (4 cases) in the endodissection group and 30% (9 cases) in the THE group (P<0.05). The rate of recurrent nerve palsy was only 6.6% (2 cases) in the endodissection group and 13.3% (4 cases) in THE group, and was not statistically significant. Radical mediastinal lymph node dissection was difficult in this technique due to the field of view.
Tangoku et al. reported that mediastinoscope-assisted transhiatal esophagectomy (MATHE) was performed in 41 patients with esophageal cancer, who were chiefly diagnosed with superficial esophageal cancer and had medical risk factors (14). The average operative time was 269 min and the average total blood loss was 449 g. Regarding complications, 10 patients (24.4%) had pulmonary infiltration. RLNP was recorded in 15 patients (36.6%). The palsy was only recognized on the left side, and with the exception of one patient, all patients recovered within 2 months. Anastomotic leakage occurred in 4 patients (9.8%) and there was one hospital death due to cerebral infarction on the 29th postoperative day.
The former procedure—radical esophagectomy through right thoracotomy—has changed to thoracoscopic esophagectomy and robot-assisted esophagectomy, which reduces the invasiveness. In the later THE procedure—so-called “blunt dissection”, which do not require thoracotomy—lymphadenectomy was more widely achieved under pneumomediastinum, which maintains the surgical field of view. Mediastinoscopic esophagectomy using pneumomediastinum has been developed and applied to clinical cases.
Mediastinoscopic esophagectomy using pneumomediastinum
Ikeda et al. reported a method of mediastinoscopic esophagostomy via a left cervical incision (30). Two 5-mm trocars for endoscopic instruments were positioned, 1 at the suprasternal notch and 1 lateral to the incision. A 12-mm trocar was inserted through the incision, and a purse-string suture was made to prevent gas leakage and dislocation of the trocar. In addition, the 106recL lymph nodes were dissected using a left cervical approach under pneumomediastinum because the surgical field of view via a left cervical incision is better than that of right thoracoscopy. In 2002, they reported on hybrid surgery using mediastinoscopic and thoracoscopic procedures (31).
Parker et al. reported on mediastinoscopic surgery using a single incision laparoscopic surgery (SILS) port via a left cervical incision (32). However, this technique was mainly used for the patients with severe dysplasia or adenocarcinoma who had poor risk factors and esophagectomy with two-field lymph node dissection was performed.
In 2015, Tokairin et al. developed and reported a method that enabled complete dissection of the upper mediastinal lymph nodes in a cadaver study because they thought that the above-mentioned lymph node dissection was still insufficient for patients with thoracic esophageal cancer, which is common in Japan (33). In 2017, they reported the application of this method in the clinical setting (34,35).
Fujiwara et al. reported the perioperative outcomes of 60 patients with thoracic esophageal cancer who underwent mediastinoscopic esophagectomy (36). The median operation time and blood loss were 363 minutes and 235 mL, respectively. There were two patients who underwent conversion to thoracotomy. Postoperatively, pneumonia was observed in four patients (CD, Grade II, 2 cases; Grade IIIb, 2 cases), although vocal cord palsy was observed relatively frequently (20 cases; 33.3%).
Mediastinoscopic procedure performed at our institute
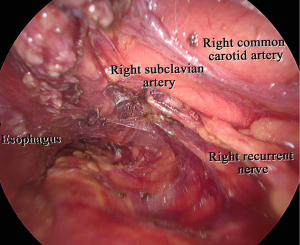
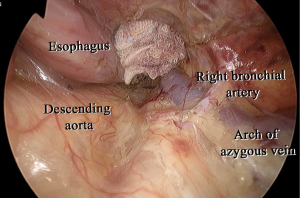
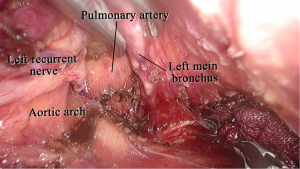
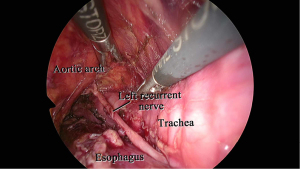
In our procedure, the right recurrent nerve is first identified using an open approach, and the right cervical paraesophageal lymph nodes and part of the right recurrent nerve lymph nodes are dissected, after which pneumomediastinum is initiated. The location of the ports and single-port laparoscopic access devices are indicated in Figure 1. and dissection via a right transcervical approach under pneumomediastinum is illustrated in Figure 2. The dorsal side of the esophagus is dissected along the visceral sheath (37), taking care to avoid thoracic duct injury, and is then dissected along the vascular sheath in front of the descending aorta (Figure 3). The esophagus is dissected from the trachea at the caudal side of the aortic arch, and then dissected along the ventral side of the left main bronchus, reaching the pulmonary artery (Figure 4). Finally, the right recurrent nerve lymph nodes around the right subclavian artery are completely retrieved. The left cervical approach is almost the same as that via the right side. The dorsal side of the esophagus is almost dissected along the visceral sheath with a right transcervical approach. The ventral side of the esophagus is dissected from the left side of the membranous portion of the trachea, the left main bronchus, and the aortic arch. The left recurrent nerve lymph nodes are dissected along the dorsal side of the left recurrent nerve. Next, the ventral side of the left recurrent nerve lymph nodes is dissected along the left recurrent nerve (Figure 5). Finally, the subaortic arch to the left tracheobronchial lymph nodes are dissected using the crossover technique (34,35,38,39). Then, laparoscopic surgery is performed to dissect the upper abdominal lymph nodes and achieve gastric conduit mobilization (Figure 1). Transhiatally, the middle to lower thoracic esophagus and mediastinal lymph nodes, including the subcarinal nodes, are dissected. The gastric conduit is pulled through the post-sternal route into the left cervical area, where esophagogastric anastomosis is performed.
Can mediastinoscopic esophagectomy become radical esophagectomy?
For esophageal resection, esophagectomy through a right thoracotomy, thoracoscopic esophagectomy, THE and mediastinoscopic esophagectomy are currently performed. The characteristics of each method of esophagectomy are shown in Table 2. Feasibility, safety, and oncological effectiveness are required for radical esophagectomy. Oncological effectiveness means the performance of complete tumor resection and regional lymphadenectomy. If the procedure can also be performed with less invasiveness, it would be an ideal procedure for esophagectomy.
Table 2
| Method | Invasiveness | Radicality | Safety |
|---|---|---|---|
| Thoracotomy | +++ | ++ | ++ |
| Thoracoscopic | ++ | ++ | + |
| Transhiatal | + | − | − |
| Mediastinoscopic | + | + or ++ | + |
Mediastinoscopic, mediastinoscopic esophagectomy; thoracoscopic, thoracoscopic esophagectomy; thoracotomy, esophagectomy through a right thoracotomy; transhiatal, transhiatal esophagectomy.
In order for the mediastinoscopic esophagectomy to be accepted as radical esophagectomy, it is required to not only be minimally invasive but to also have curative potential by allowing lymph node dissection. In this regard, our considerations are described below.
Integrity of lymph node dissection in mediastinoscopic esophagectomy
In this investigation, it is important to compare the integrity of mediastinal lymph node dissection of transthoracic esophagectomy with that of mediastinoscopic esophagectomy. However, few studies have evaluated the number of lymph nodes dissected in mediastinoscopic esophagectomy. In 2019, Tokairin et al. reported a clinical trial in which radical esophagectomy, including mediastinal lymph node dissection, was performed by a mediastinoscopic procedure and verified the integrity of mediastinal lymph node dissection, followed by thoracoscopic observation and lymph node dissection (35,39). They reported the risk of leaving some lymph nodes in 106recR and in 106tbL if a right cervical approach under pneumomediastinum is not performed. Thus, they reported that a right cervical approach under pneumomediastinum is important for complete dissection of the mediastinal lymph nodes.
On the other hand, Fujiwara et al. [2017] reported a method of mediastinoscopic esophagectomy in which the right cervical procedure is first performed using an open approach, followed by a left cervical approach and transhiatal approach under pneumomediastinum (36). The mean number of resected lymph nodes was 37.0±11.8 in the thorax and abdomen, 22.4±8.9 in the thorax, 10.3±6.0 in the upper mediastinum, 12.1±5.8 in the middle and lower mediastinum, and 14.7±6.2 in the abdomen. In 2016, Mori et al. reported that there were no differences in the numbers of harvested mediastinal lymph nodes in their a non-transthoracic esophagectomy (NTTE) and conventional transthoracic esophagectomy (TTE) groups (median, 30 vs. 29) (40). Taken together, there is no defined opinion on what kind of mediastinoscopic esophagectomy is adequate to achieve the same level of lymphadenectomy in the transthoracic esophagectomy. The accumulation of clinical cases treated with mediastinoscopic procedures is expected to solve this problem.
Minimal invasiveness and safety of mediastinoscopic esophagectomy
(I) Short-term outcomes of mediastinoscopic esophagectomy
In the aforementioned article, Mori et al. developed and reported on a non-transthoracic esophagectomy (NTTE) procedure combining a video-assisted left cervical approach for the upper mediastinum and a robot-assisted transhiatal approach for the middle and lower mediastinum (40). The authors described 22 accumulated cases of NTTE, and verified the feasibility by analyzing perioperative and histopathological outcomes. They compared this group’s short-term outcomes with the outcomes of 139 equivalent esophageal cancer cases that were surgically treated by conventional transthoracic esophagectomy (TTE) in their institution (TTE group). In the NTTE group, the mean operation time was longer (median, 524 vs. 428 minutes). The estimated blood loss of the two groups did not differ to a statistically significant extent (median, 385 vs. 490 mL). However, in the NTTE group, the postoperative hospital stay was shorter (median, 18 vs. 24 days). No postoperative pneumonia occurred in the NTTE group. The frequency of other major postoperative complications did not differ to a statistically significant extent. In 2019, Tokairin et al. reported that no postoperative pneumonia occurred in patients treated with mediastinoscopic esophagectomy and noted that the incidence of respiratory complications in these patients was reduced (39).
On the other hand, Tokairin et al. (39) and Fujiwara et al. (36) reported that RLNP was occurred in 33–40% of patients treated with mediastinoscopic essophagectomy. Takeuchi et al. (11) reported that RLNP was observed at 8.2% of patients who underwent conventional transthoracic OE and 10.1% of patients who underwent thoracoscopic esophagectomy using data from a larger number of patients in the Japanese National Clinical Database (NCD). The frequency of RNLP in mediastinoscopic esophagectomy is considered to be high in comparison to OE and thoracoscopic esophagectomy. Because the movement of the forceps is tangential to the running of the left recurrent laryngeal nerve in mediastinoscopic esophagectomy, so the left recurrent nerve is likely to be pinched between the forceps and the tracheal wall, which may lead to an increase of RLNP. In addition, the tension on the recurrent laryngeal nerve may occur in unexpected situations. As a preventive measure, NIM monitoring, previously used in thyroid surgery (41), was used in esophagectomy. This procedure was reported to be useful for reducing the incidence of RLNP (42-48).
Surgical indication for mediastinoscopic esophagectomy and problems to be solved
Mediastinoscopic esophagectomy using pneumomediastinum is a recently reported and now continuously developing procedure. Thus, the indications of this procedure should be discussed. Because the field of view via the cervical and transhiatal approach is narrow, it is difficult to observe the structure in the back if an occupying lesion is present in the foreground. Thus, esophageal cancers with bulky tumors in the upper thoracic region or with bulky lymph nodes metastasis are excluded from the indications of this procedure in our institute. It is necessary to discuss the indications of this procedure.
We herein describe the problems of mediastinoscopic esophagectomy that remain to be solved. First, RLNP occurs relatively more frequently than transthoracic esophagectomy. As a preventive measure, NIM monitoring, previously used in thyroid surgery, is used in esophagectomy. In thoracoscopic esophagectomy, the incidence of RLNP is reportedly reduced using intraoperative nerve monitoring (IONM) (44,47,49). Although a preliminary article was reported in Japan, whether the incidence of RLNP is reduced in mediastinoscopic esophagectomy has not yet been reported formally. Further detailed reports are thus expected in the future. Second, surgical devices to keep the surgical field of view in this procedure should be developed. Finally, we should develop a procedure of lymph node dissection for the subaortic arch to the left tracheobronchial lymph nodes (35).
Based on the above, we conclude that the mediastinoscopic esophagectomy has the potential to be the ultimate esophagectomy procedure, based on its reduced invasiveness and its radicality. However, the incidence of RLNP should be reduced using intraoperative NIM monitoring (50). In addition, the indication of this procedure should be discussed. Robot (daVinci SP)-assisted mediastinoscopic esophagectomy is expected to be more widely performed in the future (51).
Conclusions
We described the transition of esophagectomy, the development of mediastinoscopic esophagectomy, and the evolution and future of this procedure. Although there are problems to be solved, this procedure has the potential to achieve radical esophagectomy.
Acknowledgments
Funding: This work was partly supported by JSPS KAKENHI (Grant Number 20K09002).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-64
Peer Review File: Available at https://dx.doi.org/10.21037/dmr-21-64
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-64). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- McKeown KC. Total oesophagectomy. British Medical Association, Film No. 439. 1969.
- McKeown KC. Trends in oesophageal resection for carcinoma with special reference to total oesophagectomy. Ann R Coll Surg Engl 1972;51:213-39. [PubMed]
- Akiyama H, Tsurumaru M, Kawamura T, et al. Principles of surgical treatment for carcinoma of the esophagus: analysis of lymph node involvement. Ann Surg 1981;194:438-46. [Crossref] [PubMed]
- Akaishi T, Kaneda I, Higuchi N, et al. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg 1996;112:1533-40; discussion 1540-1. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Kawahara K, Maekawa T, Okabayashi K, et al. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc 1999;13:218-23. [Crossref] [PubMed]
- Luketich JD, Schauer PR, Christie NA, et al. Minimally invasive esophagectomy. Ann Thorac Surg 2000;70:906-11; discussion 911-2. [Crossref] [PubMed]
- Nguyen NT, Follette DM, Lemoine PH, et al. Minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2001;72:593-6. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Ozawa S, et al. Comparison of Short-Term Outcomes Between Open and Minimally Invasive Esophagectomy for Esophageal Cancer Using a Nationwide Database in Japan. Ann Surg Oncol 2017;24:1821-7. [Crossref] [PubMed]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [Crossref] [PubMed]
- Akiyama H. Esophagectomy without thoracotomy. Surg Annu 1981;13:109-21. [PubMed]
- Tangoku A, Yoshino S, Abe T, et al. Mediastinoscope-assisted transhiatal esophagectomy for esophageal cancer. Surg Endosc 2004;18:383-9. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1-36.
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus 2017;14:37-65.
- Torek F. The first successful resection of the thoracic portion of the esophagus for carcinoma: preliminary report. JAMA 1913;60:1533. [Crossref]
- Torek F. The first successful case of resection of the thoracic portion of the oesophagus for carcinoma. Surg Gynecol Obstet 1913;16:614-7.
- Adams WE, Phemister DB. Carcinoma of the Lower Thoracic Esophagus. J Thor Surg 1938;7:621-32. [Crossref]
- Garlock JH. Re-establishment of esophagogastric continuity following resection of esophagus for carcinoma of middle third. Surg Gynecol Obstet 1944;78:23-8.
- Sweet RH. Transthoracic Resection of the Esophagus and Stomach for Carcinoma: Analysis of the Postoperative Complications, Causes of Death, and Late Results of Operation. Ann Surg 1945;121:272-84. [Crossref] [PubMed]
- GARLOCK JH. KLEIN SH. The surgical treatment of carcinoma of the esophagus and cardia; an analysis of 457 cases. Ann Surg 1954;139:19-34. [Crossref] [PubMed]
- SWEET RH. The results of radical surgical extirpation in the treatment of carcinoma of the esophagus and cardia with five year survival statistics. Surg Gynecol Obstet 1952;94:46-52. [PubMed]
- Nakayama K. Esophageal Surgery. The Medical Frontline 1952;7:17-28.
- Okuma T, Kaneko H, Yoshioka M, et al. Prognosis in esophageal carcinoma with cervical lymph node metastases. Surgery 1993;114:513-8. [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Kawamura T, et al. Esophageal stripping with preservation of the vagus nerve. Int Surg 1982;67:125-8. [PubMed]
- Bumm R, Feussner H, Bartels H, et al. Radical transhiatal esophagectomy with two-field lymphadenectomy and endodissection for distal esophageal adenocarcinoma. World J Surg 1997;21:822-31. [PubMed]
- Bumm R, Hölscher AH, Feussner H, et al. Endodissection of the thoracic esophagus. Technique and clinical results in transhiatal esophagectomy. Ann Surg 1993;218:97-104. [Crossref] [PubMed]
- Ikeda Y, Niimi M, Kan S, et al. Mediastinoscopic esophagectomy using carbon dioxide insufflation via the neck approach. Surgery 2001;129:504-6. [Crossref] [PubMed]
- Ikeda Y, Niimi M, Kan S, et al. Thoracoscopic esophagectomy combined with mediastinoscopy via the neck. Ann Thorac Surg 2002;73:1329-31. [Crossref] [PubMed]
- Parker M, Bowers SP, Goldberg RF, et al. Transcervical videoscopic esophageal dissection during two-field minimally invasive esophagectomy: early patient experience. Surg Endosc 2011;25:3865-9. [Crossref] [PubMed]
- Tokairin Y, Nagai K, Fujiwara H, et al. Mediastinoscopic subaortic and tracheobronchial lymph node dissection with a new cervico-hiatal crossover approach in thiel-embalmed cadavers. Int Surg 2015;100:580-8. [Crossref] [PubMed]
- Tokairin Y, Nakajima Y, Kawada K, et al. A Mediastinoscopic Approach With Bilateral Cervicopneumomediastinum in Radical Thoracic Esophagectomy. Int Surg 2017;102:278-83. [Crossref]
- Tokairin Y, Nakajima Y, Kawada K, et al. The usefulness of a bilateral trans-cervical pneumomediastinal approach for mediastinoscopic radical esophagectomy: a right transcervical approach is an available option. Gen Thorac Cardiovasc Surg 2019;67:884-90. [Crossref] [PubMed]
- Fujiwara H, Shiozaki A, Konishi H, et al. Perioperative outcomes of single-port mediastinoscope-assisted transhiatal esophagectomy for thoracic esophageal cancer. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Tokairin Y, Nakajima Y, Kawada K, et al. Histological study of the thin membranous structure made of dense connective tissue around the esophagus in the upper mediastinum. Esophagus 2018;15:272-80. [Crossref] [PubMed]
- Tokairin Y, Nakajima Y, Kawad K, et al. Mediastinoscopic esophagectomy with lymph node dissection using a bilateral transcervical and transhiatal pneumomediastinal approach. Mini-invasive Surgery 2020. doi:
10.20517/2574-1225.2020.23 10.20517/2574-1225.2020.23 - Tokairin Y, Nakajima Y, Kawada K, et al. A feasibility study of mediastinoscopic radical esophagectomy for thoracic esophageal cancer from the viewpoint of the dissected mediastinal lymph nodes validated with thoracoscopic procedure: a prospective clinical trial. Esophagus 2019;16:214-9. [Crossref] [PubMed]
- Mori K, Yamagata Y, Aikou S, et al. Short-term outcomes of robotic radical esophagectomy for esophageal cancer by a nontransthoracic approach compared with conventional transthoracic surgery. Dis Esophagus 2016;29:429-34. [Crossref] [PubMed]
- Randolph GW, Kobler JB, Wilkins J. Recurrent laryngeal nerve identification and assessment during thyroid surgery: laryngeal palpation. World J Surg 2004;28:755-60. [Crossref] [PubMed]
- Garas G, Kayani B, Tolley N, et al. Is there a role for intraoperative recurrent laryngeal nerve monitoring during high mediastinal lymph node dissection in three-stage oesophagectomy for oesophageal cancer? Int J Surg 2013;11:370-3. [Crossref] [PubMed]
- Kanemura T, Miyata H, Yamasaki M, et al. Usefulness of intraoperative nerve monitoring in esophageal cancer surgery in predicting recurrent laryngeal nerve palsy and its severity. Gen Thorac Cardiovasc Surg 2019;67:1075-80. [Crossref] [PubMed]
- Kobayashi H, Kondo M, Mizumoto M, et al. Technique and surgical outcomes of mesenterization and intra-operative neural monitoring to reduce recurrent laryngeal nerve paralysis after thoracoscopic esophagectomy: A cohort study. Int J Surg 2018;56:301-6. [Crossref] [PubMed]
- Hikage M, Kamei T, Nakano T, et al. Impact of routine recurrent laryngeal nerve monitoring in prone esophagectomy with mediastinal lymph node dissection. Surg Endosc 2017;31:2986-96. [Crossref] [PubMed]
- Staubitz JI, van der Sluis PC, Berlth F, et al. Recurrent laryngeal nerve monitoring during totally robot-assisted Ivor Lewis esophagectomy. Langenbecks Arch Surg 2020;405:1091-9. [Crossref] [PubMed]
- Fujimoto D, Taniguchi K, Kobayashi H. Intraoperative neuromonitoring during prone thoracoscopic esophagectomy for esophageal cancer reduces the incidence of recurrent laryngeal nerve palsy: a single-center study. Updates Surg 2021;73:587-95. [Crossref] [PubMed]
- Yuda M, Nishikawa K, Takahashi K, et al. A Strategy for Using Intraoperative Nerve Monitoring During Esophagectomy to Prevent Recurrent Laryngeal Nerve Palsy. Anticancer Res 2018;38:1563-7. [PubMed]
- Yuda M, Nishikawa K, Ishikawa Y, et al. Intraoperative nerve monitoring during esophagectomy reduces the risk of recurrent laryngeal nerve palsy. Surg Endosc 2021; [Crossref] [PubMed]
- Ikeda Y, Inoue T, Ogawa E, et al. Recurrent laryngeal nerve monitoring during thoracoscopic esophagectomy. World J Surg 2014;38:897-901. [Crossref] [PubMed]
- van der Sluis P, Egberts JH, Stein H, et al. Transcervical (SP) and Transhiatal DaVinci Robotic Esophagectomy: A Cadaveric Study. Thorac Cardiovasc Surg 2021;69:198-203. [Crossref] [PubMed]
Cite this article as: Tokairin Y, Nagai K, Kawada K, Kinugasa Y. Mediastinoscopic esophagectomy for esophageal cancer: will this procedure achieve radical esophagectomy?—a narrative review. Dig Med Res 2021;4:73.


