Donor quality of life after living donor liver transplantation: a review of the literature
Introduction
Liver transplantation (LT) is the only life-saving intervention for end-stage liver disease. Despite notable innovation in graft optimization (1-3) and the organ allocation process (4,5) over the past two decades, the shortage of available donor organs remains profound and represents a prominent challenge in LT (6). Living donor liver transplantation (LDLT) is one option to address this critical shortage of liver grafts in the United States (7), yet donor risks must be critically assessed and weighed against obvious recipient benefits.
Donors achieve no medical benefits from LDLT, but are subject to the risks of an extensive surgery, which includes death. In the U.S., the donor mortality rate is 0.2–0.4% (8-10), similar to the mortality rate in elective surgeries (11). Reported donor complication rates vary significantly in the literature ranging from 24–67%, with the majority of these complications classified as minor and resolving within one year of surgery (8,12,13). Reported morbidity and mortality rates, however, do not fully capture the global donor experience, which is multifaceted and better summarized by health-related quality of life (HRQOL) (14). HRQOL can be assessed using patient interviews and patient-reported outcome (PRO) measures, which largely comprise of questionnaires such as the widely used Medical Outcomes Study Short Form 36 (SF-36) and the World Health Organization Quality of Life-BREF (WHOQOL-BREF) (15).
It is imperative that the impact on donor HRQOL be assessed rigorously and critically in order to optimize donor selection, education and post-operative care. Past systematic reviews have summarized the short-term physical and mental quality of life in donors following LDLT, with overall favorable outcomes (16-19). Yet, until recently, there were few studies on donor HRQOL more than one year after surgery. With the multi-center Adult-to-Adult Living Donor Liver Transplant Cohort Study (A2ALL) and a multitude of other recent publications, we have a better understanding of the short-term and long-term impact of LDLT on donor HRQOL. We also have new insight into the psychiatric impact, sexual dysfunction and the financial burden reported after donation. In order to synthesize the new data available, we performed an updated systematic review of the literature to supplement our review published in 2010 (16).
Materials and methods
We performed a systematic search of the MEDLINE database from 2008–2020 to identify original studies evaluating donor HRQOL after LDLT. This time interval was chosen to ensure that this systematic review included any articles that were published between 2008 and 2010, and not captured in our previous systematic review (16). In order to include all relevant articles, we searched the MEDLINE database using the following Medical Subject Headings: “Liver donors”, “liver transplantation”, and “quality of life”. In addition to these Medical Subject Headings, we included the following keywords to ensure thorough coverage: “QOL”, “HRQOL”, “HRQL”, “liver transplant”, “liver grafting”, “liver graft(s)”, “hepatic transplantation(s)”, “hepatic transplant(s)”, “living donor hepatectomy”, “living donor hepatectomies”, “LDLT”, “live donor(s)”, “living donor hepatectomy” and “living donor hepatectomies”. Inclusion criteria limited articles to human subjects and those available in English. We excluded review articles, case reports, case series, articles that only assessed HRQOL in donors prior to LDLT and articles that exclusively assessed pediatric LT. The remaining articles were evaluated for study design, cohort size, and follow up time. Studies were excluded if they included significant methodological flaws, including small sample size (n<20).
The specific HRQOL instrument utilized in the study was also critically examined in order to evaluate the clinical significance of results and compare findings across studies (Table 1). Each HRQOL survey and relevant supporting literature was assessed individually to determine if the survey had been validated prior to use in the study and if so, whether or not the survey was validated in an appropriate study population. Each instrument’s psychometric properties were evaluated thoroughly to ensure that the instrument was valid and reliable. If a survey had not been validated or if it had been created by the authors specifically for the study, survey questions were assessed when available to determine if the questions were suitable and clinically relevant to live living donors’ QOL. Studies were excluded if they included non-validated surveys without outlining specific survey questions or if the survey questions were not deemed to be clinically relevant to living donors’ QOL. Studies using surveys validated in the general population were included as living donors were thought to most closely resemble this population.
Table 1
| Author | Date of publication | Cohort size | Study design | Quality of life metric | Comparison population | Follow up time (months) |
|---|---|---|---|---|---|---|
| El-Serafy | 2009 | 30 | Cross-sectional | SF-36 | Healthy adults | 39.4 (±18.7)a |
| Schulz | 2009 | 43 | Longitudinal | SF-36, HADS, CLDQ, NDI, Caregiver burden scale, EUROTOLD questionnaire | Normative population, Potential donors | 3.3 (±0.7)a |
| Castedal | 2010 | 34 | Cross-sectional | Study specific questionnaire | None | 6 (1–12)d |
| Hsieh | 2010 | 51 | Longitudinal | SF-36, 6MWD | Normative population | 3a |
| DuBay | 2010 | 142 | Cross-sectional | SF-36, BIQ, IIRC, Confidence rating scale | Normative population | 27 (3–84)d |
| Togashi | 2011 | 35 | Longitudinal | SF-36 | Normative population | 18c |
| Noma | 2011 | 40 | Longitudinal | STAI, BDI, WHOQOL-BREF, PACT | None | 51.4 (±6.5)a |
| Azoulay | 2011 | 91 | Cross-sectional | NIDDK | Normative population | 77(±29)a |
| Kousoulas | -2011 | 55 | Cross-sectional | SF-36 | Normative population | 94a |
| Yamanouchi | 2012 | 20 | Longitudinal | SF-36 | Normative population | 12c |
| Jin | 2012 | 92 | Longitudinal | SF-36, SCL-90-R | Normative population | 12a |
| Narumi | 2012 | 33 | Cross-sectional | SF-36, Hamilton anxiety and depression scale | Normative population | 78d |
| Toyoki | 2012 | 27 | Cross-sectional | SF-36 | Normative population | 72d |
| Choi | 2012 | 150 | Longitudinal | NRS pain scale | None (LADH, ODH) | N/A |
| Takada | 2012 | 578 | Cross-sectional | SF-36 | Normative population | 81.6 (±40.8)a |
| Lei | 2013 | 300 | Longitudinal | SF-36, SCL-90-R | Normative population | 5a |
| Marubashi | 2013 | 31 | Longitudinal | SF-36 | Normative population (ODH) | 12c |
| Kroencke | 2014 | 40 | Longitudinal | SF-36, HADS | Normative population, Healthy adults, Potential donors | 12.6b |
| Kawagishi | 2014 | 84 | Cross-sectional | SF-36 | Normative population | 103.6a |
| Ladner | 2015 | 374 | Longitudinal | SF-36 | Normative population | 132c |
| Suh | 2015 | 429 | Cross-sectional | Study specific questionnaire | None | 32.6 (6.4–55.4)a |
| DiMartini | 2015 | 208 | Longitudinal | Study specific questionnaire | Potential donors | 7.5b |
| Bhatti | 2015 | 60 | Cross-sectional | SF-36, POMS-65 | Normative population | 15 (±5.1)a |
| Kimura | 2015 | 142 | Cross-sectional | Chart review of comorbidities | None | 65.3 (±38.2)a |
| Humphreville | 2015 | 107 | Longitudinal | SF-36, DSS | Normative population | 82.8d |
| Murad | 2016 | 68 | Cross-sectional | Study specific questionnaire | None | 66 (range 18–130.8)d |
| Dew | 2016 | 517 | Cross-sectional | SF-36, PTGI-SF, Study specific questionnaire | Normative population | 72a |
| Shen | 2016 | 114 | Cross-sectional | SF-36, HADS | Normative population | 48c |
| Wang | 2016 | 204 | Cross-sectional | GIQLI | None | 24a |
| DiMartini | 2017 | 271 | Longitudinal | PHQ-9, Study specific questionnaire | None | 9.6b |
| Cherian | 2017 | 64 | Cross-sectional | SF-36 | Normative population | 48a |
| Kitajima | 2017 | 153 | Longitudinal | Interview | None (ODH) | 36.6 (1.4–66.0)a |
| Chandran | 2017 | 200 | Cross-sectional | SF-36 | Normative population | 12(±0)a |
| Wang | 2017 | 60 | Cross-sectional | CES-D, WHOQOL-BREF, CHQ | Normative population | 3a |
| Butt | 2018 | 297 | Longitudinal | SF-36, PRIME-MD, PTGI-SF, Simmons Better Person Scale, Interview | Normative population | 9.6b |
| Benzing | 2018 | 104 | Longitudinal | SF-36 | Normative population | 41 (7–161)a |
| Berglund | 2018 | 176 | Longitudinal | SF-36, DSS | Normative population | 99.6a |
| Butt | 2018 | 271 | Longitudinal | SF-36, FACIT-Fatigue, BPI | Normative population | 9.6b |
| Hesimov | 2018 | 55 | Longitudinal | SF-36 | Normative population | 12a |
| Dew | 2018 | 517 | Longitudinal | SF-36, FACIT-Fatigue, BPI, NRS pain scale, Study specific questionnaire | Normative population | N/A |
| Weng | 2019 | 68 | Longitudinal | SF-36 | Normative population | 12a |
| Morooka | 2019 | 374 | Cross-sectional | SF-36, LLD-QOL | Normative population | N/A |
| Raza | 2020 | 68 | Longitudinal | SF-36, Study specific questionnaire | Normative population | 138 (±61.2)a |
a, mean follow up time (months); b, mean follow up time was not provided; a weighted mean follow up time was calculated based on number of patients lost to follow up (months); c, data not available to calculate weighted mean follow up time; maximum follow up reported (months); d, median follow up time reported (months); N/A, did not report follow up time. SF-36, Medical Outcomes Study Short Form 36; HADS, Hospital Anxiety and Depression Scale; CLDQ, Chronic Liver Disease Questionnaire; NDI, Nepean Dyspepsia Index; EUROTOLD Questionnaire, Modified European Multicenter Study of Transplantation of Organs from Living Donors Questionnaire; 6MWD, six minute walk distance; BIQ, Body Image Questionnaire; IIRS, Intimacy Subscale of the Illness Intrusiveness Rating Scale; STAI, The State-Trait Anxiety; BDI, The Beck Depression Inventory; WHOQOL-BREF, World Health Organization Quality of Life Questionnaire; PACT, The Psychosocial Assessment of Candidates for Transplantation; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases Quality of Life Survey; SCL-90-R, Symptom Checklist 90 Revised; NRS pain scale, Numeric Rating Pain Scale; POMS-65, Profile of Moods; DSS, Donor Specific Survey; PTGI-SF, Posttraumatic Growth Inventory Short Form; GIQLI, Gastrointestinal Quality of Life Index; PHQ-9, Patient Health Questionnaire-9; CES-D, Center for Epidemiologic Studies Depression Scale; CHQ, Chinese Health Questionnaire; PRIME-MD, Primary Care Evaluation of Mental Health Disorders; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy Fatigue Scale; BPI, Brief Pain Inventory; LLD-QOL, Live Liver Donor Quality of Life Questionnaire.
Results
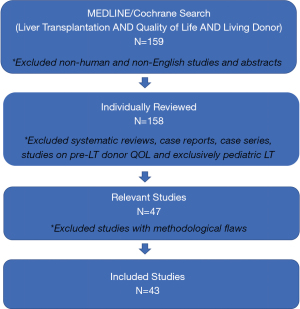
Our original MEDLINE search, restricted to the English language and human subjects, identified 159 unique articles. After evaluating each article, 43 articles met inclusion criteria and were included in the review (Figure 1).
Twenty studies (47%) had a cross-sectional design and 23 (53%) studies had a longitudinal design (Table 1). Forty-two unique HRQOL metrics were implemented across the 43 studies, the majority of which were questionnaires. The most frequently used instrument was the Medical Outcomes Study Short Form 36 (SF-36), which was used in 31 (72%) studies.
In total, 19 generic instruments, which were not designed for a specific population or disease process, were used to assess HRQOL, with the WHOQOL-BREF questionnaire, the Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue Scale, the Hospital Anxiety and Depression Scale, the Profile of Mood States (POMS-65) and the Symptom Checklist 90-Revised (SCL-90-R) used most frequently. Fifteen of the 19 (79%) generic instruments were validated in general populations, demonstrating acceptable construct validity and internal consistency reliability, but none of them were validated in live liver donors specifically. Thirteen surveys specific to chronic liver disease were used in 12 different studies. Of these thirteen surveys, only two were extensively validated in those with chronic liver disease (the Chronic Liver Disease Questionnaire and the National Institute of Diabetes and Digestive and Kidney Disease Liver Transplant Database Quality of Life questionnaire). The Living Liver Donor-QOL Survey (LLD-QOL) and the Donor Specific Survey (DSS), which were used in two different studies, were designed specifically for liver donors and validated in this specific population. Nine of the 13 (69%) liver disease specific surveys were designed for the individual study and were not validated. Moreover, two studies included unique questionnaires specific to abdominal surgeries, with a focus on cosmetic appearance and body image after surgery, neither of which were validated. A minority of studies employed alternate means of assessing HRQOL, with five (12%) studies using qualitative interviews rather than quantitative questionnaires and one study (2%) relying on chart review to assess the development of psychiatric comorbidities following donation (20).
The mean number of donors enrolled in the 47 studies was 142 (range: 8–578). Studies provided follow-up times as either mean, medians or total length of follow-up (Table 1). For the 33 studies that provided mean follow up times, the average follow-up was 31.3 months. For all included articles, follow up ranged from 3 to 132 months. Two studies did not provide follow up times or the data necessary to calculate follow up time.
Overall Physical and Mental HRQOL
In studies that implemented the SF-36, donor HRQOL including both physical (PCS) and mental composite scores (MCS) were typically higher than the general population prior to donation (21-25). Ladner et al. (23) evaluated the A2ALL cohort and found mean PCS and MCS scores to be close to one standard deviation higher than the normative means before donation. Prior to donation, potential candidate donors and eventual donors were found to have higher PCS scores (57.6 and 58.0 respectively) than the general population and the healthy reference sample (25). Eventual donors also had higher MCS scores than potential candidate donors (51.8 vs. 46.8), but these scores were not significantly different from the general population or the healthy reference sample.
Donors generally experience positive long-term outcomes in both physical and mental HRQOL after LDLT. Physical HRQOL tends to decrease and reach a nadir between 1 to 6 months directly after surgery, with PCS scores decreasing to 42.9–50.9 at 3 months (21,23,25-28). Numerous studies report rapid improvement of physical HRQOL within 3–6 months of LDLT (21,23,25-27,29). For example, in one prospective study (29), the mean PCS score decreased significantly at 1 month after surgery but fully recovered by 6 months. Togashi et al. (27) demonstrated similar findings, with PCS scores dropping significantly at 3 months post-surgery, but then returning to baseline 3 months later. Ladner et al. (23) confirmed worsening PCS scores at 3 months post-surgery and showed subsequent improvement to baseline 1 year after surgery.
In recent years, however, multiple studies that included lengthier follow up periods demonstrate that donors may take significantly longer to return to baseline physical function, ranging from 2 to 4 years post-donation. In Butt et al.’s (28) prospective study containing 271 patients, donors reported significant decrease in PCS scores and higher levels of fatigue, abdominal pain and back pain at 3 months post-donation. These physical HRQOL domains started improving 6 months after donation and continued to do so for 2 years, but failed to reach pre-donation levels at the end of the study, with significantly worse scores in the pain domain. Shen et al. (30) found a similarly prolonged recovery period, with physical domains of HRQOL taking 3 to 4 years to return to pre-operative baseline levels or levels comparable to the general population. Humphreville et al. (24) found that 11.2% patients reported their health as worse than prior to donation 7 years later, suggesting an even more prolonged recovery period than the majority of studies in this review. Among the studies included in this review, Raza et al.’s (31) study had the longest mean follow up period of 11.5 years and found PCS scores to be similar to the general U.S. population more than a decade later. Regardless of new data suggesting a longer time to recovery, our evaluation of the literature does support overall positive long-term physical quality of life amongst donors, as the majority of studies demonstrate that HRQOL returns to pre-operative levels or levels comparable to the general population after 4 years (22,32).
Physical symptoms
After surgery, the most commonly reported donor symptoms were abdominal pain, irritation or numbness of the surgical scar, and gastrointestinal complaints including irregular bowel habits, nausea and heartburn (24,25,27,33-38). In one large cross-sectional study (38), the most common post-operative symptoms were numbness and itching around the surgical site and decreased stomach tone. Approximately 6 years after surgery, 15% of donors were still endorsing donation-related medical problems, with the most common problems being hernias, nausea, diarrhea and problems relating to scar tissue and adhesions from the surgery. Berglund et al. (37) had a mean follow up period of 4.8 years after donation and found the most common physical complaints to be incisional discomfort (25% of respondents) and intolerance to fatty meals (20%). Kroencke et al. confirmed a high prevalence of gastrointestinal complaints after donation, finding that digestive symptoms were the most frequent complaint amongst donors 2 years after surgery.
A large portion of patients reported that post-operative pain was more significant than they had expected and that time to recovery was longer than they had anticipated (28,39,40). For example, in one cross-sectional study (39) containing 68 donors, 41% noted post-operative pain that was “much greater than or somewhat greater than expected.” In another study (34), 24% of patients were still having wound-related pain 10 months after surgery. Furthermore, multiple studies revealed long-lasting fatigue and limitations in physical activity following donation (28,31,35). Azoulay et al. (35) found that 22% of donors felt limited in their ability to perform vigorous activity such as running, lifting heavy weights or playing sports up to 6 years after donation. In another study (31), 48.5% of donors endorsed restricting the type of physical activity they performed when evaluated more than a decade after surgery.
LDLT was also found to impact donor’s body image. In Dubay et al.’s cross-sectional study (41), donors reported significantly lower perception of their body image and lower satisfaction with their surgical scar compared to those who underwent open-donor nephrectomy. Younger age, greater pre-donation concern regarding their own health and greater post-donation perception that the recipient engaged in behaviors that risked the new liver were independently associated with negative body image.
Mental HRQOL and psychiatric comorbidities
The existing literature demonstrates that mental HRQOL remains relatively stable after donation and comparable to the general population (27,42,43), with a few studies finding an increase in overall mental HRQOL (26,40,44). While overall MCS scores remain stable, certain domains of mental HRQOL can worsen. Hesimov et al. (21) found that MCS scores did not change post-donation, but the social functioning component of the SF-36 assessment did drop significantly 3 months after donation and gradually returned to pre-operative levels by 12 months post-donation.
The development of psychiatric comorbidities after donation was low in the majority of studies (20,28). For example, in a cross-sectional study containing 142 donors (20), only 4.2% of patients developed psychiatric complications such as depression or anxiety. Humphreville et al.’s (24) study was an outlier, finding that 22.4% of donors reported depression after surgery—a percentage far higher than the remainder of the literature, which shows the prevalence of depression in donors to be similar to the general population (22). While rates of depression were not elevated, anxiety was more prevalent post-donation, ranging from 2.6–4.9% (22) compared to 0.9–1.9% in the general population (45). Alcohol use disorder was found in 2–6% of donors after LDLT and more common in men (22,28), similar to the general population (45).
Sexual function after donation
Our review showed that LDLT and even evaluation for potential donation negatively impacts sexual function (33,35,41). In a cross-sectional study containing 91 donors (41), 9% of donors had a decrease in sexual activity following surgery. Donors with low body image scores were significantly more likely to report interference with both sexual activity and intimate relationships. While donors typically report high physical and emotional functioning at evaluation, DiMartini et al. (33) found that donors and potential candidate donors had worse sexual function at evaluation and at 3 months post-LDLT when compared to 1-year post-LDLT, suggesting that poorer sexual function at evaluation and directly after LDLT could be due to the stresses inherent to the evaluation process and the decision to donate.
Financial impact of donation
Studies revealed that a significant portion of donors felt that donation-related expenditures were burdensome and that LDLT impacted their employment and health insurance. In one illustrative example (33), 40% of donors reported that donation-related costs were burdensome at 3 months post-donation and 19% reported feeling similarly at 2 years post-donation. Cumulatively, 24% of donors found donation-related costs to be more than expected in this study. Furthermore, 34% of donors changed jobs or modified their work because of donation at 3 months post-donation, though only 1% reported changes in employment 2 years later. In this study, those with lower household incomes were at higher risk of poorer financial outcomes. Dew et al. (38) assessed financial burden approximately 6 years after donation and found that 15% of donors reported that donation expenses were burdensome; a portion of these donors changed jobs and experienced permanent income reductions. In this study, 11% of donors also experienced health or life insurance problems due to donation.
Overall donor perception of LDLT
Despite our review demonstrating that LDLT can impact short-term HRQOL and can be perceived as financially burdensome to some patients, all studies in this review found that almost all donors did not regret their decision to donate and 85–100% of participants would donate again if need be (28,35-40,46,47). The minority of patients who would not donate again were those who experienced significant post-operative complications (47) or the recipient died (28).
Predictors of post-donation quality of life
A wide variety of predictors of HRQOL were evaluated across the studies in this review, producing conflicting results. Potential predictors of HRQOL included donor gender, race, body mass index (BMI), education status, financial status, surgical approach including graft type (right versus left lobe) and incision type, post-operative complications, length of hospital admission, indication for transplant and urgency of LDLT, relationship with recipient and recipient mortality. Among the various studies, female gender (22,28,48), transplant recipient death (22,23,46) and obesity (22,46) were found to be predictors of worse post-donation physical HRQOL. Toyoki et al. (49) found that donors who underwent emergency hepatectomy had worse PCS scores. Female gender (22,28,43), longer post-donation hospitalization (22), higher financial burden (22), transplant recipient death (23) and emergency surgery (46) were found to predict worse post-donation MCS and depression in multiple studies. Narumi et al. (50) similarly found that donors who believed that they did not have sufficient time to make a decision on donation before surgery had significantly worse social functioning and higher depression and anxiety scores. There were conflicting findings on whether post-operative complications impacted HRQOL. While some studies found that more post-operative complications led to worse HRQOL (28,32,43,51), others studies (21,46,52,53) showed that complications had no impact on HRQOL. Ladner et al. (23) found that the Hispanic ethnicity and an education less than a bachelor’s degree to be predictors of poor PCS scores, yet ethnicity and education have not been studied extensively in the literature. Thus, these findings have not been replicated.
Left lobe hepatectomy was associated with fewer complications than right lobe hepatectomy, but did not impact MCS or PCS scores (32,37,53,54). When comparing a traditional open versus laparoscopic approach, the laparoscopic approach had favorable outcomes. For example, Marubashi et al. (29) found that recovery from bodily pain and physical disturbance after surgery was quicker in laparoscopy-assisted living donor hepatectomy (LADH) group than in open living donor hepatectomy (ODH). This study also found that donors who underwent LADH had shorter hospitalizations than those who underwent ODH. Kitajima et al. (55) did not find the complication rate different between LADH and ODH but reported significantly lower scar discomfort in the LAHD group than in the ODH group. Choi et al. (56)’s prospective study containing 150 donors was the only study to compare single-port laparoscopy-assisted donor right hepatectomy (SPLADRH) to LADH and ODH. This study found SPLADRH to have favorable outcomes, with similar complication rates, shorter operative time, and less blood loss than LADH and ODH. Moreover, patients described less post-operative pain in the SPLADRH group. Suh et al.’s (57) study was the only study to describe how donor satisfaction varied by incision type. In this study, upper midline incision and the transverse incision for laparoscopy resulted in higher patient satisfaction with cosmetic appearance than the inverted L incision. The upper midline incision also resulted in decreased operative time and shorter hospitalizations.
Discussion
This review of the literature demonstrates that donor physical and mental HRQOL are typically higher than the general population prior to donation. This is not surprising given the rigorous physical and psychosocial evaluation required to be considered a donor. Donor physical HRQOL decreases post-donation, reaching a nadir between 3-6 months after surgery, while mental HRQOL remains stable or equivalent to normative populations. Almost half of patients find post-operative pain to be far greater than expected and physical HRQOL can take many years to return to baseline, with a significant portion of donors experiencing long-lasting physical limitations. There is also a higher than expected prevalence of general anxiety disorder and alcohol use disorder (22,45) and significant financial strain experienced by donors after surgery, with almost one in four donors reporting donation related costs to be more than expected 3 months after donation.
It is noteworthy that 41% of donors found post-operative pain to be “much greater than or somewhat greater than expected” in one cross-sectional study containing 68 donors (39). This result suggests that donors may not be educated adequately on the spectrum of post-operative pain and the range of post-surgical recovery time. Moreover, an unexpected degree of post-operative pain likely contributes to overall worsening of donor physical HRQOL. We also found that physical recovery from LDLT can take longer than previously described. Prior studies found that donor physical HRQOL returns to baseline by 1 year after donation (58,59). Recent studies with lengthier follow up, however, reveal that PCS scores can take up to 3–4 years to return to baseline. Furthermore, 22–48.5% of donors report long-lasting fatigue and some degree of limitation in their physical activity for 5–10 years (31,35), underscoring the lingering physical impact of donation. While donors endorse long-lasting physical limitations, however, their overall physical HRQOL improves to pre-donation levels within 4 years and 96–100% of donors were able to return to their pre-donation occupation (35,60). These findings suggest that the physical limitations reported by donors might not alter daily activities to the extent that it has a profound effect on donor perceived QOL. Thus, the downstream effects of these physical limitations and persistent fatigue on donor health remain unclear and must be investigated further.
Interestingly, we found that alcohol use disorder was identified in 2–6% of donors, which is similar to the prevalence reported in the general population (45). Given the careful donor selection process aimed at identifying at risk drinkers, the prevalence of alcohol use disorder in donors is surprising and therefore more rigorous screening might need to be considered. Additionally, further research is necessary to determine the potential consequences of alcohol use on donor health following LDLT.
We also found that LDLT results in significant financial strain on donors, which is a predictor of worse mental HRQOL. Twenty-four percent of donors reported costs to be more than expected and 40% found donation-related costs to be burdensome. This financial burden was long-lasting, with 15% of donors endorsing financial concerns and changes in life insurance policies 6 years after donation (38). As one might expect, donors with lower household income at time of donation were more likely to worry about financial expenditures after donation (33), highlighting the socioeconomic disparity in the burden related to LDLT and the need for more financial aid towards living liver donors. The American Transplant Surgeons’ (ASTS) National Living Donor Assistance Center (NLDAC) was created to provide reimbursement for donation-related expenses and thus reduce the financial disincentives to living organ donation. However, it is unclear whether the donors who were financially impacted by LDLT in the included studies were directed to the NLDAC for financial support. Based on these studies, it is paramount to clearly outline the potential short- and long-term financial burden to potential donors to allow for fully informed decisions and prevent significant financial stress that could worsen mental HRQOL post-donation.
Across the studies in this review, there were conflicting results on positive and negative predictors of HRQOL. Although female gender and obesity were found to consistently predict worse HRQOL, the positive outcomes in left lobe hepatectomy and laparoscopy-assisted donor hepatectomy were especially noteworthy. Recent studies consistently show that left lobe hepatectomy has fewer surgical complications than right lobe donor hepatectomy. Moreover, laparoscopy-assisted donor hepatectomy resulted in less post-operative pain and quicker recovery.
Finally, this systematic review highlights the fact that there is no validated and standardized patient-reported outcome measure to assess HRQOL in living liver donors. The majority of studies implemented the SF-36, but a large portion of the studies used generic instruments, none of which have been validated in the liver transplantation setting and specifically with living liver donors. Although the majority of these instruments were validated in the general population, living liver donors are often healthier than the general population, with higher reported baseline HRQOL. Only two HRQOL surveys (DDS and LLD-QOL scale) were tested in living liver donors and were found to have construct validity and internal consistency reliability, but even these were only validated in small studies. The large spectrum of patient reported outcome measures used, the majority of which were not validated in living liver donors, is a weakness in the current literature on HRQOL in LDLT, which offers opportunity for further study. Moreover, the heterogeneity of outcome measures presents a significant limitation in a systematic review, as quality of life cannot be easily compared across studies.
One notable omission from the current studies on LDLT is the National Institute of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS) measures of HRQOL (61). PROMIS, which was developed between 2004 and 2014, is innovative in multiple ways. It was sponsored by the NIH “roadmap” for 21st century medical research to develop a new system of brief, psychometrically sound, and flexible measures covering physical, mental, and social HRQOL, along with multiple other symptoms. Through its use of item response theory (IRT) and computer adaptive testing (CAT), PROMIS measures can draw from large banks of items to generate efficient, reliable, and parsimonious measures of patients’ HRQOL. All PROMIS measures are scored on a user-friendly T-score metric that sets the mean value to 50 and standard deviation to 10, normed to the United States general population, so that any individual’s score on PROMIS measures can be compared to this standard. Due to its normative reference to the general population, PROMIS measures may be particularly useful for living liver donor HRQOL assessment and tracking. Moreover, because PROMIS was developed to be applicable to a wide variety of patient populations, ranging from patients who are severely impaired to those who are high functioning, it can be applied to any study population. Hence, future studies of HRQOL in LDLT should consider using PROMIS measures.
Conclusion
LDLT is an emerging treatment for end-stage liver disease in the United States and offers one strategy to alleviate the shortage of liver grafts. Donors achieve no medical benefits from LDLT, but are exposed to the risks of an extensive surgery. Thus, the impact of LDLT on donor health-related quality of life must be assessed critically and comprehensively in order to optimize donor selection and to better educate donors on the risks involved in the procedure. Our review confirms that donors tolerate LDLT well and eventually return to baseline function. However, longer follow up in recent studies has revealed that some patients experience prolonged physical limitations, develop alcohol dependence and are burdened by healthcare expenditures after the surgery. Recently published studies also show that potential donors may need more comprehensive education on the spectrum of post-operative pain and the range of post-surgical recovery time. Further studies are needed to better elucidate predictors of poor HRQOL in order to improve clinical outcomes and educate potential donors accurately prior to LDLT. Moreover, the use of a standardized and extensively validated patient reported outcomes measure, such as PROMIS, could allow for direct comparison of outcomes across studies and provide further insight into the impact of LDLT on donor HRQOL.
Acknowledgments
Funding: This work was supported by R01 DK090129 and the National Institute of Health T32 Training Grant 5T32DK077662-14.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuliano Testa, Greg McKenna, and Johanna Bayer) for the series “Living Donor Liver Transplantation” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-20-151). The series “Living Donor Liver Transplantation” was commissioned by the editorial office without any funding or sponsorship. AJT and DPR reports T32 NIH Grant from Transplant Surgery Scientist Program, Northwestern University. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Croome KP, Lee DD, Pungpapong S, et al. What are the outcomes of declining a public health service increased risk liver donor for patients on the liver transplant waiting list? Liver Transpl 2018;24:497-504. [Crossref] [PubMed]
- Bohorquez H, Seal JB, Cohen AJ, et al. Safety and Outcomes in 100 Consecutive Donation After Circulatory Death Liver Transplants Using a Protocol That Includes Thrombolytic Therapy. Am J Transplant 2017;17:2155-64. [Crossref] [PubMed]
- Ravikumar R, Jassem W, Mergental H, et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant 2016;16:1779-87. [Crossref] [PubMed]
- Enhancing Liver Distribution. An overview of the historical and current effort by the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. 2017.
- Croome KP, Lee DD, Burns JM, et al. Intraregional model for end-stage liver disease score variation in liver transplantation: Disparity in our own backyard. Liver Transpl 2018;24:488-96. [Crossref] [PubMed]
- Kwong A, Kim WR, Lake JR, et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant 2020;20:193-299. [Crossref] [PubMed]
- Abu-Gazala S, Olthoff KM. Current Status of Living Donor Liver Transplantation in the United States. Annu Rev Med 2019;70:225-38. [Crossref] [PubMed]
- Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant 2012;12:1208-17. [Crossref] [PubMed]
- Adcock L, Macleod C, Dubay D, et al. Adult living liver donors have excellent long-term medical outcomes: the University of Toronto liver transplant experience. Am J Transplant 2010;10:364-71. [Crossref] [PubMed]
- Iida T, Ogura Y, Oike F, et al. Surgery-related morbidity in living donors for liver transplantation. Transplantation 2010;89:1276-82. [Crossref] [PubMed]
- Mullen MG, Michaels AD, Mehaffey JH, et al. Risk Associated With Complications and Mortality After Urgent Surgery vs Elective and Emergency Surgery: Implications for Defining "Quality" and Reporting Outcomes for Urgent Surgery. JAMA Surg 2017;152:768-74. [Crossref] [PubMed]
- Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transpl 2002;8:110-7. [Crossref] [PubMed]
- Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology 2008;135:468-76. [Crossref] [PubMed]
- . The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 1995;41:1403-9. [Crossref] [PubMed]
- Jay CL, Butt Z, Ladner DP, et al. A review of quality of life instruments used in liver transplantation. J Hepatol 2009;51:949-59. [Crossref] [PubMed]
- Parikh ND, Ladner D, Abecassis M, et al. Quality of life for donors after living donor liver transplantation: a review of the literature. Liver Transpl 2010;16:1352-8. [Crossref] [PubMed]
- Zhong J, Lei J, Wang W, et al. Systematic review of the safety of living liver donors. Hepatogastroenterology 2013;60:252-7. [PubMed]
- Kim-Schluger L, Florman S. Quality of life after living donor liver transplantation: what we have learned and how we can do better. Liver Transpl 2012;18:1272-3. [Crossref] [PubMed]
- Chen PX, Yan LN. Health-related quality of life in living liver donors after transplantation. Hepatobiliary Pancreat Dis Int 2011;10:356-61. [Crossref] [PubMed]
- Kimura H, Onishi Y, Sunada S, et al. Postoperative Psychiatric Complications in Living Liver Donors. Transplant Proc 2015;47:1860-5. [Crossref] [PubMed]
- Hesimov I, Kirimker EO, Duman B, et al. Health-related Quality of Life of Liver Donors: A Prospective Longitudinal Study. Transplant Proc 2018;50:3076-81. [Crossref] [PubMed]
- Dew MA, Butt Z, Liu Q, et al. Prevalence and Predictors of Patient-Reported Long-term Mental and Physical Health After Donation in the Adult-to-Adult Living-Donor Liver Transplantation Cohort Study. Transplantation 2018;102:105-18. [Crossref] [PubMed]
- Ladner DP, Dew MA, Forney S, et al. Long-term quality of life after liver donation in the adult to adult living donor liver transplantation cohort study (A2ALL). J Hepatol 2015;62:346-53. [Crossref] [PubMed]
- Humphreville VR, Radosevich DM, Humar A, et al. Longterm health-related quality of life after living liver donation. Liver Transpl 2016;22:53-62. [Crossref] [PubMed]
- Kroencke S, Nashan B, Fischer L, et al. Donor quality of life up to two years after living donor liver transplantation: a prospective study. Transplantation 2014;97:582-9. [Crossref] [PubMed]
- Hsieh CB, Tsai CS, Chen TW, et al. Correlation between SF-36 and six-minute walk distance in liver donors. Transplant Proc 2010;42:3597-9. [Crossref] [PubMed]
- Togashi J, Sugawara Y, Tamura S, et al. Donor quality of life after living donor liver transplantation: a prospective study. J Hepatobiliary Pancreat Sci 2011;18:263-7. [Crossref] [PubMed]
- Butt Z, DiMartini AF, Liu Q, et al. Fatigue, Pain, and Other Physical Symptoms of Living Liver Donors in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study. Liver Transpl 2018;24:1221-32. [Crossref] [PubMed]
- Marubashi S, Wada H, Kawamoto K, et al. Laparoscopy-assisted hybrid left-side donor hepatectomy. World J Surg 2013;37:2202-10. [Crossref] [PubMed]
- Shen CJ, Huang HL, Chen KH, et al. Comparison of Liver Function, Emotional Status, and Quality of Life of Living Liver Donors in Taiwan. Transplant Proc 2016;48:1007-11. [Crossref] [PubMed]
- Raza MH, Kim MH, Ding L, et al. Long-Term Financial, Psychosocial, and Overall Health-Related Quality of Life After Living Liver Donation. J Surg Res 2020;253:41-52. [Crossref] [PubMed]
- Kawagishi N, Takeda I, Miyagi S, et al. Donors' quality of life evaluated by short form-36 analysis after living donor liver transplantation in a single-center experience. Transplant Proc 2014;46:675-7. [Crossref] [PubMed]
- DiMartini A, Dew MA, Liu Q, et al. Social and Financial Outcomes of Living Liver Donation: A Prospective Investigation Within the Adult-to-Adult Living Donor Liver Transplantation Cohort Study 2 (A2ALL-2). Am J Transplant 2017;17:1081-96. [Crossref] [PubMed]
- Ishizaki M, Kaibori M, Matsui K, et al. Change in donor quality of life after living donor liver transplantation surgery: a single-institution experience. Transplant Proc 2012;44:344-6. [Crossref] [PubMed]
- Azoulay D, Bhangui P, Andreani P, et al. Short- and long-term donor morbidity in right lobe living donor liver transplantation: 91 consecutive cases in a European Center. Am J Transplant 2011;11:101-10. [Crossref] [PubMed]
- Castedal M, Andersson M, Polanska-Tamborek D, et al. Long-term follow-up of living liver donors. Transplant Proc 2010;42:4449-54. [Crossref] [PubMed]
- Berglund D, Kirchner V, Pruett T, et al. Complications after Living Donor Hepatectomy: Analysis of 176 Cases at a Single Center. J Am Coll Surg 2018;227:24-36. [Crossref] [PubMed]
- Dew MA, DiMartini AF, Ladner DP, et al. Psychosocial Outcomes 3 to 10 Years After Donation in the Adult to Adult Living Donor Liver Transplantation Cohort Study. Transplantation 2016;100:1257-69. [Crossref] [PubMed]
- Darwish Murad S, Fidler JL, Poterucha JJ, et al. Longterm clinical and radiological follow-up of living liver donors. Liver Transpl 2016;22:934-42. [Crossref] [PubMed]
- Wang SH, Lin PY, Wang JY, et al. Mental health status after living donor hepatectomy. Medicine (Baltimore) 2017;96:e6910 [Crossref] [PubMed]
- DuBay DA, Holtzman S, Adcock L, et al. Cosmesis and body image after adult right lobe living liver donation. Transplantation 2010;89:1270-5. [Crossref] [PubMed]
- Raza MH, Aziz H, Kaur N, et al. Global experience and perspective on anonymous nondirected live donation in living donor liver transplantation. Clin Transplant 2020;34:e13836 [Crossref] [PubMed]
- Morooka Y, Umeshita K, Taketomi A, et al. Long-term donor quality of life after living donor liver transplantation in Japan. Clin Transplant 2019;33:e13584 [Crossref] [PubMed]
- Schulz KH, Kroencke S, Beckmann M, et al. Mental and physical quality of life in actual living liver donors versus potential living liver donors: a prospective, controlled, multicenter study. Liver Transpl 2009;15:1676-87. [Crossref] [PubMed]
- American Psychiatric Association. DSM-5 Diagnostic Classification. In: Diagnostic and Statistical Manual of Mental Disorders. 2013.
- Chandran B, Bharathan VK, Shaji Mathew J, et al. Quality of life of liver donors following donor hepatectomy. Indian J Gastroenterol 2017;36:92-8. [Crossref] [PubMed]
- Lei J, Yan L, Wang W. Donor safety in living donor liver transplantation: a single-center analysis of 300 cases. PLoS One 2013;8:e61769 [Crossref] [PubMed]
- Jin SG, Xiang B, Yan LN, et al. Quality of life and psychological outcome of donors after living donor liver transplantation. World J Gastroenterol 2012;18:182-7. [Crossref] [PubMed]
- Toyoki Y, Ishido K, Kudo D, et al. Donor quality of life after living donor liver transplantation: single-institute experience. Transplant Proc 2012;44:341-3. [Crossref] [PubMed]
- Narumi S, Umehara M, Toyoki Y, et al. Importance of awareness of perioperative social and physical situations of living donors for liver transplantation. Transplant Proc 2012;44:328-31. [Crossref] [PubMed]
- Weng LC, Huang HL, Lee WC, et al. Health-related quality of life of living liver donors 1 year after donation. Hepatobiliary Surg Nutr 2019;8:1-9. [Crossref] [PubMed]
- Bhatti AB, Zia H, Dar FS, et al. Quality of Life After Living Donor Hepatectomy for Liver Transplantation. World J Surg 2015;39:2300-5. [Crossref] [PubMed]
- Takada Y, Suzukamo Y, Oike F, et al. Long-term quality of life of donors after living donor liver transplantation. Liver Transpl 2012;18:1343-52. [Crossref] [PubMed]
- Cherian PT, Mishra AK, Mahmood SM, et al. Long-term health-related quality of life in living liver donors: A south Asian experience. Clin Transplant 2017;31. [Crossref] [PubMed]
- Kitajima T, Kaido T, Iida T, et al. Short-term outcomes of laparoscopy-assisted hybrid living donor hepatectomy: a comparison with the conventional open procedure. Surg Endosc 2017;31:5101-10. [Crossref] [PubMed]
- Choi HJ, You YK, Na GH, et al. Single-port laparoscopy-assisted donor right hepatectomy in living donor liver transplantation: sensible approach or unnecessary hindrance? Transplant Proc 2012;44:347-52. [Crossref] [PubMed]
- Suh SW, Lee KW, Lee JM, et al. Clinical outcomes of and patient satisfaction with different incision methods for donor hepatectomy in living donor liver transplantation. Liver Transpl 2015;21:72-8. [Crossref] [PubMed]
- Verbesey JE, Simpson MA, Pomposelli JJ, et al. Living donor adult liver transplantation: a longitudinal study of the donor's quality of life. Am J Transplant 2005;5:2770-7. [Crossref] [PubMed]
- Karliova M, Malagó M, Valentin-Gamazo C, et al. Living-related liver transplantation from the view of the donor: a 1-year follow-up survey. Transplantation 2002;73:1799-804. [Crossref] [PubMed]
- Kousoulas L, Emmanouilidis N, Klempnauer J, et al. Living-donor liver transplantation: impact on donor's health-related quality of life. Transplant Proc 2011;43:3584-7. [Crossref] [PubMed]
- Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63:1179-94. [Crossref] [PubMed]
Cite this article as: Thuluvath AJ, Peipert J, Berkowitz R, Siddiqui O, Whitehead B, Thomas A, Levitsky J, Caicedo-Ramirez J, Ladner DP. Donor quality of life after living donor liver transplantation: a review of the literature. Dig Med Res 2021;4:49.