
Endoscopic biliary drainage for distal biliary stenosis: a narrative review of current status and future prospects
Introduction
Distal bile duct stenosis is a condition that causes obstructive jaundice and occurs not only in people with malignant diseases but also in people with benign diseases. With respect to malignant diseases, distal bile duct stenosis is usually caused by biliary tract cancers such as cholangiocarcinoma and tumors of the papilla of Vater and pancreatic head; however, distal bile duct stenosis due to dissemination or metastasis of cancer arising in other organs has also been observed. Obstructive jaundice may occur in 70% of patients with pancreatic cancer (1). The benign conditions that cause distal bile duct stenosis include immunoglobulin G4-related sclerosing cholangitis, chronic pancreatitis and the presence of inflammatory lesions; such stenosis can also occur postoperatively (2-4). Endoscopic biliary drainage is the gold standard technique for the treatment of distal bile duct stenosis; however, in difficult cases or cases in which treatment with other techniques was unsuccessful, percutaneous transhepatic biliary drainage (PTBD) has been selected as an alternative technique.
In recent years, endoscopic ultrasound (EUS)-guided biliary drainage (EUS-BD) has been used as an alternative technique for treatment. The approach to biliary drainage differs between benign and malignant diseases, and the current drainage method is controversial. There is also no consensus on whether transpapillary biliary drainage should be selected or EUS-BD should be performed. Based on the literature reports, this review was conducted for the purpose of clarifying one index for the selection method of biliary drainage for benign and malignant diseases. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-55).
Methods
The literature search was done thorough the PubMed database. We searched the literature on published with the keywords biliary stenosis/biliary obstruction/ERCP/EUS-guided fine-needle aspiration (EUS-FNA)/EUS-BD/EUS-guided hepaticogastrostomy (EUS-HGS)/EUS-guided choledochoduodenostomy (EUS-CDS)/EUS-guided hepaticojejunostomy (EUS-HJS) and EUS-guided antegrade stenting (EUS-AS) from year 1976 to 2021. Literature was selected from retrospective studies/prospective studies/case reports/reviews and meta-analysis studies.
Biliary stenosis
The diseases that cause distal bile duct stenosis are diverse. While it is often observed in cases of biliary tract cancers and pancreatic cancer, bile duct stenosis can also occur due to infiltration of cancer arising in other organs (1,5,6). It results in obstructive jaundice and often requires biliary drainage.
EUS-FNA, bile duct biopsy, and bile duct brushing cytology are useful for the histological diagnosis of malignant bile duct stenosis. Results for evaluations of the diagnostic accuracy of EUS-FNA for pancreatic tumors have been widely reported, with a reported sensitivity of approximately 90% and an average of specificity of 95% (7-11). Regarding transpapillary histological diagnoses of bile duct tumors, it has been found that intraductal biopsy has a sensitivity of 48%, and biliary brush cytology has a slightly lower sensitivity (45%); however, the sensitivity of a combination of both modalities (brush cytology and intraductal biopsy) has been reported to be 59% (12). In another study, direct biliary biopsies with peroral cholangioscopy (POCS) were found to have a sensitivity of 68.2%, which was higher than the sensitivity of endoscopic retrograde cholangiopancreatography (ERCP)-guided brushing, which was 21.4%; the overall accuracy of POCS-guided biopsies was 87.1%, and that for ERCP-guided brushing was 65.5% (13).
On the other hand, bile duct stenosis can also develop because of benign diseases. In clinical practice, bile duct obstruction due to common bile duct stones is often observed; however, few cases of bile duct stenosis have been reported. The benign diseases that most commonly cause distal bile duct stenosis include chronic pancreatitis and immunoglobin G4-related sclerosing cholangitis associated with autoimmune pancreatitis (AIP), and bile duct lesions have been reported to occur in 13–21% of cases of chronic pancreatitis (2,3,14). Although it is necessary to confirm the lack of coexistence of malignant diseases, the usefulness of EUS-FNA for the diagnosis AIP has been reported (15,16). In cases of benign distal bile duct stenosis, it is necessary to rule out the possible coexistence of malignant disease through histological examinations. Often, with respect to stent choices and techniques used for biliary drainage, there are differences between the treatment methods selected for malignant and benign disorders; such differences have been discussed later in this review.
Endoscopic retrograded cholangiopancreatography
ERCP is the gold standard technique for biliary drainage. It involves the use of a transpapillary approach in which drainage is performed using plastic stents (PSs) or self-expandable metallic stents (SEMSs). The use of PSs or SEMSs for malignant biliary strictures has been reported several times; in particular, the indwelling of SEMSs has been reported to be very useful (17,18) (Figure 1). Chemotherapy is expected to prolong the survival of patients with biliary pancreatic cancer. The patency period of SEMSs is longer than that of PSs, and through the use of SEMSs, frequent re-interventions can be avoided; however, PSs are commonly used for benign distal bile duct stenosis, and their usefulness has also been reported (19-21). Although a long patency period of SEMSs can be expected, no fatal adverse events associated with SEMS placement have been observed. It has also been reported that SEMS removal can be performed without the occurrence of adverse events (19,20). In transpapillary biliary drainage, both PS and SEMS are used as effective biliary drainage at many facilities. However, it is not possible to perform it in all cases, and it is necessary to discuss the drainage method for patients with difficult transpapillary biliary drainage.
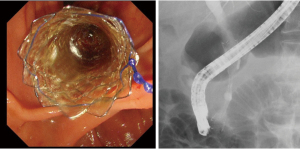
EUS-BD
The transition to EUS-BD began in 1996, when Wiersema et al. reported their novel technique of performing endosonography-guided cholangiopancreatography (22). In 2001, Giovannini et al. first reported the use of EUS-BD for EUS-guided bilioduodenal anastomosis (23). Since then, EUS-BD has been developed as an alternative to PTBD for cases in which the performance of ERCP is unsuccessful or considered difficult (Figure 2).
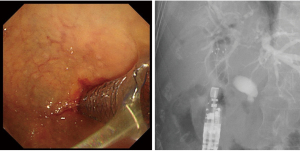
EUS-BD includes EUS-HGS, which involves the anastomosis of the stomach to an intrahepatic bile duct, EUS-CDS, which involves the anastomosis of the duodenum to an extrahepatic bile duct, and EUS-guided hepaticoenterostomy or EUS-HJS (EUS-HES/HJS), which involves the anastomosis of the small intestine to an intrahepatic bile duct.
EUS-CDS is mainly performed for malignant biliary strictures, and although its performance with PSs has been reported, currently, it is usually performed with SEMSs (24-26). Its use is indicated for cases in which there is distal bile duct stenosis without stenosis in the duodenal bulb; currently, it is used for primary drainage and as an alternative to PTBD; in this regard, randomized control trials have also been conducted (27-30). Reportedly, the technical success rate is 90.9–100%, and the incidence rate is 7–23%. Adverse events include biliary peritonitis, stent migration, and bleeding; however, the incidence of fatal adverse events is not high. The performance of EUS-CDS with lumen-apposing metallic stents has also been reported, and the simplification of the procedure is expected to cause an improvement in the success rate and decrease in the incidence of adverse events (31-33).
EUS-HGS may be performed not only in cases of malignant bile duct stenosis but also in cases of benign bile duct stenosis. PSs are also widely used for EUS-HGS. With respect to the use of EUS-HGS with SEMSs for malignant bile duct stenosis, success rates >90% have been reported; further, the occurrence of adverse events such as bleeding, peritonitis and cholangitis, has been reported (34-37). The use of EUS-HGS as well as EUS-CDS for primary drainage has been reported (38).
With the development of new stents and devices with small-diameter devices, the performance of EUS-HGS without fistula dilation has also been reported (39-41). Additionally, the use of a 22-gauge EUS-FNA needle and 0.018-inch guidewire for EUS-HGS has been reported (42,43). EUS-HGS without fistula dilation or EUS-HGS with small-diameter needles may be considered for the prevention of bile leakage. In this regard, Yamamoto et al. performed a study to determine risk factors for adverse events associated with bile leakage during EUS-HGS (44).
EUS-HES/HJS is a technique for carrying out drainage by puncturing the intrahepatic bile duct. The technique is similar to EUS-HGS; however, in EUS-HES/HJS the punctured side is the postoperative intestinal tract. For postoperative autopsy, the approach from the duodenal papilla to distal bile duct stenosis requires balloon-assisted endoscopy and can sometimes be difficult. EUS-HES allows for simple bile duct drainage by puncturing the intrahepatic bile duct from a modified anatomy. Regarding EUS-HES, the use of a method in which the distal bile duct was approached through a fistula formed by EUS-HES has been reported; this method has been described later in this review.
The development of dedicated devices for EUS-BD is expected to lead to an increase in the procedure’s success rate and a decrease in the incidence of associated adverse events; furthermore, it is expected that it will be possible in many facilities.
The outcomes of previously reported EUS-BD are from the tertiary hospital, and the discussing point is that the procedure has not been generalized. The lack of a dedicated device is also considered to be a factor hindering the generalization of the procedure.
EUS-AS
EUS-AS is a method of approaching distal bile duct stenosis through a fistula formed by EUS-HGS or EUS-HES/HJS. It is suggested that EUS-AS, which is performed during EUS-HES/HJS, may be effective (45,46). Unlike the retrograde approach from the duodenal papilla, with the antegrade approach, sphincterotomy or balloon dilation of the papilla of Vater is expected to be difficult in some cases. In cases of malignant distal bile duct stenosis, stent deployment may often be the main purpose of treatment techniques. Therefore, the occurrence of post-procedural pancreatitis may be unavoidable when stents are deployed across the papilla of Vater. However, there have been few reports of the occurrence of pancreatitis after EUS-AS. In a study in which the use of EUS-AS and PTBD for malignant distal bile duct obstruction in patients with surgically altered anatomy were compared, with respect to safety and efficacy, EUS-AS was found to be similar to PTBD; thus, EUS-AS is considered an effective method (47). EUS-AS requires not only EUS-BD but also ERCP, and it should ideally be performed by skilled endoscopists.
Conclusions
We reviewed reports on the method and current status of endoscopic biliary drainage. We will discuss strategies for endoscopic biliary drainage for benign and malignant stenosis in this part.
In cases of malignant biliary strictures, the methods used for endoscopic biliary drainage may differ depending on whether the strictures are resectable or unresectable. In cases of malignant biliary strictures, EUS-BD is not guaranteed to be safe, and in such cases, biliary drainage with ERCP should be selected. For cases involving resectable malignant biliary strictures in which performing ERCP is difficult, PTBD, the safety of which has been established, should be selected. On the other hand, EUS-BD is considered an option for unresectable malignant biliary strictures; however, despite the safety and success rate of EUS-BD, it is unlikely to be used commonly for primary drainage. EUS-BD is considered to be a very effective technique for patients for whom the performance of biliary drainage with ERCP is difficult (Figure 3).
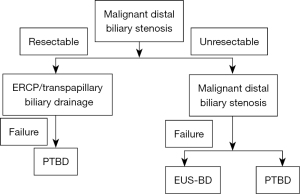
ERCP is considered the first choice for treating benign distal bile duct stenosis. The use of EUS-BD for benign biliary stenosis has been reported many times, and EUS-HGS and EUS-HES/HJS are considered to be particularly effective for cases in which transpapillary treatment is difficult due to postoperatively altered anastomosis. However, it is safe to limit the use of EUS-BD to cases of benign biliary strictures in which, with respect to safety, the performance of biliary drainage with ERCP is difficult (Figure 4).
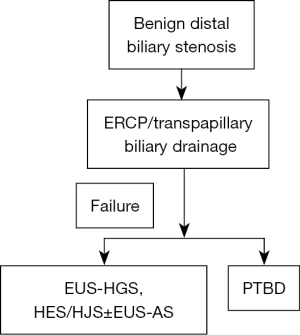
The development of endoscopic biliary drainage has led to an increase in the number of available options for biliary drainage in cases of distal bile duct stenosis. However, there is no dedicated device for EUS-BD, and generalization of the procedure remains questionable in EUS-BD. For each case, the most appropriate technique for biliary drainage should be selected after necessary considerations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-55
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-55). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kruse EJ. Palliation in pancreatic cancer. Surg Clin North Am 2010;90:355-64. [Crossref] [PubMed]
- Lévy P, Barthet M, Mollard BR, et al. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006;30:838-44. [Crossref] [PubMed]
- Wang LW, Li ZS, Li SD, et al. Prevalence and clinical features of chronic pancreatitis in China: a retrospective multicenter analysis over 10 years. Pancreas 2009;38:248-54. [Crossref] [PubMed]
- Gómez CM, Dumonceau JM, Marcolongo M, et al. Endoscopic management of biliary complications after adult living-donor versus deceased-donor liver transplantation. Transplantation 2009;88:1280-5. [Crossref] [PubMed]
- Yamaguchi K, Okusaka T, Shimizu K, et al. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society: A Synopsis. Pancreas 2017;46:595-604. [Crossref] [PubMed]
- Miyazaki M, Yoshitomi H, Miyakawa S, et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci 2015;22:249-73. [Crossref] [PubMed]
- Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012;75:319-31. [Crossref] [PubMed]
- Chen G, Liu S, Zhao Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a meta-analysis. Pancreatology 2013;13:298-304. [Crossref] [PubMed]
- Kuraoka N, Hashimoto S, Matsui S, et al. Effectiveness of EUS-Guided Fine-Needle Biopsy versus EUS-Guided Fine-Needle Aspiration: A Retrospective Analysis. Diagnostics (Basel) 2021;11:965. [Crossref] [PubMed]
- Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc 2016;30:2431-41. [Crossref] [PubMed]
- Banafea O, Mghanga FP, Zhao J, et al. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: a meta-analysis of diagnostic accuracy studies. BMC Gastroenterol 2016;16:108. [Crossref] [PubMed]
- Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 2015;81:168-76. [Crossref] [PubMed]
- Gerges C, Beyna T, Tang RSY, et al. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: a prospective, randomized, multicenter trial (with video). Gastrointest Endosc 2020;91:1105-13. [Crossref] [PubMed]
- Warshaw AL, Schapiro RH, Ferrucci JT Jr, et al. Persistent obstructive jaundice, cholangitis, and biliary cirrhosis due to common bile duct stenosis in chronic pancreatitis. Gastroenterology 1976;70:562-7. [Crossref] [PubMed]
- Kanno A, Masamune A, Fujishima F, et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: a prospective multicenter study. Gastrointest Endosc 2016;84:797-804.e1. [Crossref] [PubMed]
- Morishima T, Kawashima H, Ohno E, et al. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest Endosc 2016;84:241-8. [Crossref] [PubMed]
- Isayama H, Nakai Y, Kawakubo K, et al. Endoscopic retrograde cholangiopancreatography for distal malignant biliary stricture. Gastrointest Endosc Clin N Am 2012;22:479-90. [Crossref] [PubMed]
- Saleem A, Leggett CL, Murad MH, et al. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc 2011;74:321-327.e1-3.
- Lakhtakia S, Reddy N, Dolak W, et al. Long-term outcomes after temporary placement of a self-expanding fully covered metal stent for benign biliary strictures secondary to chronic pancreatitis. Gastrointest Endosc 2020;91:361-369.e3. [Crossref] [PubMed]
- Devière J, Nageshwar Reddy D, Püspök A, et al. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology 2014;147:385-95; quiz e15. [Crossref] [PubMed]
- Siiki A, Helminen M, Sand J, et al. Covered self-expanding metal stents may be preferable to plastic stents in the treatment of chronic pancreatitis-related biliary strictures: a systematic review comparing 2 methods of stent therapy in benign biliary strictures. J Clin Gastroenterol 2014;48:635-43. [Crossref] [PubMed]
- Wiersema MJ, Sandusky D, Carr R, et al. Endosonography-guided cholangiopancreatography. Gastrointest Endosc 1996;43:102-6. [Crossref] [PubMed]
- Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001;33:898-900. [Crossref] [PubMed]
- Yamao K, Bhatia V, Mizuno N, et al. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy 2008;40:340-2. [Crossref] [PubMed]
- Hara K, Yamao K, Niwa Y, et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol 2011;106:1239-45. [Crossref] [PubMed]
- Hara K, Yamao K, Hijioka S, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy 2013;45:392-6. [Crossref] [PubMed]
- Kuraoka N, Hara K, Okuno N, et al. Outcomes of EUS-guided choledochoduodenostomy as primary drainage for distal biliary obstruction with covered self-expandable metallic stents. Endosc Int Open 2020;8:E861-8. [Crossref] [PubMed]
- Nakai Y, Isayama H, Kawakami H, et al. Prospective multicenter study of primary EUS-guided choledochoduodenostomy using a covered metal stent. Endosc Ultrasound 2019;8:111-7. [Crossref] [PubMed]
- Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc 2018;88:9-17. [Crossref] [PubMed]
- Paik WH, Lee TH, Park DH, et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol 2018;113:987-97. [Crossref] [PubMed]
- Tsuchiya T, Teoh AYB, Itoi T, et al. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: a prospective multicenter study. Gastrointest Endosc 2018;87:1138-46. [Crossref] [PubMed]
- Anderloni A, Fugazza A, Troncone E, et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc 2019;89:69-76. [Crossref] [PubMed]
- El Chafic AH, Shah JN, Hamerski C, et al. EUS-Guided Choledochoduodenostomy for Distal Malignant Biliary Obstruction Using Electrocautery-Enhanced Lumen-Apposing Metal Stents: First US, Multicenter Experience. Dig Dis Sci 2019;64:3321-7. [Crossref] [PubMed]
- Nakai Y, Isayama H, Yamamoto N, et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016;48:1125-8. [Crossref] [PubMed]
- Paik WH, Park DH, Choi JH, et al. Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J Gastroenterol 2014;20:5051-9. [Crossref] [PubMed]
- Artifon EL, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: is there any difference? Gastrointest Endosc 2015;81:950-9. [Crossref] [PubMed]
- Ogura T, Chiba Y, Masuda D, et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy 2016;48:156-63. [PubMed]
- Okuno N, Hara K, Mizuno N, et al. Efficacy of the 6-mm fully covered self-expandable metal stent during endoscopic ultrasound-guided hepaticogastrostomy as a primary biliary drainage for the cases estimated difficult endoscopic retrograde cholangiopancreatography: A prospective clinical study. J Gastroenterol Hepatol 2018;33:1413-21. [Crossref] [PubMed]
- Park DH, Koo JE, Oh J, et al. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol 2009;104:2168-74. [Crossref] [PubMed]
- Kuraoka N, Hashimoto S, Matsui S. Endoscopic ultrasound-guided hepaticogastrostomy using a new fully covered metallic stent without fistula dilatation. Dig Endosc 2020;32:e122-3. [Crossref] [PubMed]
- Maehara K, Hijioka S, Nagashio Y, et al. Endoscopic ultrasound-guided hepaticogastrostomy or hepaticojejunostomy without dilation using a stent with a thinner delivery system. Endosc Int Open 2020;8:E1034-8. [Crossref] [PubMed]
- Kanno Y, Ito K, Sakai T, et al. Novel combination of a 0.018-inch guidewire, dedicated thin dilator, and 22-gauge needle for EUS-guided hepaticogastrostomy. VideoGIE 2020;5:355-8. [Crossref] [PubMed]
- Hara K, Okuno N, Haba S, et al. How to perform EUS-guided hepaticogastrostomy easier and safer. J Hepatobiliary Pancreat Sci 2020;27:563-4. [Crossref] [PubMed]
- Yamamoto Y, Ogura T, Nishioka N, et al. Risk factors for adverse events associated with bile leak during EUS-guided hepaticogastrostomy. Endosc Ultrasound 2020;9:110-5. [Crossref] [PubMed]
- Ogura T, Masuda D, Imoto A, et al. EUS-guided hepaticogastrostomy combined with fine-gauge antegrade stenting: a pilot study. Endoscopy 2014;46:416-21. [Crossref] [PubMed]
- Kumbhari V, Tieu AH, Khashab MA. EUS-guided biliary drainage made safer by a combination of hepaticogastrostomy and antegrade transpapillary stenting. Gastrointest Endosc 2015;81:1015-6. [Crossref] [PubMed]
- Iwashita T, Uemura S, Mita N, et al. Endoscopic ultrasound guided-antegrade biliary stenting vs percutaneous transhepatic biliary stenting for unresectable distal malignant biliary obstruction in patients with surgically altered anatomy. J Hepatobiliary Pancreat Sci 2020;27:968-76. [Crossref] [PubMed]
Cite this article as: Kuraoka N, Hashimoto S, Matsui S, Terai S. Endoscopic biliary drainage for distal biliary stenosis: a narrative review of current status and future prospects. Dig Med Res 2021;4:50.


