
Neoadjuvant double metal stent placement in a patient with locally unresectable cancer of the panceatic head—a case report
Introduction
More than 20 years after the advent of the first options for chemotherapy prognosis for pancreatic cancer is still poor. Only surgical resection combined with adjuvant chemotherapy may result in long-term survival. However, only a minority of patients present with localized and surgically resectable tumors. In general, patients with metastatic disease are not considered for surgery. Other patients present with locally advanced disease that may become resectable following neoadjuvant therapy. Novel concepts to achieve surgical resection include pre-operative chemotherapy following the combined chemotherapy regime “FOLFIRINOX” and this strategy may provide a survival benefit for patients with locally advanced pancreatic cancers. However, patients may develop complications such as jaundice and duodenal obstruction that need to be resolved prior to chemotherapy. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-31).
Case presentation
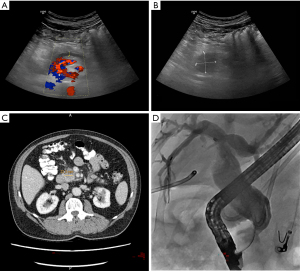
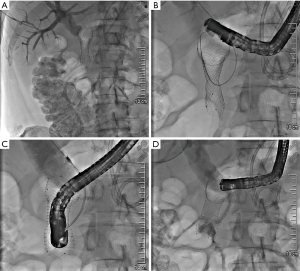
A 72-year-old patient presented to the emergency department of our hospital with upper abdominal discomfort and nausea. The past medical history and family history were unremarkable. Physical examination revealed upper abdominal tenderness but was unremarkable otherwise. Routine laboratory finding including liver function tests were within normal limits. Abdominal ultrasound showed a dilated pancreatic duct (Figure 1A) and a mass in the pancreatic head with low echogenicity (Figure 1B). The common bile duct was not dilated at the time of presentation and there were no distant metastases detected. CT scan confirmed a mass of the pancreatic head/uncinated process with broad contact to the superior mesenteric artery (Figure 1C). The findings were compatible with cancer of the pancreatic head and the interdisciplinary tumor conference deemed the tumor borderline resectable due to possible arterial involvement and recommended exploration. Surgery confirmed tumor contact with the superior mesenteric artery >180° and therefore, resection was not possible at the time. Adenocarcinoma of the uncinated processus (cT3 cNx cM0) was histologically confirmed following partial diagnostic tumor resection. Unfortunately, post-surgery the patient developed acute pancreatitis with extensive fluid collections, which in the further course required internal and external drainage due to bacterial superinfection. Four weeks after surgery, the patient became jaundiced due to distal bile duct obstruction; endoscopic retrograde cholangiopancreatography (ERCP) was performed and following sphincterotomy a plastic stent was placed (Figure 1D). An additional 4 weeks later the patient developed postprandial nausea and vomiting. Gastroscopy confirmed gastric outlet obstruction due to duodenal compression stenosis, which could only be passed with a small diameter nasal endoscope. Options to relieve both biliary and gastric outlet obstruction were discussed and we decided to perform a two-step procedure: first, the biliary plastic stent was removed employing the nasal gastroscope and a standard short uncovered duodenal stent was placed (Cook Medical, 6 cm). Unfortunately, the stent blocked access to the papilla, which we were unable to identify through the metal mesh. Therefore, we waited for 6 days for cholestasis to develop. We then established percutaneous transhepatic access to the bile duct system (Figure 2A) and passed a guide wire through the distal bile duct stenosis and the metal mesh inside the stent in the duodenum (Terumo Guidewire). We grasped the wire with forceps and withdrew it through the duodenoscope (Figure 2B). Subsequently, a standard diagnostic ERCP catheter was inserted over the Terumo guidewire, stable position with the duodenoscope within the duodenal stent was achieved, and the catheter was inserted over the external guidewire through the metal mesh into the common bile duct (Figure 2C). The guidewire was exchanged and a stiff wire (Visiglide, Olympus) was placed through the duodenoscope into the common bile duct. We dilated the distal bile duct stenosis and the metal mesh with a balloon (6 mm) and placed a metals stent over the wire into the distal bile duct with the distal end of the stent within the duodenal stent (Cook, 10 mm, 6 cm). Contrast media passage through both the bile duct and the duodenal stent was satisfactory (Figure 2D). The patient reported that he did well after the rendez-vous procedure, jaundice did not reoccur as confirmed by laboratory tests and oral ingestion was possible with no limitation. He started neoadjuvant chemotherapy 4 weeks later and continuous to do well after 5 cycles of chemotherapy with Gemcitabine (1,000 mg/m2) and nab-Paclitaxel (125 mg/m2) days 1, 8, 15. A recent CT scan showed no distant metastases and we plan a repeat-exploration with possible pancreaticoduodenectomy. Written informed consent was obtained from the patient for publication of this case report and the accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Discussion
Despite progress in the understanding of the molecular pathology, imaging, surgery and chemotherapy, pancreatic cancer is still associated with a poor prognosis (1). Surgery is the only curative treatment and results in longer survival compared with other therapies (1). However, only a minority of patients can receive tumor resection due to metastatic disease, performance status or locally advanced tumors (2). In addition, locally advanced tumors commonly cause local complications including biliary and gastric outlet obstruction. Advances in chemotherapy for pancreatic cancer enable neoadjuvant treatment for locally advanced cancers (2). In our case, the tumor was not resectable due to contact with the superior mesenteric artery >180°. Neoadjuvant chemotherapy may render locally advanced tumors resectable and when resection is achieved, resection results in improved prognosis (3). Importantly, following chemotherapy, radiology is not a predictor of resectability and therefore, patients without progress and distant metastases require surgical exploration. For our patient, we planned neoadjuvant chemotherapy after histological confirmation. Unfortunately, due to postoperative pancreatitis this was delayed and he required stenting of the common bile duct for obstructive jaundice. At the time of stenting there was no duodenal obstruction present and we decided to place a plastic stent, which we routinely exchange every three months. Infection of peripancreatic fluid collections delayed chemotherapy further and the patient developed duodenal obstruction. Concomitant biliary and gastric obstruction is a common dilemma in patients with cancer of the pancreatic head (4). In general, treatment options include double bypass surgery (hepaticojejunostomy and gastrojejunostomy), transpapillary bile duct stenting and duodenal stenting as well as endoscopic ultrasound-guided stenting and duodenal stenting (4). We avoided the surgical approach so as not to delay chemotherapy any further and ultrasound-guided stenting due to remaining fluid collections. Placement of a permanent transpapillary metal stent first was not achieved because passage of the stenosis with a duodenoscope was not possible. Therefore, we placed the uncovered duodenal metal stent first and, due to blockage of the papilla by the metal stent, we performed a complex rendez-vous procedure to place a transpapillary metal stent. Following the “neoadjuvant” double-stenting, the patient does very well and received 5 cycles of neoadjuvant chemotherapy with Gemcitabine and nab-Paclitaxel (5). We avoided more aggressive chemotherapy with the modified FOLFIRINOX combination chemotherapy owing to the patients’ performance status. In conclusion, improved surgical and medical tools for the treatment of pancreatic cancer require strategies for complication management in order to enable patients to receive these therapies. Here we describe a “neoadjuvant” double-stenting approach involving a rendez-vous procedure that resulted in complete relief from biliary and gastric obstruction and enabled the patient to receive timely chemotherapy.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Nahum Méndez-Sánchez) for the series “Current Status and Future Expectations in the Management of Gastrointestinal Cancer” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-31
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-31). The series “Current Status and Future Expectations in the Management of Gastrointestinal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 2019;16:11-26. [Crossref] [PubMed]
- Macedo FI, Ryon E, Maithel SK, et al. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg 2019;270:400-13. [Crossref] [PubMed]
- Nakai Y, Hamada T, Isayama H, et al. Endoscopic management of combined malignant biliary and gastric outlet obstruction. Dig Endosc 2017;29:16-25. [Crossref] [PubMed]
- Dhir M, Zenati MS, Hamad A, et al. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann Surg Oncol 2018;25:1896-903. [Crossref] [PubMed]
Cite this article as: Wittenburg H, Kirchner T, Partecke LI. Neoadjuvant double metal stent placement in a patient with locally unresectable cancer of the panceatic head—a case report. Dig Med Res 2021;4:58.


