
Minimally invasive treatment of congenital peritoneal encapsulation: a case report
Introduction
Congenital peritoneal encapsulation (CPE) is a rare malformation, characterized by a supplementary peritoneal membrane. It is consequence of abnormal peritoneal development which leads to an accessory peritoneal sac partially or totally covering the small intestine (1,2).
CPE is a condition that can remain asymptomatic or cause small bowel obstruction. In a recent work, the authors reviewed the literature systematically and found that since its first description over 100 years ago, CPE has been described in less than 50 reports worldwide (3). Interestingly, in all the reports published over the last 20 years (1-4), the therapeutic approach has involved exclusively open surgery. We describe the case of a patient presenting with asymptomatic CPE discovered accidentally during an operation conducted for a diverticulitis complicated by a covered perforation and treated for the first time with a minimally invasive surgical approach.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-27).
Case presentation
A 72-year-old woman was referred from the emergency department for acute abdominal pain in the lower quadrants along with low-grade fever, clinical condition deterioration and constipation. Additionally, the patient described abdominal pain not associated to any red flag symptoms over the previous three months.
Past medical history included mild obesity, hypercholesterolemia, diabetes mellitus and open appendicectomy. Physical examination revealed abdominal tenderness with pain on deep palpation at the lower left quadrant and signs of peritoneal irritation. Blood test showed increased levels of C-reactive protein (CRP) 6.61 mg/dL (<0.75), white blood cells (WBC) 12.28×109/L(range, 4.5–10.5×109/L) and platelets 615×109/L (range, 150–350×109/L).
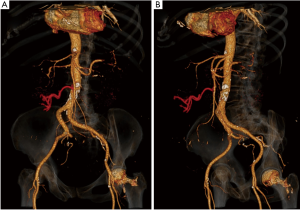
An abdominal contrast computer tomography (CT) scan showed heterogeneous thickening of the sigmoid colon closely adjacent to the left ovary. This finding was compatible with a picture of complicated diverticulitis or suspected ovarian lesion with possible colonic infiltration. Interestingly, the transverse colon was located in the pelvis (Figure 1A), the inferior mesenteric vein was dislocated to the right (Figure 1B), and the middle colonic vessels showed a peculiar course, being displaced caudally and to the right (Figure 2).
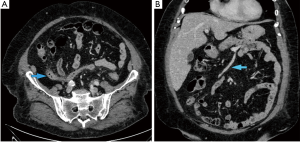
In order to complete the diagnostic pathway, a sigmoidoscopy was performed, and this confirmed an acute sigmoid diverticulitis with rigidity and stricture of the bowel.
Given the clinical picture, (diverticulitis with bowel lumen stricture together with the radiological suspicion of left ovary malignancy), after 5 days of conservative therapy (intravenous antibiotics, intravenous fluids and fasting) the indication for surgical treatment was provided. As in our service a minimally invasive approach is standard of care for sigmoid colon diverticulitis, an explorative laparoscopy was performed. The camera trocar was placed in the right paraombelical region with Hasson’s technique and pneumoperitoneum was achieved. Two further operative trocars were added in right iliac fossa (12 mm) and epigastrium (5 mm). A 3D flex camera was used (ENDOEYE FLEX 3D, Olympus).
The exploration of the abdominal cavity, following cranial luxation of the greater omentum, revealed a transparent accessory peritoneal membrane, which encapsulated the small bowel entirely. This sheet extended from the inferior pancreatic edge to the transverse colon and laterally was attached to the left parietal peritoneum (Figure 3).
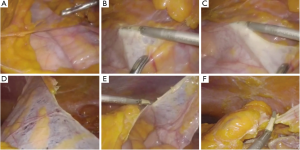
As observed in the CT scan, the transverse colon was ptosic, located into the pelvis, alongside the ascending colon. As a consequence, the splenic flexure was placed in the pelvis, next to the sigmoid colon. In order to gain access to the medial aspect of the descending colon, the accessory peritoneal layer was removed after sectioning its insertions (Figure 3). This maneuver freed the underlying small intestine which appeared vital and trophic without identifiable wall suffering. Given the unusual course of the transverse colon, the middle colic vessels appeared stretched downwards and displaced towards the right side, as described in the CT scan.
Despite these anatomical variations, the laparoscopic left hemicolectomy plus left ovarian resection and resection of the redundant peritoneal sheet was completed successfully. Given the consistent inflammation, the left ovary had to be sacrificed, since no cleavage from the sigmoid colon was present. No post-operative complications occurred, and the patient started a light diet on the first postoperative day (POD) as per ERAS (enhanced recovery after surgery) protocol. She opened her bowel on third POD and was discharged home on fifth POD.
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
CPE is a rare condition, firstly described by Cleland in 1868 (1) and reported only in a few cases over the years (3).It is a congenital abnormality with an unclear etiology, characterized by an aberrant embryological development of the abdominal organs and the mesentery, leading to the creation of an additional peritoneal membrane (2).
Dave et al. recently attempted a classification, based on the etiology, of different encapsulated bowel diseases. In fact, CPE enters in the differential diagnosis with the Fibrotic Peritoneal Encapsulation (FPE), which includes the idiopathic cocoon syndrome, and the sclerosing encapsulating peritonitis (SEP), secondary to well defined inflammatory causes (3). The term FPE has been introduced to distinguish CPE from the acquired encapsulated bowel diseases. Additionally, FPE is characterized by a thick fibro-collagenous layer encasing the small and large bowel (1,5), and thus the associated membrane differs for morphology and histology from the peritoneal sheet encountered in the CPE. In fact, the membrane present in CPE is typically thin, semi-transparent and vascularized, differing consistently from the thick, white and fibrotic membrane typical of FPE.
Peritoneal encapsulation is usually a silent condition, found incidentally during surgical procedures undertaken for other reasons, as reported by our experience. However, CPE has been occasionally reported as primary cause of small bowel obstruction (4,6-18).
In the majority of cases, the pre-operative diagnosis is extremely challenging, since there are no pathognomonic clinical signs. As a matter of fact, fixed, asymmetrical distension of the abdomen and abnormal consistency of the abdominal wall on palpation have been described (1,19). Naraynsingh et al. (2) supposed that these signs are secondary to the fact that the accessory peritoneal sac does not change its position with peristaltic activity; in addition the flat abdominal area is more firm at palpation because of the fibrous consistency of the CPE, while the softer area corresponds to the intestinal loops extended and not covered by the accessory peritoneal sheet.
As reported in our experience, the clinical examination may not be helpful, especially in patients with high BMI, as plain abdominal radiography is often normal or might reveal nonspecific distention of small bowel loops. On the other hand, CT scan might have additional diagnostic value. In fact, a radiological aspect of the small intestine suggestive of CPE and named the helix-sign has been previously observed and described (13).
In our case, the helix sign was not present. However, we noticed other indirect signs of CPE, such as the aberrant location of the transverse colon in the pelvis with low insertion of the transverse mesocolon. This explains the atypical posterior-medial course of the inferior mesenteric vein (Figure 3). As previously described, the middle colic vessels were pulled towards the transverse colon in the pelvis (Figure 2) and consequently they could have easily been confused with the inferior mesenteric vessels, increasing the risk of their iatrogenic injury with possible blood-flow decrease in a healthy colonic segment. These anatomical variations of the vessels together with the abnormal position of the transverse colon, identified with the CT-scan could help in the future in the diagnosis of PE, especially if associated with intestinal obstruction without other obvious etiological factors. Interestingly, other authors reported already the association of CPE with vascular mesenteric abnormalities (6) or incomplete situs inversus and epigastric hernia (20). This reinforces the theory that CPE is the result of an embryological malformation involving the midgut/hindgut, therefore it might associate with additional developmental abnormalities of the abdominal organs. Those indirect signs are easier to detect with the current diagnostic radiological modalities, such as CT scan and might be helpful in raising the suspicion of CPE.
Treatment of CPE can be conservative or surgical. Conservative management has only been described in a single case of CPE. Surgical management consists in peritonectomy, adhesiolysis and enterolysis (3), in order to resolve or prevent small bowel occlusion (13). PE has a high survival rate after operation and a very low recurrence rate (19).However, in all the reported cases treated with surgical therapy, a laparotomy was performed. Our report represents the first case present in the literature, in which laparoscopic surgery was carried on to successfully treat CPE.
In the past, other authors supported the need to dissect and remove the accessory peritoneal sac found during laparotomy, since they speculated that this condition predisposes to small bowel occlusion (10). In our experience, during the left hemicolectomy, opening the accessory peritoneal membrane was necessary to access the vessels. Moreover, the entire membrane was removed to avoid postoperative complications, such as internal hernias. We believe that the mini-invasive approach represents an added value in comparison to the open one. In fact, the pneumoperitoneum might be used, as we noticed during our procedure, as aid in recognition and lysis of this accessory peritoneal sac. For this reason, CPE should not be considered as an argument for conversion to open surgery. It must be underlined that CPE has been occasionally reported as a bowel obstruction cause, in this case a minimally invasive approach could result more challenging, since the intraabdominal space might be limited due to bowel distention. However, according to our clinical experience laparoscopic bowel obstruction management is feasible and it should be attempted, provided that acceptable intraabdominal space is gained through pneumoperitoneum.
Despite the low incidence, the knowledge of this rare condition is important for the surgeon, both for the management of CPE-dependent small bowel occlusion or in case of accidental finding during other surgical procedures. Additionally, it is important to be aware that CPE can be associated with other congenital conditions such as mesenteric malformations or abnormalities of the mesenteric vascular anatomy, which might be easily detected by CT scan and might result helpful in setting a diagnostic suspicion of CPE.
In conclusion, CPE is a rare consequence of abnormal peritoneal development. Only 46 cases have been reported in literature (3) and with our experience we demonstrated that it can be successfully treated using a minimally invasive approach.
Acknowledgments
The authors are grateful to Silvia Luceri for her kind contribution in providing the radiological images.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-27
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-27). MB serves as an unpaid editorial board member of Digestive Medicine Research from Jan 2021 to Dec 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teixeira D, Costa V, Costa P, et al. Congenital peritoneal encapsulation. World J Gastrointest Surg 2015;7:174. [Crossref] [PubMed]
- Naraynsingh V, Maharaj D, Singh M, et al. Peritoneal encapsulation: a preoperative diagnosis is possible. Postgrad Med J 2001;77:725-6. [Crossref] [PubMed]
- Dave A, McMahon J, Zahid A. Congenital peritoneal encapsulation: A review and novel classification system. World J Gastroenterol 2019;25:2294. [Crossref] [PubMed]
- Renko AE, Mirkin KA, Cooper AB. Peritoneal encapsulation: a rare cause of small bowel obstruction. BMJ Case Rep 2019;12:e228594 [Crossref] [PubMed]
- Al-Taan OS, Evans MD, Shami JA. An asymptomatic case of peritoneal encapsulation: case report and review of the literature. Cases J 2010;3:13. [Crossref] [PubMed]
- Adedeji OA, McAdam WA. Small bowel obstruction due to encapsulation and abnormal artery. Postgrad Med J 1994;70:132-3. [Crossref] [PubMed]
- Arumugam PK, Dalal AK. Peritoneal encapsulation - an unexpected cause of acute intestinal obstruction. J Visc Surg 2017;154:303-5. [Crossref] [PubMed]
- Awasthi S, Saraswat VA, Kapoor VK. Peritoneal encapsulation of the small bowel: a rare cause of intestinal obstruction. Am J Gastroenterol 1991;86:383. [PubMed]
- Basu A, Gupta AK, Biswas S, et al. An interesting case of a left paraduodenal hernia with peritoneal encapsulation presenting as acute intestinal obstruction. Hell Cheirourgike 2014;86:109-11. [Crossref]
- Griffith D, Boal M, Rogers T. Peritoneal encapsulation; a rare cause of bowel obstruction. Ann R Coll Surg Engl 2017;99:e11-2. [Crossref] [PubMed]
- Huddy SP, Bailey ME. Small bowel obstruction due to peritoneal encapsulation. Br J Surg 1988;75:262. [Crossref] [PubMed]
- Lee S, Kim JC, Youn SS, et al. A Case of Small Bowel Obstruction due to Peritoneal Encapsulation. The Korean Journal of Gastroenterology 2000;35:122-5.
- Mitrousias V, Alexiou E, Katsanas A, et al. The helix sign in the peritoneal encapsulation syndrome: a new sign in a rare cause of bowel obstruction. J Gastrointestin Liver Dis 2015;24:144. [Crossref] [PubMed]
- Mordehai J, Kleiner O, Kirshtein B, et al. Peritoneal encapsulation: a rare cause of bowel obstruction in children. J Pediatr Surg 2001;36:1059-61. [Crossref] [PubMed]
- Shamsuddin S, Bilal M, Rehman B, et al. Peritoneal encapsulation presenting as small bowel obstruction in a 16 year old girl. J Ayub Med Coll Abbottabad 2012;24:215. [PubMed]
- Sherigar JM, McFall B, Wali J. Peritoneal encapsulation: presenting as small bowel obstruction in an elderly woman. Ulster Med J 2007;76:42-4. [PubMed]
- Shioya T, Shibuya T, Tokunaga A, et al. Intestinal Obstruction due to Peritoneal Encapsulation of the Small Bowel. Nihon Gekakei Rengo Gakkaishi 2005;30:625-8. (Journal of Japanese College of Surgeons). [Crossref]
- Wani I, Wani KA, Wani M, et al. Peritoneal encapsulation, left paraduodenal hernia with retroperitonealization of inferior mesenteric vein leading to triple obstruction. Int J Case Rep Images 2013;4:169-74. [Crossref]
- Rajagopal AS, Rajagopal R. Conundrum of the cocoon: report of a case and review of the literature. Dis Colon Rectum 2003;46:1141-3. [Crossref] [PubMed]
- Ince V, Dirican A, Yilmaz M, et al. Peritoneal encapsulation in a patient with incomplete situs inversus. J Coll Physicians Surg Pak 2012;22:659-60. [PubMed]
Cite this article as: Giaracuni G, Gregori M, Barbieri V, Altamura A, D’Errico G, Mita MT, Rubichi F, Pizzicannella M, Barberio M, Viola MG. Minimally invasive treatment of congenital peritoneal encapsulation: a case report. Dig Med Res 2021;4:37.


