
Real-life experience with avatrombopag
Introduction
In chronic liver disease thrombocytopenia generally defines the presence of cirrhosis and is mostly due to splenic sequestration (1). Thrombocytopenia is defined as a platelet count below 150.0×109/L and severity of thrombocytopenia can be further subdivided as mild, moderate and severe if the platelet count is between 100.0×109/L to 150.0×109/L, 50.0×109/L to 95.0×109/L and <50.0×109/L, respectively (2). Because thrombocytopenia can predict the likelihood of bleeding, several guidelines recommend increasing the platelet count prior to invasive procedures in patients with cirrhosis and severe thrombocytopenia (3-8).
Management strategies for correcting thrombocytopenia in patients with cirrhosis generally include platelet transfusion and pharmacological treatment using thrombopoietin receptor agonists (9,10). Platelet transfusion has been established as the gold standard in the management of thrombocytopenia (11). Despite advances in reducing risk of adverse events such as improvement in collection and storage techniques platelet transfusion is not without complications such as infection, alloimmunization, febrile illness, and non-hemolytic reactions (11,12). Furthermore, in advanced liver disease newly produced platelets, as a result of portal hypertension leading to splenomegaly, are sequestered in the spleen potentially leading to suboptimal response to platelet transfusion (13).
Avatrombopag is an oral thrombopoietin receptor agonist which regulates megakaryocyte proliferation and differentiation into platelets thus increasing platelet count (14). It has been demonstrated that after starting avatrombopag platelet counts start to increase by day 4, peak between days 10–13, and return to baseline at day 35 post-initiation. (15). Avatrombopag has been shown to be an effective treatment of thrombocytopenia (16,17). Two international randomized, placebo-controlled, phase 3 trials (ADAPT-1 and ADAPT-2) found that amongst patients with chronic liver disease and thrombocytopenia undergoing procedures, avatrombopag was superior to placebo in raising platelet count and reducing the need for platelet transfusion or need for rescue procedure for bleeding up to 7 days after a scheduled procedure (15). In addition, a recent systematic review and meta-analysis concluded that avatrombopag was an effective treatment for thrombocytopenia (18). Avatrombopag is well tolerated with most common adverse events described in clinical trials being abdominal pain, dyspepsia, nausea, pyrexia, dizziness, and headache. In 2018, the Food and Drug Administration approved avatrombopag for the treatment of thrombocytopenia amongst chronic liver disease patients with thrombocytopenia scheduled to undergo a procedure (19).
Randomized controlled trials (RCTs) are the gold standard study design to assess the safety and efficacy of drugs (20). In RCTs a variety of inclusion and exclusion criteria are used to select a well-defined cohort that are likely to benefit from a particular treatment. As a result, studies assessing real-world data (RWD) may benefit from greater generalizability and external validity compared to RCTs (21). Efficacy of therapeutic intervention may not be absolute and may not have favorable outcomes in real-life conditions thus creating a gap between efficacy and effectiveness of treatment (22,23). Therefore, we sought to assess our real-world experience of the utility of avatrombopag as a treatment option for thrombocytopenia amongst patients with cirrhosis scheduled to undergo an invasive procedure. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/dmr-21-10).
Methods
We identified all patients who received avatrombopag dosing between July 2018 and December 2020 at the University of California Los Angeles Pfleger Liver Institute in Los Angeles, California. Patients were prescribed avatrombopag according to recommended dosing (19). Procedures were performed between 9 and 13 days after starting avatrombopag, and the dose of avatrombopag was dependent on baseline platelet count. Patients with a baseline platelet count of <40.0×109/L (low baseline) were prescribed avatrombopag 60 mg by mouth daily for 5 days, and those patient with platelet counts >40.0×109/L (high baseline) were prescribed avatrombopag 40 mg daily for 5 days.
This study was approved by the institutional review board of University of California at Los Angeles (UCLA). Inclusion criteria were: (I) patients with cirrhosis undergoing an invasive procedure and prescribed avatrombopag; (II) baseline platelet count <50.0×109/L within 5 months of the procedure avatrombopag; (III) and record of platelet transfusion and/or rescue therapy recorded for at least 7 days after the procedure. In the case a patient was prescribed multiple courses of avatrombopag, only the first episode was recorded. Patients were excluded if they were prescribed but did not take avatrombopag, or if there was any question as to if they took the drug.
Our cohort was subdivided into two subgroups: patients who had peri-procedure platelet count defined as platelet count collected within 72 hours of procedure, and patients who had imaging done post procedure.
Data was obtained through the electronic medical record and included gender, age, body mass index (BMI), race/ethnicity, pertinent co-morbidities, laboratory results and treatment details. Records were reviewed for adverse effects and need for rescue therapy. Patients were observed and monitored for complications 2–3 hours after invasive procedure. The presence of portal vein thrombosis was determined by the presence of thrombus in the portal vein by either abdominal ultrasound, MRI or computed tomography (CT).
Data collection and statistical analysis
Study data were collected and managed using the Research Electronic Data Capture (REDCap) application hosted at UCLA. Changes in platelet count were calculated. Descriptive statistics were reported using mean and standard deviation (SD) or percentage where appropriate.
Our primary outcome assessed efficacy as defined as the proportion of patients who did not require a platelet transfusion or rescue procedure for bleeding up to 7 days after a procedure. Secondary end points assessed adverse events as defined as the proportion of patients with adverse events and proportion of patients who developed portal vein thrombosis after receiving avatrombopag. Sensitivity analysis was performed on platelet response to avatrombopag.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board (No. 20-002122). Informed consent was not taken from the patients because it was performed retrospectively.
Results
All patients who made the inclusion criteria were included in our study and in our study period we assessed 29 patients with cirrhosis and severe thrombocytopenia who were prescribed avatrombopag before an invasive procedure (Table 1). The mean age was 62 years of age, and most patients were male (59%). The most common indication for avatrombopag was esophagogastroduodenoscopy (EGD) with planned esophageal band ligation (86%). The most common etiologies of cirrhosis were hepatitis C virus infection (HCV), non-alcoholic steatohepatitis, and alcohol-related liver disease. All patients with HCV had been cured with direct acting agents prior to initiation of avatrombopag.
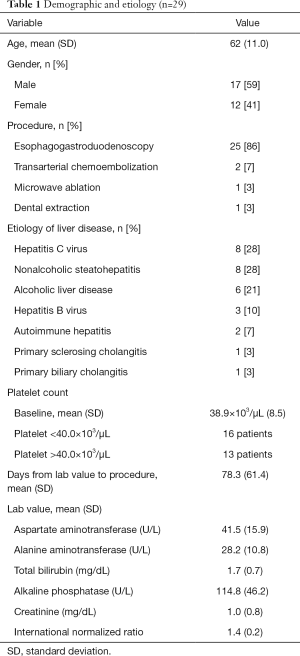
Full table
Mean liver associated tests are shown in Table 1. The overall mean (SD) baseline platelet count in the entire study cohort was 38.9×109/L (8.5×109/L). Sixteen patients had baseline platelet counts below 40.0×109/L and were prescribed 60 mg avatrombopag for 5 days, while 13 patients had had baseline platelet counts above 40.0×109/L and were prescribed 40 mg avatrombopag for 5 days. The overall mean (SD) time for baseline platelet count to procedure date was 78.3 (61.4) days.
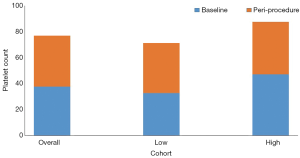
Of the 29 patients, 20 had peri-procedure platelet counts defined as collected within 72 hours of the invasive procedures. The platelet count increased 2-fold avatrombopag in the 20 patients; the mean (SD) baseline platelet count was 37.4×109/L (8.0×109/L) and the peri-procedure platelet count was 76.9×109/L (34.0×109/L). Specifically, the mean platelet count increased 2.2 folds in the low baseline platelet group, and 1.8 folds in the high baseline platelet group. Specifically, the mean platelet count increased from 32.0×109/L (3.0×109/L) to 71.0×109/L in the low baseline platelet group, and 47.0×109/L (4.8×109/L) to 87.6×109/L (30.9×109/L) in the high baseline platelet group.
There were no reports of adverse reactions to any of the 29 study patients. Twenty-four study patients had abdominal imaging after being prescribed avatrombopag, of which ten of these patients had evidence of portal vein thrombosis. However, all ten had documented portal vein thrombosis also prior to being prescribed avatrombopag. The mean (SD) time from imaging to invasive procedure was 146.2 (104.9) days, and from invasive procedure to follow up abdominal imaging mean of 116.8 (160.8) days.
Discussion
Thrombocytopenia is a common hematological complication of cirrhosis and its presence in patients with liver disease is a useful noninvasive indicator of the development fibrosis and portal hypertension (24,25). Due to the nature of the disease, patients with chronic liver disease undergo numerous diagnostic and therapeutic procedures, which may be limited in the setting of severe thrombocytopenia. Although guidelines vary in their recommendations for minimal platelet level prior to performing a procedure, many providers use platelet count of 50.0×109/L as a threshold to perform an invasive procedure (3-6). Platelet transfusion has been the gold standard for the treatment of thrombocytopenia but it is associated with complications such as transfusion reactions, infections, and refractory thrombocytopenia (11,26). Recently, thrombopoietin agonists such as avatrombopag have emerged as an alternative method to raise platelet count.
Avatrombopag is approved by the FDA for two indications: (I) to treat thrombocytopenia in patients with chronic liver disease who are scheduled to undergo an invasive elective procedure and (II) to treat thrombocytopenia in adult patients with chronic immune thrombocytopenia who have had an insufficient response to a previous treatment (15,17,19). However, avatrombopag is not FDA approved for the treatment of chemotherapy-induced thrombocytopenia because the results of a recent phase 3 trial demonstrated a lack of efficacy with avatrombopag (27).
The results of our study are similar to that seen in two randomized, double-blind, placebo-controlled phase 3 trials, ADAPT-1 (n=231) and ADAPT-2 (n=204) (15). In those two studies, the mean platelet count nearly doubled with avatrombopag. In ADAPT-1, the mean platelet count increased from 44.0×109/L to 81.0×109/L and 31.0×109/L to 63.0×109/L in the high and low baseline platelet groups, respectively. Similarly, in the ADAPT-2, the mean platelet count increased from 31.0×109/L to 63.0×109/L and 33.0×109/L to 64.0×109/L in the high and low baseline platelet groups, respectively. In our study, the platelets in patients in the high baseline platelet increased 1.8 folds from 47.1×109/L to 87.6×109/L. Similarly, the mean platelets in patients in the low baseline platelet increased 2.2 folds from 32.6×109/L to 71.2×109/L.
As with all thrombopoietin agonists, there is a potential risk of thromboembolism that must be monitored (19). In ADAPT-1 and ADAPT-2, adverse effects including portal vein thrombosis were uncommon (15). Similarly, we found avatrombopag to be safe and tolerable in patients with cirrhosis and thrombocytopenia who underwent invasive procedures. There were no reports of any adverse events including constitutional symptoms, and no patient required rescue therapy.
There are several important limitations to our study. This was an observational retrospective study with limitations inherent to this type of study design for evaluating therapeutic effects. Our study design highlights the generalizability of the results. The results of our study further support an update in the management of thrombocytopenia in patients undergoing invasive procedures Another limitation was the small sample size, nevertheless to date this is the only real-life experience studies published on avatrombopag. In addition, large number of patients excluded from the analysis largely because the procedure was cancelled or missing peri-procedure data (Figure 1). This observation highlights pragmatic challenges with procedures being rescheduled or cancelled in clinical practice. This is particularly true during the COVID-19 pandemic where there may be concerns of infection and the extra step of Covid testing prior to procedure is required. In regards to missing peri-procedure data, the most common reason was that the laboratory test was not ordered. Given our initial experience, we felt comfortable not waiting for repeat platelet count prior to performing invasive procedure. Although we are able to determine the date that the patient was prescribed the study drug, another limitation is that we are unable to confirm exact dates when the patient took avatrombopag. We were also unable to determine if the study subjects took the full prescribed course of the avatrombopag. Nevertheless, these are barriers that exist in common day real world practices. To mitigate these factors a dedicated staff is needed to coordinate care with patient, pharmacy, and procedure scheduling.
It is important to note that lusutrombopag is another thrombopoietin receptor agonist approved by the FDA to treat pre-procedure thrombocytopenia in patients with chronic liver disease scheduled to undergo a procedure (28). We are unable to comment on advantage of avatrombopag as there are no head-to-head studies comparing the two drugs. The respective trials differed in their base population and dosing regimen.
Avatrombopag is a safe, tolerable, and effective alternative to platelet transfusion in patients with thrombocytopenia and chronic liver disease who are scheduled to undergo a procedure. The results of our real-world study are consistent with two randomized controlled double blinded trials ADAPT-1 and ADAPT-2 which compared avatrombopag to placebo. Our RWD shows a favorable benefit-risk profile of avatrombopag, and provides an effective alternative to the status quo platelet transfusion for the treatment of thrombocytopenia in patients with chronic liver disease scheduled to undergo a procedure.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/dmr-21-10
Data Sharing Statement: Available at http://dx.doi.org/10.21037/dmr-21-10
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-10). SS serves as an unpaid editorial board member of Digestive Medicine Research from Apr 2020 to Mar 2022. SS reports he has received a speaker honorarium from Dova, and he is a consultant for Dova, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board (No. 20-002122). Informed consent was not taken from the patients because it was performed retrospectively.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int 2017;37:778-93. [Crossref] [PubMed]
- Williamson DR, Albert M, Heels-Ansdell D, et al. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest 2013;144:1207-15. [Crossref] [PubMed]
- Rockey DC, Caldwell SH, Goodman ZD, et al. American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009;49:1017-44. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707-18. [Crossref] [PubMed]
- Patel IJ, Davidson JC, Nikolic BStandards of Practice Committee, et al. with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol 2012;23:727-36. [Crossref] [PubMed]
- Nilles KM, Caldwell SH, Flamm SL. Thrombocytopenia and procedural prophylaxis in the era of thrombopoietin receptor agonists. Hepatol Commun 2019;3:1423-34. [Crossref] [PubMed]
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205-13. [Crossref] [PubMed]
- Szczepiorkowski ZM, Dunbar NM. Transfusion guidelines: when to transfuse. Hematology Am Soc Hematol Educ Program 2013;2013:638-44. [Crossref] [PubMed]
- Saab S, Brown RS Jr. Management of thrombocytopenia in patients with chronic liver disease. Dig Dis Sci 2019;64:2757-68. [Crossref] [PubMed]
- Maan R, de Knegt RJ, Veldt BJ. Management of thrombocytopenia in chronic liver disease: Focus on Pharmacotherapeutic Strategies. Drugs 2015;75:1981-92. [Crossref] [PubMed]
- Poordad F. Review article: thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther 2007;26:5-11. [Crossref] [PubMed]
- Murphy MF, Waters AH. Clinical aspects of platelet transfusions. Blood Coagul Fibrinolysis 1991;2:389-96. [Crossref] [PubMed]
- Kurokawa T, Ohkohchi N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol 2017;23:3228-39. [Crossref] [PubMed]
- Wolber EM, Jelkmann W. Thrombopoietin: the novel hepatic hormone. News Physiol Sci 2002;17:6-10. [PubMed]
- Terrault N, Chen YC, Izumi N, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 2018;155:705-18. [Crossref] [PubMed]
- Kuter DJ, Allen LF. Avatrombopag, an oral thrombopoietin receptor agonist: results of two double-blind, dose-rising, placebo-controlled Phase 1 studies. Br J Haematol 2018;183:466-78. [Crossref] [PubMed]
- Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol 2018;183:479-90. [Crossref] [PubMed]
- Li C, Li X, Huang F, et al. Efficacy and safety of avatrombopag in patients with thrombocytopenia: A systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 2019;10:829. [Crossref] [PubMed]
- Doptelet (Avatrombopag) [package insert]. Durham, North Carolina: Dova Pharmaceuticals, Inc., 2018.
- Barnish MS, Turner S. The value of pragmatic and observational studies in health care and public health. Pragmat Obs Res 2017;8:49-55. [Crossref] [PubMed]
- Maissenhaelter BE, Woolmore AL, Schlag PM. Real-world evidence research based on big data: Motivation-challenges-success factors. Onkologe (Berl) 2018;24:91-8. [Crossref] [PubMed]
- Eichler HG, Abadie E, Breckenridge A, et al. Bridging the efficacy-effectiveness gap: a regulator's perspective on addressing variability of drug response. Nat Rev Drug Discov 2011;10:495-506. [Crossref] [PubMed]
- Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf 2017;26:1033-9. [Crossref] [PubMed]
- Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol 2000;95:2936-9. [Crossref] [PubMed]
- Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis c-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726-36. [Crossref] [PubMed]
- Gangireddy VG, Kanneganti PC, Sridhar S, et al. Management of thrombocytopenia in advanced liver disease. Can J Gastroenterol Hepatol 2014;28:558-64. [Crossref] [PubMed]
- Sobi announces topline phase 3 data of avatrombopag for the treatment of chemotherapy-induced thrombocytopenia. News release. Swedish Orphan Biovitrum AB. October 9, 2020. Accessed October 9, 2020. Available online: https://bit.ly/2GNtb8C
- Peck-Radosavljevic M, Simon K, Iacobellis A, et al. Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures (L-PLUS 2). Hepatology 2019;70:1336-48. [Crossref] [PubMed]
Cite this article as: Verma D, Yum JJ, LeRoy K, McDaniel TK, Saab S. Real-life experience with avatrombopag. Dig Med Res 2021;4:27.


