
Endoscopic innovations in gastric and pyloric disease
Introduction
Surgeons have been innovating from the beginning. The concept of creating endoscopic solutions to manage previously open surgical problems, is not new. In 1980, Drs. Ponsky and Dr. Gauderer innovated the percutaneous endoscopic gastrostomy tube in pediatric patients, from the previously required laparotomy (1). Just under 30 years later, Dr. Inoue began to use the intramural space to perform the per oral endoscopic myotomy (POEM) procedure, a procedure for achalasia previously performed in both open and laparoscopic fashions (2). The ability to provide patients who suffer from a variety of foregut pathologies with incisionless solutions has been a decades long feat in trials of surgical innovation. Operating through the endoscope requires various instruments, both standard and therapeutic endoscopes, endoscopic instruments to traverse the channels of the scopes, knowledge of the anatomy and physiology of the area of the gastrointestinal (GI) tract you are operating on including the layers of the wall, as well as the extraluminal anatomy surrounding you, the ability to convert to a laparoscopic or open approach as needed, and patience. At times, the procedures performed require multiple surgeons and assistants. This review will cover some of the more recent events in intramural and other endoluminal surgery.
Intramural gastric surgery
The recent history of intramural surgery
Intramural surgery requires dissection and expansion of the tissue layer between the mucosa and the muscularis propria. During natural orifice transluminal endoscopic surgery, otherwise known as NOTES, a flap valve is created in this intramural space to offset any leakage into the peritoneum. Dr. Inoue utilized this discovered space, to innovate the Heller myotomy for achalasia. This resulted in the per oral endoscopic myotomy procedure, or “POEM”, created and described by Inoue et al. during the early 2000s (2). This procedure involved creating a submucosal bleb, by injection of saline, a mucosotomy, dissection of the submucosal space, with creation of a submucosal tunnel, and a myotomy. Dr. Inoue presented his procedural video at a SAGES conference in 2008 (2). It didn’t take long for innovative surgeons to expand this technique to other foregut disorders. Kawai et al. went on to described the endoscopic pyloromyotomy in 2012 (3). The investigators found that by using the technique of Inoue to dissect the submucosal space through a mucosotomy made proximal to the pylorus, allowed for the endoscopic delineation of the circular and longitudinal muscle fibers at the pylorus, allowing for a precise, and safe endoscopic myotomy. Their study, however, was performed on pigs. Less than a year later, Khashab et al. submitted a video manuscript of the first human endoscopic pyloromyotomy for gastroparesis, and in 2017 reported the first multicenter study (4,5).
Per-oral pyloromyotomy (POP)
From the time that it was realized that the gastric emptying procedure, laparoscopic pyloroplasty, was an effective stand-alone treatment for medically refractory gastroparesis (6)—it became feasible to perform the innovative endoscopic only gastric emptying procedure, POP as a first, stand-alone treatment, as well.
The indication for a POP is gastroparesis, most commonly diabetic, post-surgical, autoimmune or idiopathic gastroparesis. Prior to performance of POP the diagnosis of gastroparesis must be confirmed using the American society of gastroenterology guidelines—confirming symptoms of gastroparesis, without gastric obstruction or ulcer and delayed emptying demonstrated on gastric emptying study. The gastroparesis cardinal symptom index can be used to assess pre procedural symptomatology, this can also be used as a post procedural comparison tool. Patients should ideally undergo informed consent inclusive of a discussion of a laparoscopic pyloroplasty versus endoscopic pyloromyotomy. The details of both procedures and the need for general endotracheal anesthesia should be discussed with the patient. Patients with gastroparesis are at high risk for aspiration during induction of anesthesia. Therefore, the pre-operative plan should include a full liquid diet 2 days before the procedure, a clear liquid diet the day before, and the patient should be directed to remain nothing per os the midnight prior to the procedure.
At the authors institution, the POP has been performed hundreds of times for medically refractory gastroparesis and the short-term results have been successful, the long-term results are currently being studied (7). Our standard work up includes a formal solid 4-hour gastric emptying study, diagnostic endoscopy and a pre-operative gastroparesis cardinal symptom index. The general steps of the procedures follow, the specific of each step with accompanying photos and videos are published previously (8).
As with any procedure, the appropriate equipment availability should be confirmed prior to procedure start. For POP this will include a standard diagnostic endoscope, an overcap, an electrosurgical endoscopic knife and electrosurgical unit, an injection needle with retractable tip, and endoscopic hemostatic clips. It is the authors practice to place the patient supine on the operating room table. The surgeon and assistant stand on the left of the patient. A stage, typically made up of at least six steps in a three by two row fashion, is created for the surgeon and assistant to stand upon. The patient undergoes general endotracheal anesthesia for the procedure. Pre-operative antibiotics are up to surgeon discretion. The general steps of the procedure include the following:
Step 1 (Figure 1A): submucosal injection: an injection needle is used to deliver a submucosal injection of a solution containing blue dye. The site of injections is chosen based on the anatomy of the stomach. The authors prefer a site along the lesser curve 3–5 cm proximal to the pylorus.
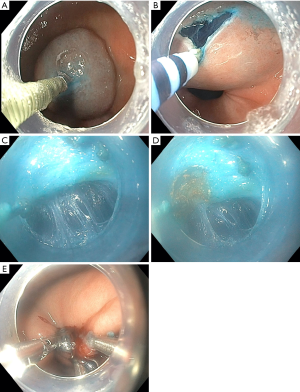
Step 2 (Figure 1B): mucosal incision: once the bleb is formed, a transverse incision is made using an endoscopic knife connected to an energy source with cut current. It is important to maintain an even inferior mucosotomy flap as the transverse mucosotomy is made.
Step 3 (Figure 1C): tunneling: once the endoscope is inserted into the submucosal plane, tunneling towards the pylorus is performed. Once the muscle fibers are identified, dissection is continued distally at the junction of the muscularis and submucosa. The mucosa of the duodenum becomes perpendicular to the tunnel just distal to the pylorus.
Step 4 (Figure 1D): division of the pylorus: the pylorus can then be divided using the electrosurgical knife on either cut or coagulation current.
Step 5 (Figure 1E): closure of the mucosotomy: hemostatic clips are a great and simple tool to re approximate the mucosal edges. This usually requires between three and five hemostatic clips.
Our institution started to perform the POP in January 2016 and reported on the first 100 patients in 2018. In this series, 85 of the patients were female and 56 suffered from idiopathic gastroparesis, with the majority of the remaining patients having diabetic and post-surgical gastroparesis. In this series, the short-term results were excellent. Significant changes were noted in terms of objective and subjective measures of gastroparesis symptoms and gastric emptying. The mean gastroparesis cardinal symptom index prior to POP was 3.8, which improved to 2.4, or an absolute difference of 1.4 points which was statistically significant. Changes in gastric emptying were also statistically significant. The mean percent retention at 4 hours was 39.9% prior to POP, which improved to a mean of 16.3% at 90-day follow-up. The long-term results are currently being studied (8). Several other institutions have published their results. The overwhelming results of currently published patient series are improvement in gastroparesis cardinal symptom index, gastric emptying studies, with a low morbidity profile.
Endoscopic management of strictures and stenoses
A stricture can be benign, malignant or post-operative. For the purpose of this chapter the authors will focus on benign and post-operative strictures and stenoses. The mainstay for endoscopic management of strictures is endoscopic dilation, which will be briefly described here. More recently endoscopic stricturoplasty has been utilized for stricture disease, especially at anastomotic sites, and will be described in detail. It must be kept in mind that previous to these endoscopic innovations, an anastomotic stricture, or gastric sleeve stenoses required either open or laparoscopic revisional surgery. The following interventions, up to a certain point, allow for incisionless, same day management of upper GI post-operative complications. The endoscopic surgeon, however must be prepared for the possibility of need for eventual operative intervention, whether due to failure of multiple endoscopic attempts or due to a complication of endoscopic intervention such as full thickness perforation unable to be repaired endoscopically.
A benign stricture of the gastro-esophageal junction is more often than not caused by reflux disease, while a benign stricture of the pylorus/duodenum is most likely related to peptic ulcer disease. The first step in treatment of benign strictures is balloon dilation. The second step is dilation with injection of steroids, if it is a gastro-esophageal stricture (9). A standard gastroscope can be used for balloon dilation. The surgical endoscopist advances the scope to the stricture, and will assess the size of the stricture to choose the first balloon size. If the standard scope can traverse the stricture, this determines the stricture to be at least 12 mm in diameter. The gastroscope view is held proximal to the stricture. The balloon catheter is advanced through the working channel beyond the stricture. A pressure gun is connected to the balloon catheter. Saline is injected to establish a controlled radial expansion. The various pressures are held for 60 seconds. The stricture dilation itself can be visualized by the endoscopist by pulling back on the balloon catheter, diverting the left-right wheel in towards the balloon, and creating a water interface by use of the blue irrigation button (Figure 2). The dilation commonly causes bleeding which can be noticed through the balloon while actively dilating. If a “dark space” is noted through the balloon during dilation, this indicates a full thickness perforation. This technique is frequently used for sleeve gastrectomy stenosis, as well (10).
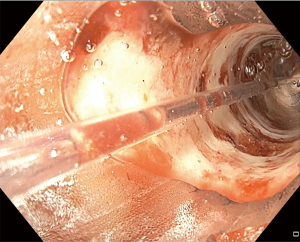
Strictures and stenoses as a complication of foregut surgery, whether it be following paraoesophageal hernia repair with fundoplication, anastomosis after partial or total gastrectomy for benign or malignant disease, or following bariatric surgery with gastrojejunal anastomosis or gastric sleeve stenosis can also typically be first treated with balloon dilation. The technique is the same as the description above, in the case of anastomotic stricture the balloon catheter is advanced across the anastomosis. One of the most frequent complications of bariatric surgery is stricture/stenosis. After Roux-en-Y gastric bypass the stricture is typically at the gastrojejunal anastomosis, and after gastric sleeve is stenosis at the level of the incisura. If it is a stricture after gastrojejunal anastomosis for bariatric surgery, the intended dilation of the anastomosis will be smaller (up to 18 mm), to avoid weight regain. A video of a balloon dilation and stent placement for stricture for a patient status post pancreaticoduodenectomy with a gastrojejunal anastomosis stricture accompanies this review, provided by Dr. Matthew Allemang and Dr. John Rodriguez (Cleveland Clinic).
Anastomotic strictures typically occur 3–4 weeks post operatively, but can present earlier or later. Symptoms include bloating, reflux, regurgitation, emesis and weight loss (11). When repeated balloon dilation fails (usually attempted at least three times with injection of steroids as an adjunct), the step the authors choose to employ is endoscopic stricuturoplasty (Video 1).
Endoscopic stricturoplasty
An endoscopic stricturoplasty is done under general anesthesia in the supine position. The procedure requires an endoscopic electrosurgical knife, an electrosurgical unit, and a standard gastroscope. The authors prefer to use a gold probe coagulation catheter to mark the sites of incision and again to make a stage to the left of the patient and to perform the procedure with an assistant. The gastroscope is advanced to the stricture, and the gold probe catheter is utilized to mark out the intended stricture incision sites. The electrosurgical needle knife is then used to create the stricturoplasty which is comprised of making multiple radial cuts in a circumferential manner to cut through the scar tissue. Post stricturoplasty stent placement can enhance and maintain the results by decreasing the chance of re-scarring down to the original stricture size.
Post sleeve gastrectomy stenosis occurs typically at the level of the incisura. Patients present with nausea, epigastric pain, inability to tolerate oral intake, and failure to thrive. Typically, an upper GI swallow study is performed for diagnosis and an upper endoscopy follows. The first steps in management are similar to the description above for benign gastro-esophageal strictures and anastomotic strictures. The balloon dilation, however, can safely be up to 30 mm with an achalasia balloon. If these steps fail, a recently described procedure for sleeve stenosis is tunneled stricturotomy, first reported by De Moura et al., which employs techniques from intramural surgery, including mucosotomy and submucosal tunneling (12).
Tunneled endoscopic stricturotomy
This innovative procedure is new and there is currently no data to support its use, however the results of the initial reports are successful and the concept and techniques are sensical and derived from known endoscopic procedures. The required instruments are a standard gastroscope, a beveled overcap, an injection needle with saline, an endoscopic electrosurgical needle knife, and hemostatic clips or an endoscopic suturing device.
Step 1: endoscopic identification of the stenosis with a standard gastroscope.
Step 2: submucosal injection with creation of a bleb 3–5 cm proximal to the stenosis.
Step 3: mucosotomy using an endoscopic electrosurgical needle knife.
Step 4: submucosal tunneling with continued injections of saline during tunneling to decrease the risk of perforation. This tunneling can be difficult because of the level of fibrosis and scarring. It is important to exit the tunnel as needed to confirm the direction of tunneling.
Step 5: stricturotomy by incising the scar tissue and the underlying muscularis with the electrosurgical endoscopic needle knife.
Step 6: closure of the mucosotomy using hemostatic clips or an endoscopic suturing device.
A video abstract by de Moura et al. is available in VideoGIE and is referenced below (13).
Endoscopic submucosal dissection and endoscopic mucosal resection for gastric masses
Endoscopic submucosal dissection may be considered for treatment of superficial premalignant and malignant lesions of the GI tract in the absence of lymph node or distant metastases. It is currently recommended in the National Comprehensive Cancer Network guidelines for T1a and Tis lesions, with a less than 2% chance of lymph node metastasis. A T1b lesions has a lymph node metastasis rate starting at 8.4% (14). Early-stage gastric cancers are much more commonly diagnosed in Japan than in the United States, and much of the data for endoscopic submucosal dissection and advances in the technique have occurred there. In general, this technique is under-utilized in the United states, with the majority of endoscopic surgeons employing endoscopic mucosal resection. The advantage of endoscopic mucosal dissection is the ability to obtain and R0 resection on larger lesions, those greater than 1.5 to 2 cm. With endoscopic mucosal resection, a snare is used, and when resecting larger lesions, a piecemeal fashion is required, limiting the ability to get a clear R0 resection and to analyze the pathologic specimen accurately which can result in need for formal resection (15). Again, these innovations have given patients with a diagnosis of early-stage gastric cancer the ability to undergo curative, incisionless resection. The technique for endoscopic submucosal dissection, alternatively, shares instruments and techniques of endoscopic procedures described earlier in this chapter.
Endoscopic submucosal dissection technique
Numerous therapeutic gastroscopes are available for endoscopic submucosal dissection, although it is possible to use a standard gastroscope. There are various methods used to enhance the ability to obtain R0 resection such as chromoendoscopy, narrow band imaging, and most recently magnifying endoscopy with allowance for histopathological evaluation of margins in real-time (16). Description of these techniques is beyond the scope of this review. Endoscopic submucosal dissection is performed in the supine position, under general anesthesia. An electrosurgical unit it required. The technique is characterized by the following steps:
Step 1: identification of the lesion, using a therapeutic or standard gastroscope.
Step 2: marking of the lesion circumferentially with endoscopic electrocautery, such as the gold probe.
Step 3: submucosal injection of fluid to separate the lesion from the underlying muscular layers.
Step 4: mucosal incision circumferentially with electrosurgical knife where marked.
Step 5: submucosal tunneling between the lesion and the muscular layers using some type of endoscopic electrosurgical knife (triangle knife, insulated tip, scissors, etc.).
Step 6: hemostasis, coagulation and/or clipping of any visible bleeding vessels.
This technique has been enhanced by creation of longer lasting injectable fluids such as hyaluronic acid solutions, glycerin gels, as well as introduction of methods for retraction of the lesion upwards during the dissection (17). Recent innovation, such as the versatile robotic tool presented by Mylonas et al. at the SAGES conference in 2017, allows for even greater dexterity for endoscopic submucosal dissection (18). This tool includes a universal overcap with robotic arms controlled by the surgeon at a separate platform, allowing for bimanual dexterity, triangulation and erases the need for the surgeon to control the wheels on the endoscope. Simpler solutions to improve retraction and visualization during endoscopic submucosal dissection include a beveled overcap, and caps with irrigation ports or snare ports however many of the distal attachments are unavailable in the United States (19). Another interesting solution to retraction is the suture-pulley method, in which an endoscopic suturing device is used to take bites of the edges of the mucosa of the lesion, the sutures pulled through the mouth, and an assistant retracts the lesion away from the underlying muscularis. This tents the submucosal fibers, putting the submucosa on tension, and allowing for an easier submucosal dissection under the desired lesion (20).
Endoscopic management of benign gastric polyps
Gastric polyps are most frequently identified incidentally during diagnostic endoscopy for other concerns. Hyperplastic polyps are a pre-malignant condition of the gastric mucosa, and are associated with an increased risk of gastric cancer. The most recent American Society of Gastrointestinal Endoscopy guidelines recommend polypectomy for any hyperplastic polyp greater than 0.5 cm. Endoscopic mucosal resection can be definitive for hyperplastic polyps. Adenomatous polyps have a higher pre malignant potential and should ideally be treated with endoscopic submucosal dissection to decrease the chance of local recurrence. Follow up endoscopy should be performed to ensure no recurrence. There have been several reports of large or multiple antral polyps causing gastric outlet obstruction. Sometimes these patients present with iron deficiency anemia, sometimes with intermittent obstructive symptoms and weight loss, and in one report with biochemical pancreatitis from a pedunculated polyp prolapsing into the duodenum and compressing the ampulla of Vater (21). Multiple antral polyps are most commonly hyperplastic and related to underlying inflammation either bile reflux gastritis, heliobacter pylori gastritis, or reactive gastritis. Any symptomatic polyp must be resected and sent for pathologic evaluation. In addition, the underlying inflammatory mechanism must be managed (22). The techniques described above for endoscopic mucosal resection and endoscopic submucosal dissection can be reviewed in reference to benign polyp management.
Endoscopic management of gastric outlet obstruction
Gastric outlet obstruction can be benign or malignant and is characterized by epigastric abdominal pain and postprandial vomiting, eventually associated with nausea, abdominal bloating, early satiety, and weight loss. For benign obstructions due to scarring/stricturing from peptic ulcer disease, please review the description of balloon dilation above. Secondarily, stenting can be performed either through a therapeutic endoscope or through a standard gastroscope with fluoroscopic guidance. For malignant disease, or some benign indications, a relatively new technique utilizing magnets for creation of an endoscopic gastrojejunal anastomosis will be described here.
The preferred method of treating gastric outlet obstruction, with longest term patency, is a surgical gastrojejunostomy, whether it be open or laparoscopic. However, in the setting of malignancy, with acute or chronic gastric outlet obstruction, with malnutrition and other metabolic consequences of obstruction the adverse event rate post operatively can be quite high. Endoscopic surgeons have innovated several methods to provide a more minimally invasive treatment for this group of patients, which allows for relief of the obstruction and ability for oral intake, even if in the setting of palliation.
Stenting across the obstruction is an option, if either a scope or a wire can traverse the lesion. Stents however may not fully expand, and experience eventual ingrowth, which will the majority of the time require repeat intervention. In general these stents have a 50–60% patency rate at 6 months (23). More recent endoscopic methods for bypass of gastric outlet obstruction include lumen apposing metal stents, and endoscopic magnetic gastro-jejunal anastomoses. Lumen apposing metal stents are endoscopic ultrasound-guided. There are two methods of placement, assisted and unassisted. The assisted version includes placement of a wire and then catheter across the obstruction. Following this an endoscopic ultrasound is advanced into the stomach. Contrast is injected into the catheter in the jejunal lumen, in order to identify the jejunal loop with two methods, fluoroscopically and with endoscopic ultrasound. The jejunum is access with a needle, a wire is placed into the jejunum, and then after serial dilations of the tract the lumen apposing metal stent system is deployed. The stent is then balloon dilated. There is a direct puncture technique using endoscopic ultrasound alone to identify the jejunal loop. In addition, natural orifice transluminal endoscopic surgery can be utilized to traverse the gastric wall, into the peritoneum to identify a jejunal loop just beyond the ligament of treitz to deploy the lumen apposing stent into. This remains an endoscopic ultrasound guided procedure and does utilize wire placement across the gastric wall prior to accessing the peritoneum (24). An advantage of these lumen apposing metal stents compared to typical enteral stents placed directly across obstructing lesions, is the lack of tissue ingrowth and potential for long term patency.
An innovation, the concept of which is not new but has been recently revived, for malignant gastric outlet obstruction is endoscopic magnetic gastrojejunal anastomosis. Magnetic compression anastomoses were first reported by Yamanouchi et al. in 2002, for biliary enteric anastomoses (25). To perform a magnetic anastomosis, two rare-earth magnets (such as neomydium or samarium cobalt) are placed within the intended proximal and distal bowel sites inducing necrosis at the compression site between the two lumens, by allowing them to adsorb. These magnetic anastomoses have been formed in the upper and lower GI tracts, and as mentioned in the biliary system. The gastrojejunal anastomosis may be of most ease, in terms of magnetic compression, because delivery of the magnets to the intended bowel is the most difficult part of the procedure for more distal anastomoses (25). The “magnamosis” device was first presented in humans as a small bowel-small bowel anastomosis, with open method of placement at the SAGES conference in 2017 (26). The following technique of magnetic compression anastomosis is derived from a case report from 2019, the authors created a gastrojejunal anastomosis for superior mesenteric artery (SMA) syndrome in an elderly patient who could not tolerate a major operation (27).
Endoscopic gastrojejunal magnetic compression anastomosis technique (26)
Step 1: choose the magnet type and size.
Step 2: identify target lumens using axial imaging, fluoroscopic imaging, and endoscopy.
Step 3: advance the endoscope to the intended distal target lumen (proximal jejunum).
Step 4: advance a guidewire through the scope, remove the endoscope under fluoroscopic guidance, maintaining location of the distal wire tip.
Step 5: place the magnet over the wire, and push forward with a standard front viewing gastroscope, to the intended distal target site.
Step 6: deliver the other magnet to the intended gastric site using biopsy forceps.
Step 7: observe the meeting of the magnets under fluoroscopy.
The anastomoses created by magnets take 5–10 days to mature and do frequently require post procedural dilations. Data must still accrue with continued in human magnetic compression anastomoses.
Conclusions
There are advances being made daily towards the most minimally invasive solutions for the upper GI problems that our patients face. The endoscopic armamentarium is growing rapidly. It is likely that some of the procedures in this review will become obsolete over the next decade. The problems—gastric motility disorders, post foregut and bariatric surgery complications, early-stage gastric cancer diagnoses will persist and potentially increase in frequency. Despite the continued advances, endoscopic surgeons must always be prepared to convert to a laparoscopic or open procedure if the endoscopic approach fails. This review, certainly not comprehensive of all of the innovations in endoscopic surgery of the stomach and pylorus, focused on the endoscopic management of gastroparesis, endoscopic submucosal dissection for gastric masses, and post-operative management of foregut surgery complications including strictures and stenoses, and the current endoscopic management of gastric outlet obstruction. An area of rapid expansion that is of great interest is in robotic natural orifice transluminal endoscopic surgery. The triangulation and dexterity allowed by these systems will likely enhance the performance of many of the techniques discussed in this review.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alfredo Daniel Guerron) for the series “Advanced Laparoscopic Gastric Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-3). The series “Advanced Laparoscopic Gastric Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg 1980;15:872-5. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Kawai M, Peretta S, Burckhardt O, et al. Endoscopic pyloromyotomy: a new concept of minimally invasive surgery for pyloric stenosis. Endoscopy 2012;44:169-73. [Crossref] [PubMed]
- Khashab MA, Stein E, Clarke JO, et al. Gastric peroral endoscopic myotomy for refractory gastroparesis: first human endoscopic pyloromyotomy (with video). Gastrointest Endosc 2013;78:764-8. [Crossref] [PubMed]
- Khashab MA, Ngamruengphong S, Carr-Locke D, et al. Gastric per-oral endoscopic myotomy for refractory gastroparesis: results from the first multicenter study on endoscopic pyloromyotomy (with video). Gastrointest Endosc 2017;85:123-8. [Crossref] [PubMed]
- Hibbard ML, Dunst CM, Swanström LL. Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg 2011;15:1513-9. [Crossref] [PubMed]
- Rodriguez J, Strong AT, Haskins IN, et al. Per-oral pyloromyotomy (POP) for medically refractory gastroparesis: short term results from the first 100 patients at a high volume center. Ann Surg 2018;268:421-30. [Crossref] [PubMed]
- Lundgren M, Rodriguez JH. Peroral pyloromyotomy. Surg Clin North Am 2020;100:1193-200. [Crossref] [PubMed]
- van Boeckel PG, Siersema PD. Refractory esophageal strictures: what to do when dilation fails. Curr Treat Options Gastroenterol 2015;13:47-58. [Crossref] [PubMed]
- Deslauriers V, Beauchamp A, Garofalo F, et al. Endoscopic management of post-laparoscopic sleeve gastrectomy stenosis. Surg Endosc 2018;32:601-9. [Crossref] [PubMed]
- Ardila-Gatas J, Pryor A. Endoscopic approach for the treatment of bariatric surgery complications. Mini-invasive Surg 2020;4:16. [Crossref]
- De Moura EGH, de Moura DTH, Sakai CM, et al. Endoscopic tunneled stricturotomy with full-thickness dissection in the management of a sleeve gastrectomy stenosis. Obes Surg 2019;29:2711-2. [Crossref] [PubMed]
- de Moura DTH, Jirapinyo P, Aihara H, et al. Endoscopic tunneled stricturotomy in the treatment of stenosis after sleeve gastrectomy. VideoGIE 2018;4:68-71. [Crossref] [PubMed]
- Gastric Cancer (version 1.2018). NCCN Clinical Practice Guidelines in Oncology 2018. Available online: https://www.nccn. org/professionals/physician_gls/PDF/gastric.pdf
- Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16-25.e1. [Crossref] [PubMed]
- Kim JW. Usefulness of narrow-band imaging in endoscopic submucosal dissection of the stomach. Clin Endosc 2018;51:527-33. [Crossref] [PubMed]
- Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol 2008;14:2962-7. [Crossref] [PubMed]
- Mylonas GP, Vitiello V, Cundy TP, et al. CYCLOPS: A versatile robotic tool for bimanual single-access and natural-orifice endoscopic surgery. In: 2014 IEEE International Conference on Robotics and Automation (ICRA). IEEE, 2014:2436-42.
- Maple JT, Abu Dayyeh BK, Chauhan SS, et al. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311-25. [Crossref] [PubMed]
- Aihara H, Kumar N, Ryou M, et al. Facilitating endoscopic submucosal dissection: the suture-pulley method significantly improves procedure time and minimizes technical difficulty compared with conventional technique: an ex vivo study (with video). Gastrointest Endosc 2014;80:495-502. [Crossref] [PubMed]
- Chahla E, Kim MA, Beal BT, et al. Gastroduodenal intussusception, intermittent biliary obstruction and biochemical pancreatitis due to a gastric hyperplastic polyp. Case Rep Gastroenterol 2014;8:371-6. [Crossref] [PubMed]
- Schmocker RK, Lidor AO. Management of non-neoplastic gastric lesions. Surg Clin North Am 2017;97:387-403. [Crossref] [PubMed]
- Phillips MS, Gosain S, Bonatti H, et al. Enteral stents for malignancy: a report of 46 consecutive cases over 10 years, with critical review of complications. J Gastrointest Surg 2008;12:2045-50. [Crossref] [PubMed]
- Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open 2016;4:E276-81. [Crossref] [PubMed]
- Yamanouchi E, Kumano R, Kobayashi K, et al. Treatment for bowel or biliary obstruction by magnetic compression anastomosis development of Yamanouchi's method and its clinical evaluation. J Nippon Med Sch 2002;69:471-5. [Crossref] [PubMed]
- Pichakron KO, Jelin EB, Hirose S, et al. Magnamosis II: Magnetic compression anastomosis for minimally invasive gastrojejunostomy and jejunojejunostomy. J Am Coll Surg 2011;212:42-9. [Crossref] [PubMed]
- Kamada T, Ohdaira H, Takeuchi H, et al. New technique for magnetic compression anastomosis without incision for gastrointestinal obstruction. J Am Coll Surg 2021;232:170-7.e2. [Crossref] [PubMed]
Cite this article as: Lundgren M, Rodriguez J. Endoscopic innovations in gastric and pyloric disease. Dig Med Res 2021;4:9.


