
Risk stratification of the cancer patient: a narrative review
Cancer morbidity
Cancer surgery carries significant morbidity risk, especially given the increasing age of patients undergoing treatment. Colorectal cancer, one of the commonest malignancies in the UK, has a reported morbidity rate in the range of 15–25% with some centres reporting rates over 50% (1-3). This is significant as 25% of all colorectal cancer deaths in the first year are attributed to postoperative complications (4).
Postoperative complications not only affect short term outcomes but there is evidence, for example in colorectal and pancreatic cancer, that morbidity following surgery may be associated with reduced long term survival and increased disease recurrence (5,6). The reason for the association between postoperative morbidity and long-term outcomes is not well understood. Plausible explanations would include: postoperative complications alter the behaviour of the cancer, that they are both a result of inadequate surgery, or postoperative morbidity delays adjuvant therapy (5). Where infective complications occur, the associated systemic inflammatory response may suppress cell-mediated immunity and promote tumour progression (7). Regardless of the aetiology, the development of postoperative complications has been shown to be more predictive of survival following major surgery than baseline characteristics and intraoperative events, and therefore efforts should be directed towards minimisation of postoperative morbidity by appropriate choice of cancer treatment, preoperative optimisation and postoperative destination (8).
Preoperative risk stratification
Preoperative risk stratification supports clearer communication between clinicians as well as better allocation of resources and planning of postoperative care. It enables patient understanding of postoperative morbidity and promotes discussions between patients and clinicians to aid shared decision making.
The “Choosing Wisely” Initiative is a global initiative that promotes shared decision making, whereby doctors and patients work together to select a management plan based on the patient’s informed preferences (9). Several cancer treatment options may be available. For example in gastrointestinal cancer surgery: local resection, radical resection with anastomosis or resection with stoma, or chemoradiotherapy. All carry different risks, and an accurate description of the estimated risks and benefits of potential management options allows informed decision making. That further helps the patients take ownership of their treatment, engage actively in optimisation and better tolerate potential postoperative complications.
Preoperative risk stratification needs to assess more than just the predicted mortality, as the threat of prolonged postoperative disability may have a far greater influence on a patient’s treatment preferences than dying itself. Objective risk stratification prior to elective surgery provides a baseline assessment that can be repeated over time, for example in order to evaluate deconditioning often associated with chemotherapy or response to an exercise programme. It is particularly important for cancer patients due to the comorbidity burden associated with certain types of malignancy as well as the effects of either the treatment or the cancer itself.
The objective of this article is to review the tools that may be used to assist the risk stratification of patients undergoing cancer surgery. PubMed was searched using combinations of the terms ‘preoperative assessment,’ ‘cancer’, ‘risk tool’, ‘frailty’, ‘cardiopulmonary exercise test’ and ‘biomarkers’ from 1990 through March 2020. References of selected articles were also searched. We present the following article in accordance with the Narrative Review reporting Checklist (available at http://dx.doi.org/10.21037/dmr-20-69).
Risk scoring systems
Several preoperative risk assessment tools have been described. The Surgical Outcome Risk Tool (SORT) was developed by analysis of data from the NCEPOD database. It requires input of 6 variables and predicts 30-day mortality. Lee’s revised cardiac index also includes 6 variables and estimates the risk of 30-day mortality, MI or cardiac arrest (10,11). Both tools are quick and simple to use, however, they do not predict morbidity and are, therefore, of limited value to patients’ decision making.
Postoperative morbidity prediction tools include the POSSUM (Physiological and Operative Severity Score for the enumeration of Mortality and morbidity) and ACS NSQIP (American College of Surgeons National Surgical Quality Improvement Project) risk calculators (12,13).
The POSSUM score gives an estimated risk of mortality and morbidity following the input of 12 physiological and 6 surgical parameters. It was originally developed by Copeland et al. in 1991 based on data obtained from 1,372 patients who underwent elective or emergency surgery between 1988 and 1989 at Walton Hospital in Liverpool, UK and was later modified to Portsmouth POSSUM or P-POSSUM by Prytherch et al. in 1998 to give a more accurate prediction of mortality (12,14). The surgical parameters required include some intraoperative data and, therefore, it can only be reliably used retrospectively. The score has been modified and validated for numerous subtypes of surgery including colorectal, oncologic gastric and hepatectomy. However, it may significantly overpredict morbidity and mortality, particularly in low risk patients (15). Wakabayashi et al. found a ratio of observed to predicted morbidity for patients undergoing gastrointestinal cancer surgery of 0.23. The observed to predicted mortality ratios from the POSSUM and P-POSSUM scores were 0.15 and 0.38 respectively (16). Moreover, the score’s validity in abdominal surgery for malignant disease may be less than that for benign disease (17).
The ACS NSQIP risk calculator is an online tool that uses an algorithm and validated data derived from over 500 hospitals and 2.7 million operations performed in the United States to predict the likelihood of 12 postoperative outcomes. It requires the input of a surgery-specific Current Procedure Terminology code along with 20 demographic and comorbidity variables in order to predict the risk of the 12 postoperative outcomes within 30 days after surgery (Figure 1) (18). Large internal studies have shown that the risk calculator is accurate at predicting postoperative complications, however external validation in several surgical subspecialties, including cancer surgery, has yielded variable results. As described by Rivard for gynaecological oncology laparotomy, the calculator performed well for predicting death, renal failure and cardiac complications, but did not accurately predict most other complications (19). Similarly, following surgery for gastrointestinal neuroendocrine tumour, Armstrong et al. showed that the calculator provided reasonable estimates of the risks for pneumonia, cardiac complication, urinary tract infection and discharge to nursing/rehabilitation facility [area under the curve (AUC) >0.7], but performed poorly (AUC <0.7) for other complications such as surgical site infection, return to theatre and readmission (20).
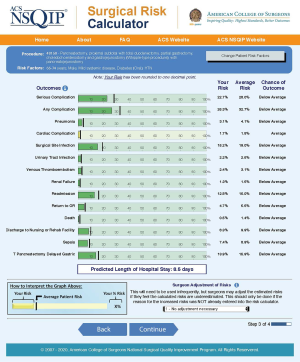
The Oncology NSQIP National Cancer Centre Collaborative in 2016 attempted to add oncology related variables (previous surgery in the operative region, previous radiotherapy and previous chemotherapy) to assess whether that would improve the accuracy of the ACS NSQIP risk prediction tool. However, the added variables failed to improve the modelling (21).
It is important to note that attempts at external validation of the risk calculator are usually limited to a single centre and a single surgical subspecialty; they suffer therefore, from high case-mix homogeneity and in combination with a relatively small number of cases, it is not surprising that they have shown poor discrimination of the ACS NSQIP calculator (22). The risk prediction model is a useful tool that can be used as an aid in preoperative assessment to support shared decision making across different specialties but should not be the only method employed.
Frailty indices
Frailty is a distinctive health state related to the ageing process in which multiple body systems gradually lose their in-built reserves. Around 10% of people aged over 65 years have frailty, rising to 25–50% of those aged over 85 (23). Particularly prevalent in patients with cancer, the median reported incidence being 42%, it is associated with both increased morbidity and mortality (24). Frailty is potentially reversible, and an objective measure of frailty is a useful tool that can be used in the decision-making process as well as to guide prehabilitation programmes.
The modified frailty index (mFI-11) was initially developed by Obeid et al. in 2012 and assessed 11 variables identified from the NSQIP database (25). The index is equal to the (number of variables present)/(number of variables assessed) and takes a value from 0 to 1 with values >0.36 indicating frailty. In 2015 it was simplified to a 5-factor predictive index (mFI-5) by Chimukangara et al. that has been shown to be equally predictive of mortality and postoperative complications within all surgical specialties (26). The five factors assessed are: functional status, diabetes, hypertension, history of cardiac failure and history of COPD. The index is calculated by adding the variables present for each patient with 0–5 points possible.
Using the mFI-11, frail patients demonstrated higher 30-day mortality rates compared with non-frail patients undergoing surgery for pancreatic (6.3% vs. 2.7%), head and neck (11.9% vs. 0.2%), and bladder cancers (3.5% vs. 1.8%) (27). This was echoed in a meta-analysis by Handforth et al. in 2015 looking at outcomes of older cancer patients; frail patients had over double the risk of death at 30 days postoperatively compared to the non-frail patients (28).
Frailty has also been associated with higher complication rates. Using the mFI-11, Vermillion et al. showed that patients with mFI-11 >0.27 undergoing surgery for gastrointestinal cancer had a 29% incidence of major complications versus 18% for those with mFI-11 ≤0.27 (29). Mogal et al. showed that frailty, as assessed by mFI-11, is an independent predictor of postoperative morbidity (OR 1.54; 95% CI: 1.29–1.85) and mortality (OR 1.54; 95% CI: 1.05–2.25) in patients undergoing pancreaticoduodenectomy for cancer. The incidence of a major complication was approximately 41% for those with mFI-11 >0.27 and 28% for those with mFI-11 ≤0.27 (30).
Using the simpler mFI-5, Hodari et al. and showed that patients undergoing oesophagectomy with frailty scores of 0, 1, 2, 3, 4 and 5 had associated morbidity of 18%, 25%, 31%, 34%, 44% and 62% respectively (31). Increased morbidity has also been shown for frail patients undergoing surgery for colorectal, head and neck, and bladder cancer (32-34).
Functional assessment and cardiopulmonary exercise test
The development of complications following surgery is closely related to the physiological impact of the surgical insult. Major surgery triggers a systemic inflammatory response that results in a rise in oxygen requirements from approximately 3.5 mL/kg/min in the resting state (1 metabolic equivalent or MET) to 5 mL/kg/min following body cavity surgery. If this increased oxygen demand cannot be met, an inevitable oxygen supply and demand imbalance will result in tissue ischaemia and a consequent rise in complication rates (35).
The ability to increase oxygen delivery to match demand is described by the term functional capacity. Cardiopulmonary exercise testing (CPET) represents an objective measurement of functional capacity and is considered the gold standard in this regard. It is a comprehensive preoperative assessment tool that allows for identification of undiagnosed respiratory and/or cardiac disease, and can direct patients to further specialist investigation or intervention. It is, therefore, increasingly being used preoperatively with approximately 30,000 tests being performed annually in surgical patients within the UK (36).
Anaerobic threshold (AT) describes the point at which energy requirements can no longer be sustained with aerobic respiration alone. It is the point at which the rate of change in arterial lactate and ventilation rapidly increase and usually occurs at about 50–60% of maximum predicted oxygen consumption (37). In 1993, Older et al. first identified an AT of <11 mL/kg/min as predictive for high risk of postoperative cardiovascular mortality in elderly patients undergoing major abdominal surgery. Patients with AT <11 mL/kg/min had over 20 times the risk of postoperative cardiovascular death compared to patients with AT >11 mL/kg/min (38). Specifically, the mortality risk for patients with AT of 11 to less than 14, 8 to less than 11 and <8 was approximately 1%, 16% and 50% respectively, these thresholds corresponding to low, intermediate and high risk for postoperative mortality (38).
Nagamatsu et al. later investigated the CPET outcomes of 91 patients undergoing oesophageal resection for cancer. They identified a peak oxygen consumption (peak VO2) value <800 mL/min/m2 as high risk for significant cardiopulmonary morbidity following oesophagectomy. Peak VO2 is the highest oxygen uptake attained at end-exercise, averaged over about a 20-second period and is reflective of the patient’s best effort (37). Patients with peak VO2 >800 mL/min/m2 had less than 10% risk of cardiopulmonary complications and were considered to belong in the low risk group. Peak VO2 of 700 to less than 800 mL/min/m2 was associated with a 44% incidence of cardiopulmonary complications and was classified as intermediate risk, whereas the rate of complications for peak VO2 <700 mL/min/m2 was over 80% and this threshold was considered to be predictive of high postoperative risk (39).
In 2014, West et al. investigated the association between CPET variables and postoperative outcomes in 136 patients undergoing elective colorectal surgery, mostly for cancer (40). AT, peak VO2 and ventilatory equivalent for CO2 (VE/VCO2) were associated with day 5 morbidity. The cut-off points identified were 10.1 mL/kg/min for AT, 16.7 mL/kg/min for peak VO2 and 32.9 for VE/VCO2 at AT (40). VE/VCO2 describes the patient’s ventilatory efficiency, i.e., the volume of air that needs to be ventilated in order to exhale a total of 1 L of carbon dioxide. A high VE/VCO2 indicates the presence of ventilation perfusion mismatch, for example in heart failure, chronic lung disease or pulmonary hypertension (37).
In 2013, Chandrabalan et al. studied the CPET outcomes of 100 patients that underwent pancreatectomy. The median AT was 10.3 mL/kg/min (IQR, 8.8–11.6). They identified an AT of <10 mL/kg/min as predictive of high risk for the development of postoperative surgical complications (pancreatic fistula and major intra-abdominal abscesses) as well as prolonged hospital stay and lower likelihood of receiving adjuvant therapy. AT, however, was not predictive of mortality or cardiopulmonary complications (41). More recently, Patel et al. investigated the association between CPET outcomes and prospectively collected postoperative morbidity data in 120 patients undergoing oesophagectomy within an enhanced recovery programme (42). Patients with peak VO2 and AT less than 17 and 10.5 mL/kg/min respectively, were twice as likely to develop major morbidity compared to the patients with values above these cut-offs.
Assessment of cardiopulmonary reserve in 105 patients undergoing radical cystectomy by Tolchard et al. in 2015 concluded that values of AT <11 mL/kg/min and/or VE/VCO2 ≥33 had a greater than fivefold increase in the risk of postoperative all-cause morbidity and prolonged length of stay (43).
The METS study by Wijeysundera et al. assessed the prognostic accuracy of CPET in a multicentre international cohort study (44). They showed that a subjective assessment of fitness has a very poor sensitivity for identifying the unfit patient. It correctly identified only 19% of patients who achieved a peak VO2 less than 14 mL/kg/min, which is consistent with less than 4 METS; its specificity, however, was higher at 94.7%. The authors also found that the addition of peak VO2, but not AT, to a baseline model that included age, sex and high-risk surgery, increased the model’s ability to predict moderate to severe postoperative complications: respiratory, surgical site infection, intensive care admission and re-operation. However, neither peak VO2 nor AT were predictive of postoperative myocardial infarction (MI), myocardial injury or death.
The Duke Activity Status Index (DASI) questionnaire, on the other hand, a simple assessment tool for functional capacity, improved prediction of postoperative myocardial injury or infarction and death within 30 days after surgery. The DASI questionnaire was initially developed by Hlatky et al. in 1989 and includes 12 self-administered questions, each of which carries a specific weight, resulting in a total score of between zero and 58.2 (45). The mean DASI scores in the METS study were 36.9±14.5 vs. 41.6±14.8 in the patients that suffered myocardial injury or death vs. those that did not (44).
The reason for the lack of association between CPET and myocardial injury is not clear. Similar to other recent studies, the incidence of postoperative MI and 30-day death were low at 2% and <1% respectively. It is possible that peak VO2 and AT failed to show a significant predictive ability due to the low incidence of the outcomes of interest. However, that does not explain the lack of association with the development of myocardial injury as that had an incidence of 13%, again similar to other recent studies. The authors speculated that other CPET variables may be better predictors of postoperative outcomes, such as the heart rate (HR) response to exercise.
Indeed, a secondary analysis of two prospective multicentre studies investigating the association between impaired HR recovery after exercise (HR at peak exercise – HR 1 min later) and postoperative morbidity, concluded that HR recovery ≤12 beats/min at 1 minute post-cessation of CPET was independently associated with higher morbidity within 5 days of surgery with a relative risk of 1.38 (95% CI: 1.14–1.67). This was independent of resting heart rate, and included not only cardiovascular but also pulmonary, infective, renal, neurological and pain morbidity (46).
Cardiac biomarkers
Troponin
Cardiac troponins are released by cardiomyocytes into the bloodstream following injury to the myocardium. The VISION study published in 2012, was a large international cohort study that measured postoperative troponin levels in approximately 15,000 patients undergoing non-cardiac surgery. It showed that even slightly raised troponin levels are associated with higher mortality, and the risk increases as the level of troponin increases (47). The same investigators repeated the study with 5th generation high sensitivity troponin T (hsTropT) measurements, the latest available troponin assays that carry the least imprecision, in over 21,000 patients from 13 countries undergoing elective or emergency non-cardiac surgery and found the same association. Bloods for hsTropT were collected at 6–12 h postoperatively and on days 1, 2 and 3. Over 75% of the patients had a hsTropT value over 5 ng/L (the lowest reading of the assay) whereas over 35% had a value over 14 ng/L (considered abnormal by the manufacturer). Even peak levels of hsTropT less than the manufacturer’s normal cut-off value were associated with at least 3 times increased risk of 30-day mortality and the risk increased as the level of troponin increased. The presence of a clinical ischaemic feature such as clinical symptoms, ECG changes or imaging findings further increased the risk to fivefold. Any change in perioperative hsTropT values of at least 5 ng/L was also associated with over 3 times the risk of 30-day mortality (48).
In 2019, Humble et al. published a meta-analysis of the prognostic value of preoperative troponin for adverse postoperative outcome, defined as major adverse cardiovascular events or all-cause mortality in patients undergoing non-cardiac surgery. They included 20 studies with a total sample size of 13,386 patients and concluded that preoperative troponin was a significant predictor of short-term (OR 5.87, 95% CI: 3.24–10.65, adjusted for cardiovascular morbidity) and long-term adverse outcome (adjusted hazards ratio 2.0, 95% CI: 1.4–3.0) (49).
We did not identify any studies evaluating the association between preoperative troponin and postoperative non-cardiac morbidity in non-cardiac surgery. There is some evidence, however, that such an association exists with postoperative troponin. Noordzij et al. investigated the significance of elevated high sensitivity troponin values in 200 patients undergoing elective major abdominal surgery and found that postoperative troponin increases ≥100% compared to baseline, in addition to its association with increased mortality, also had a four-fold increased risk of non-cardiac complications such as sepsis, anastomotic leak, respiratory insufficiency and wound infection (50). Similarly, there is evidence in cardiac surgery that preoperative troponin is associated with postoperative non-cardiac morbidity. Beller et al. showed that patients with positive preoperative troponin had higher risk of major morbidity (such as pneumonia, acute kidney injury), increased length of intensive care and hospital stay, and both operative and long term mortality. It is important to note, however, that, despite the described positive association, risk adjustment with clinical risk scoring systems failed to show preoperative troponin as an independent predictor of postoperative morbidity in this study and, therefore, whether it adds significantly to preoperative risk assessment in cardiac surgery is uncertain (51).
Brain natriuretic peptide (BNP)
Preoperative measurement of BNP or N-terminal fragment of proBNP (NT-proBNP) is included in the 2017 Canadian Cardiovascular Society Guidelines on Perioperative Cardiac Risk assessment prior to non-cardiac surgery. The authors recommend measuring BNP or NT-proBNP before surgery to enhance perioperative cardiac risk estimation in patients who are ≥65 or ≥45 years old and have significant cardiovascular disease or a Revised Cardiac Risk Index score ≥1 (52).
A cumulative meta-analysis involving 28 studies and over 9,000 patients undergoing non-cardiac surgery by Ryan et al. in 2015 concluded that an elevated preoperative BNP, as defined by the authors of the included studies using a variety of assays, was associated with increased all-cause mortality and adverse cardiac events with a cumulative OR of 5.66 (53). Similarly, Ma et al. in 2015, in a study group of over 2,500 patients undergoing emergency non-cardiac surgery, showed that preoperative NT-proBNP level >917 pg/mL was significantly and independently associated with major adverse cardiovascular events (OR 4.81, 95% CI: 3.446–6.722, P<0.001) after adjustment for confounding factors (54).
Duceppe et al. recently published an international cohort study including over 10,000 patients undergoing non-cardiac surgery and found that increased preoperative NT-proBNP levels were associated with increased risk of vascular death and myocardial injury following non-cardiac surgery, and that adding NT-proBNP thresholds to the Revised Cardiac Risk Index resulted in a net absolute reclassification improvement of 258 per 1,000 patients. The authors found that preoperative NT-proBNP levels of 100 pg/mL to less than 200 pg/mL, 200 pg/mL to less than 1,500 pg/mL and 1,500 pg/mL or greater were associated with adjusted hazard ratios of 2.27 (95% CI: 1.9–2.7), 3.63 (95% CI: 3.13–4.21) and 5.82 (95% CI: 4.81–7.05) respectively (55).
The authors of the METS study found only a slight to fair correlation between NT-proBNP and other measures of exercise capacity (Spearman correlation coefficient between NT pro-BNP and DASI of −0.25), which suggests that NT-proBNP is a distinct entity and, as such, may enhance preoperative assessment when used in conjunction with other measures of functional capacity (44).
Conclusions
Prevention of complications following cancer surgery is likely to reduce disease recurrence and optimise both duration and quality of survival. Preoperative risk stratification will add to this aim by allowing patient optimisation and better planning of care. It is also vital for decision making and informed consent prior to a major surgery that may significantly impact on postoperative quality of life. CPET is still considered the gold standard for patients undergoing high-risk surgery or those with significant comorbidities. In the absence of resources or lower risk surgery, the DASI questionnaire may be a suitable alternative and can be used alongside clinical risk scoring systems. The ACS-NSQIP risk calculator is freely available and can be used preoperatively to give an estimate of postoperative morbidity including length of stay and risk of discharge to nursing/rehabilitation facility. Given the ageing population of the patients with cancer, consideration should be given to routine objective assessment of frailty and, in cases where cardiac morbidity is of particular concern, cardiac biomarkers may offer additional information.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Chris Jones and Leigh Kelliher) for the series “Perioperative Care of the Cancer Patient” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-69
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-69). The series “Perioperative Care of the Cancer Patient” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Addae JK, Gani F, Fang SY, et al. A comparison of trends in operative approach and postoperative outcomes for colorectal cancer surgery. J Surg Res 2017;208:111-20. [Crossref] [PubMed]
- Henneman D, Ten Berge MG, Snijders HS, et al. Safety of elective colorectal cancer surgery: Non-surgical complications and colectomies are targets for quality improvement. J Surg Oncol 2014;109:567-73. [Crossref] [PubMed]
- Ali O, Awad F, Gill K, Bhangu A. Impact of early postoperative complications on disease free survival after major resection of colorectal cancer. Int J Surg 2016;36:S38. [Crossref]
- Dekker JWT, Gooiker GA, Bastiaannet E, et al. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol 2014;40:1481-7. [Crossref] [PubMed]
- Artinyan A, Orcutt ST, Anaya DA, et al. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann Surg 2015;261:497-505. [Crossref] [PubMed]
- Aoyama T, Murakawa M, Katayama Y, et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res 2015;35:2401-9. [PubMed]
- McSorley ST, Black DH, Horgan PG, et al. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr 2018;37:1279-85. [Crossref] [PubMed]
- Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326-41. [PubMed]
- Choosing Wisely | Promoting conversations between providers and patients [Internet]. [cited 2020 Mar 1]. Available online: https://www.choosingwisely.org/
- Protopapa KL, Simpson JC, Smith NCE, et al. Development and validation of the Surgical Outcome Risk Tool (SORT). Br J Surg 2014;101:1774-83. [Crossref] [PubMed]
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043-9. [Crossref] [PubMed]
- Copeland GP, Jones D, Walters M. POSSUM: A scoring system for surgical audit. Br J Surg 1991;78:355-60. [Crossref] [PubMed]
- ACS Risk Calculator - Home Page [Internet]. [cited 2020 May 3]. Available online: https://riskcalculator.facs.org/RiskCalculator/
- Prytherch DR, Whiteley MS, Higgins B, et al. POSSUM and Portsmouth POSSUM for predicting mortality. Br J Surg 1998;85:1217-20. [Crossref] [PubMed]
- Eamer G, Al-Amoodi MJH, Holroyd-Leduc J, et al. Review of risk assessment tools to predict morbidity and mortality in elderly surgical patients. Am J Surg 2018;216:585-94. [Crossref] [PubMed]
- Wakabayashi H, Sano T, Yachida S, et al. Validation of risk assessment scoring systems for an audit of elective surgery for gastrointestinal cancer in elderly patients: An audit. Int J Surg 2007;5:323-7. [Crossref] [PubMed]
- Hayashi H, Kawabata Y, Fujii T, et al. Validation of POSSUM scoring system in abdominal surgery for patients with malignant diseases: A multi-institutional analysis. Eur J Surg Oncol 2014;40:S169. [Crossref]
- ACS Risk Calculator - Home Page [Internet]. [cited 2020 Feb 11]. Available online: https://riskcalculator.facs.org/RiskCalculator/
- Rivard C, Nahum R, Slagle E, et al. Evaluation of the performance of the ACS NSQIP surgical risk calculator in gynecologic oncology patients undergoing laparotomy. Gynecol Oncol 2016;141:281-6. [Crossref] [PubMed]
- Armstrong EA, Beal EW, Lopez-Aguiar AG, et al. Evaluating the ACS-NSQIP risk calculator in primary GI neuroendocrine tumor: Results from the United States neuroendocrine Tumor Study Group. Am Surg 2019;85:1334-40. [Crossref] [PubMed]
- Liu JB, Weber SM, Berian JR, et al. Role of operative complexity variables in risk adjustment for patients with cancer. JAMA Surg 2016;151:1084-6. [Crossref] [PubMed]
- Cohen ME, Liu Y, Ko CY, et al. An Examination of American College of Surgeons NSQIP Surgical Risk Calculator Accuracy. J Am Coll Surg 2017;224:787-795.e1. [Crossref] [PubMed]
- British Geriatrics Society. Fit for Frailty - consensus best practice guidance for the care of older people living in community and outpatient settings - a report from the British Geriatrics Society 2014. Nurs Times 2001;97:41-4.
- Mrdutt MM, Papaconstantinou HT, Robinson BD, et al. Preoperative Frailty and Surgical Outcomes Across Diverse Surgical Subspecialties in a Large Health Care System. J Am Coll Surg 2019;228:482-90. [Crossref] [PubMed]
- Obeid NM, Azuh O, Reddy S, et al. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: Exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg 2012;72:878-83. [Crossref] [PubMed]
- Chimukangara M, Helm MC, Frelich MJ, et al. A 5-item frailty index based on NSQIP data correlates with outcomes following paraesophageal hernia repair. Surg Endosc 2017;31:2509-19. [Crossref] [PubMed]
- Ethun CG, Bilen MA, Jani AB, et al. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin 2017;67:362-77. [Crossref] [PubMed]
- Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann Oncol 2015;26:1091-101. [Crossref] [PubMed]
- Vermillion SA, Hsu FC, Dorrell RD, et al. Modified frailty index predicts postoperative outcomes in older gastrointestinal cancer patients. J Surg Oncol 2017;115:997-1003. [Crossref] [PubMed]
- Mogal H, Vermilion SA, Dodson R, et al. Modified Frailty Index Predicts Morbidity and Mortality After Pancreaticoduodenectomy. Ann Surg Oncol 2017;24:1714-21. [Crossref] [PubMed]
- Hodari A, Hammoud ZT, Borgi JF, et al. Assessment of morbidity and mortality after esophagectomy using a modified frailty index. Ann Thorac Surg 2013;96:1240-5. [Crossref] [PubMed]
- Tan KY, Kawamura YJ, Tokomitsu A, et al. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg 2012;204:139-43. [Crossref] [PubMed]
- Pitts KD, Arteaga AA, Stevens BP, et al. Frailty as a Predictor of Postoperative Outcomes among Patients with Head and Neck Cancer. Otolaryngol-Head Neck Surg 2019;160:664-71. [Crossref] [PubMed]
- Sathianathen NJ, Jarosek S, Lawrentschuk N, et al. A Simplified Frailty Index to Predict Outcomes After Radical Cystectomy. Eur Urol Focus 2019;5:658-63. [Crossref] [PubMed]
- Minto G, Biccard B. (PDF) Assessment of the high-risk perioperative patient [Internet]. [cited 2020 May 4]. Available online: https://www.researchgate.net/publication/273040946_Assessment_of_the_high-risk_perioperative_patient
- Otto JM, Levett DZH, Grocott MPW. Cardiopulmonary Exercise Testing for Preoperative Evaluation: What Does the Future Hold? Curr Anesthesiol Rep 2020;10:1-11. [Crossref]
- Levett DZH, Jack S, Swart M, Carlisle J, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth 2018;120:484-500. [Crossref] [PubMed]
- Older P, Smith R, Courtney P, et al. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest 1993;104:701-4. [Crossref] [PubMed]
- Nagamatsu Y, Shima I, Yamana H, et al. Preoperative evaluation of caroiopulmonary reserve with the use of expired gas analysis during exercise testing in patients with squamous cell carcinoma of the thoracic esophagus. J Thorac Cardiovasc Surg 2001;121:1064-8. [Crossref] [PubMed]
- West MA, Lythgoe D, Barben CP, et al. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: A prospective blinded observational study. Br J Anaesth 2014;112:665-71. [Crossref] [PubMed]
- Chandrabalan V V, McMillan DC, Carter R, et al. Pre-operative cardiopulmonary exercise testing predicts adverse post-operative events and non-progression to adjuvant therapy after major pancreatic surgery. HPB 2013;15:899-907. [Crossref] [PubMed]
- Patel N, Powell AG, Wheat JR, et al. Cardiopulmonary fitness predicts postoperative major morbidity after esophagectomy for patients with cancer. Physiol Rep 2019;7:e14174. [Crossref] [PubMed]
- Tolchard S, Angell J, Pyke M, et al. Cardiopulmonary reserve as determined by cardiopulmonary exercise testing correlates with length of stay and predicts complications after radical cystectomy. BJU Int 2015;115:554-61. [Crossref] [PubMed]
- Wijeysundera DN, Pearse RM, Shulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018;391:2631-40. [Crossref] [PubMed]
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989;64:651-4. [Crossref] [PubMed]
- Ackland GL, Abbott TEF, Minto G, et al. Heart rate recovery and morbidity after noncardiac surgery: Planned secondary analysis of two prospective, multi-centre, blinded observational studies. PLoS One 2019;14:1-15.
- Devereaux PJ, Chan MTV, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307:2295-304. [Crossref] [PubMed]
- Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642-51. [Crossref] [PubMed]
- Humble CAS, Huang S, Jammer I, et al. Prognostic performance of preoperative cardiac troponin and perioperative changes in cardiac troponin for the prediction of major adverse cardiac events and mortality in noncardiac surgery: A systematic review and meta-analysis. PLoS One 2019;14:e0215094. [Crossref] [PubMed]
- Noordzij PG, Van Geffen O, Dijkstra IM, et al. High-sensitive cardiac troponin T measurements in prediction of non-cardiac complications after major abdominal surgery. Br J Anaesth 2015;114:909-18. [Crossref] [PubMed]
- Beller JP, Hawkins RB, Mehaffey JH, et al. Does Preoperative Troponin Level Impact Outcomes After Coronary Artery Bypass Grafting? Ann Thorac Surg 2018;106:46-51. [Crossref] [PubMed]
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society Guidelines on Perioperative Cardiac Risk Assessment and Management for Patients Who Undergo Noncardiac Surgery. Can J Cardiol 2017;33:17-32. [Crossref] [PubMed]
- Ryan L, Rajah C, Simmers D, et al. Preoperative B-type natriuretic peptides in patients undergoing noncardiac surgery: A cumulative meta-analysis. South African J Anaesth Analg 2015;21:12-22.
- Ma J, Xin Q, Wang X, et al. Prediction of perioperative cardiac events through preoperative NT-pro-BNP and cTnI after emergent non-cardiac surgery in elderly patients. PLoS One 2015;10:e0121306. [Crossref] [PubMed]
- Duceppe E, Patel A, Chan MTV, et al. Preoperative n-terminal pro-b-type natriuretic peptide and cardiovascular events after noncardiac surgery: A cohort study. Ann Intern Med 2020;172:96-104. [Crossref] [PubMed]
Cite this article as: Papadopoulou A, Mathers E. Risk stratification of the cancer patient: a narrative review. Dig Med Res 2020;3:27.


