Analgesia for open abdominal surgery
Introduction
Analgesia forms a crucial part of the perioperative management of patients undergoing open abdominal surgery. It is now well established that optimal pain management not only facilitates patient well-being but also enhanced recovery after surgery (ERAS) milestones including early mobilisation and enteral feeding which may improve outcomes (1). One of the key aims of ERAS is to attenuate the physiological stress response following surgery, thereby reducing morbidity and mortality and analgesic technique plays an important role in this (2).
The value of multi-modal opioid-sparing analgesia in abdominal surgery has been long established (3). This encompasses differing analgesics targeting different nociceptive mechanisms with the aim of minimising excess opioid usage and its associated adverse effects including but not limited to reduced gastrointestinal motility leading to ileus, cough and respiratory depression, sedation, urinary retention and postoperative nausea and vomiting (PONV). In spite of these shortcomings, opioids remain extensively used in post-anaesthetic care units and wards for breakthrough pain and are generally universally accessible for rescue analgesia.
Although the pre-ERAS evidence base advocated thoracic epidural analgesia as the gold standard for open abdominal surgery, with the increasing adoption of ERAS pathways, recent evidence now further highlights the potential pitfalls with this technique as well as promoting alternative analgesic techniques that will be discussed.
Neuraxial analgesia
Thoracic epidural analgesia
Historically, thoracic epidural analgesia has the strongest evidence base for open abdominal surgery. They have been well studied for over 20 years and provide excellent static and dynamic analgesia provided they work (4,5). There are many published benefits including mitigation of the surgical stress response, in particular neuroendocrine (sympathetic and pituitary) and metabolic (hyperglycaemia and protein catabolism) effects, reduced pulmonary complications (hypoxia, atelectasis, infection, thromboembolism) and some evidence of reduced incidence of myocardial infarction (MI) and acute kidney injury. Also, recent evidence reports superior analgesia compared to patient controlled analgesia (PCA) and continuous wound infiltration in a variety of surgical populations including major colorectal, abdominal aortic aneurysm and open liver resection surgery (6). In colorectal surgery specifically, ERAS guidelines in both 2005 and 2008 advocated the use of epidurals.
However, epidural analgesia is not without its own setbacks and these have been garnering increasing attention that potentially challenges the view that they are the gold standard for open abdominal surgery. Epidural failure for whatever reason (incorrect primary placement, secondary catheter migration and suboptimal dosing) is one of the most common complications and has been quoted as occurring in 27–32% of cases (7). Interestingly, if this failure is proactively managed, the result is almost universally successful (8). Hypotension and motor block may lead to excessive intravenous fluid administration and impaired mobilisation respectively. There is a significantly increased risk of pruritus and urinary retention and the NAP 3 Project highlights the need for constant vigilance for more serious albeit rare complications such as epidural haematoma and abscess formation (9).
The current available evidence tends to highlight more benefits than risks. A recent meta-analysis of 9,044 patients looking as far back as 1985 highlights a 40% reduction in mortality, a reduced risk of atrial fibrillation, deep vein thrombosis, respiratory depression, atelectasis, pneumonia, ileus, PONV and an improved recovery of bowel function (1). However, this is predominantly looking at a pre-ERAS evidence base. In contrast, there was an increased risk of certain adverse events including hypotension, pruritus and urinary retention (1).
A recent Cochrane review analysing 15 trials over a 20-year period showed a reduction in risk of MI, a reduced risk of post-op respiratory failure and a reduction in post-operative pain VAS scores (5). However, the trial identified no difference in 30-day mortality, and there were high failure rates and also recurring issues with hypotension, excessive IV fluid administration and ease of mobilisation, but again the majority of these trials were non-ERAS studies. A systematic review from 2014 comparing analgesic strategies for open surgery within an ERAS programme identified no difference in 30-day morbidity between epidural and non-epidural treatment arms (6). Pain scores were reduced in the epidural groups at both 24 and 48 hours at rest and on movement (6). There was also a more rapid return of gut function in epidural groups. There was no difference between treatment groups in terms of mobilisation, length of stay and overall complication rates.
Intrathecal analgesia
Intrathecal or spinal analgesia has well documented benefits in the setting of laparoscopic surgery (10). In particular, they are well tolerated, early pain scores are superior to PCA use and they are similar when compared to epidural use. They also have a good opioid sparing effect (11). There is less postoperative fluid administration and weight gain when compared to epidurals. Spinal analgesia also facilitates a quicker return of bowel function, improved mobilisation and reduced length of stay (12).
However, it is also associated with complications. Similar to epidural use, it leads to perioperative hypotension which can be profound, and it is associated with serious neurological complications as with all neuraxial techniques. Given its limited duration of action as it is a single shot injection, it is currently generally perceived to be unsuitable for major open abdominal surgery. Consequently, there is a relative paucity of evidence supporting its use in the context of open abdominal surgery. Small randomised controlled trials have shown some evidence of benefit in the form of a reduced length of stay, however larger trials are certainly needed to confirm this benefit (13,14). In future, it may have a role when combined with other techniques as part of a multi-model approach (15).
Non-neuraxial regional analgesia
The transversus abdominis plane (TAP) block is a well-established peripheral plane block which aims to analgese the anterior abdominal wall. There are multiple meta-analyses including a Cochrane review which demonstrate reduced pain and opioid requirement in the first 24 hours following both open and laparoscopic abdominal surgery (16). They provide comparable short-term analgesia to simple wound infiltration in the setting of a multi-modal regimen. Interestingly, there is evidence that if administered pre-operatively, there is a superior analgesic effect when compared with post-operative administration (17).
Rectus sheath blocks have a more limited evidence base compared with TAP blocks however they do demonstrate an opioid-sparing effect and they avoid, like other truncal blocks, the potential immobility and hypotension associated with thoracic epidurals. A recent randomised controlled trial comparing three types of continuous anterior abdominal wall block, Wound catheter, Rectus sheath catheter and TAP block, for midline laparotomy following gynaecological oncology surgery found no difference in morphine requirements at 48 hours and no difference in length of stay (18). However, when comparing TAP block with wound catheters, there was a reduced use of morphine in recovery with TAP blocks, and reduced pain scores and antiemetic use at 24 hours, although no difference at 48 hours (18). A number of randomised controlled trials comparing rectus sheath catheters with thoracic epidural analgesia have shown a significantly reduced time to ambulation, however no difference in pain scores or length of stay (19,20).
Intra-peritoneal infiltration of local anaesthetic, despite not being routinely used in open abdominal surgery, has been shown to reduce pain scores and facilitate a quicker return of bowel function, but not reduce opioid consumption (21). A systematic review of 8 randomised controlled trials identified the above findings as well as a blunting of postoperative hyperglycaemia, suggestive of modulation of the stress response (21). It is a safe technique, however more studies in the perioperative setting are required to further evaluate its potential benefits.
The use of wound catheters for surgical site analgesia in open abdominal surgery is also widespread. This involves the placement of a catheter either supra-fascial or subfascial and allows either the bolus or continuous infusion of local anaesthetic into the wound. Systematic reviews have shown comparable pain scores at both 24 and 48 hours following surgery with a lower incidence of urinary retention and reduced opioid consumption (22,23). However, thoracic epidural analgesia showed a non-significant trend towards reduced pain scores on movement and reduced opiate requirements, systemic opioids often still being required for visceral pain in wound catheter groups (22).
Systemic analgesics
Acetaminophen (paracetamol) and non-steroidal anti-inflammatories (NSAIDs) are very well-established simple analgesics and a cornerstone of the WHO analgesic ladder. They are commonly used in open abdominal surgery to good effect and have an opioid-sparing effect. Appropriate dosing and course-length must be ensured to avoid adverse effects, including potential hepatotoxicity with paracetamol, and nephrotoxicity, impaired platelet function and concerns over wound and anastomotic healing with NSAIDs (24).
Lidocaine is a safe, readily available and widely used local anaesthetic. Its use as an analgesic has been established for many decades and there has been increasing interest in its use as an effective analgesic adjunct over the past several years. Meta-analyses have demonstrated evidence of benefit with its use as a bolus and infusion regime in the perioperative period (25-28). These include a reduction in analgesic requirements, reduced post-operative ileus and PONV. Opioid consumption was reduced by up to 60% and there was a reduced length of hospital stay. There was also a reduced effect on the stress response as measured by a reduction in IL-6, IL-8 and C3a activation. It is typically administered initially as a bolus (1.5–2.0 mg/kg) followed by an infusion of 1.0–3.0 mg/kg/h continued through till the end of surgery and for up to 24 hours perioperatively, although the requirement for cardiovascular monitoring limits this use. Currently, the ALLEGRO trial is a randomised controlled trial looking at the administration of lidocaine and its effect on accelerating gastrointestinal recovery following major colorectal surgery.
N-methyl-D-aspartate (NMDA) receptor antagonists in clinical use include both Ketamine and Magnesium sulphate. Ketamine use has undergone a resurgence recently, approximately 50 years after it was first synthesised. It appears to have a synergistic and additive effect when combined with morphine sulphate and it may reduce the incidence of opioid induced hyperalgesia and certain chronic pain syndromes. It is particularly useful in opioid-tolerant patients however it is important to have an awareness of its potential adverse effects, including sedation, delusions, hallucinations and nightmares. However, these effects were not manifest when used intraoperatively and via a 2 mcg/kg/min infusion for 48 hours postoperatively following a 0.5 mg/kg bolus, and morphine consumption was halved in patients having major abdominal surgery (29). It has also been used to good effect in thoracic, upper GI and major orthopaedic surgery as both PCA and bolus and infusion (30).
Systemic infusions of perioperative magnesium sulphate may also have a beneficial effect on postoperative pain scores and opioid usage. There isn’t a consensus on optimal dosing regime: there are protocols for loading dose followed by infusion and infusion without loading dose. However, typical boluses are 30–50 mg/kg followed by an infusion rate range of 4–15 mg/kg/h. Although meta-analyses didn’t report any significant clinical toxicity, its potential effects on cardiac conduction and interaction with neuromuscular blocking agents may curb its use (31,32).
Gabapentinoids also display promising benefits in the setting of acute pain management, including reduced postoperative pain, a good opioid sparing effect and reduced PONV. There is also emerging evidence that they reduce the risk of development of chronic post-surgical pain (33). However, their utility is limited by adverse effects such as sedation and visual disturbances, particularly at higher doses.
Alpha-2 adrenoreceptor agonists in clinical use include clonidine and dexmedetomidine and both have analgesic effects. They blunt central sympathetic outflow and noradrenaline release which has inhibitory effects on both central and peripheral pain pathways. However, evidence of significant adverse effects associated with their use have prevented their adoption into widespread clinical practice. The POISE-2 trial highlighted these specifically with clonidine, including a significant increase in clinically important hypotension and nonfatal cardiac arrest (34). Dexmedetomidine, which has not been established for as long, warrants similar concerns and further clinical trials need to evaluate its potential utility in acute perioperative pain management (35).
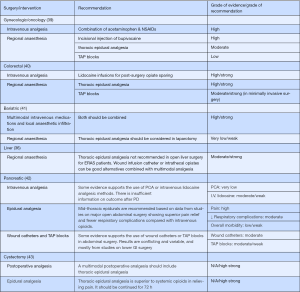
Current ERAS guidelines vary in their advocation for analgesic techniques. For open liver surgery, routine thoracic epidural analgesia is no longer recommended, and alternatives such as wound infusion catheters or intrathecal opiates combined with multi-modal techniques should be used (36). The Liver 1 and Liver 2 trials are two randomised controlled trials comparing epidural analgesia following open liver resection with continuous wound infiltration (37,38). In the Liver 1 trial, although 48 hour pain scores were reduced in the thoracic epidural analgesia group, there were otherwise no differences between the two treatment groups in complications, length of stay and no difference in time to first mobilisation (although more steps were taken in the first 48 hours in the wound infiltration group). In the Liver 2 trial, which also included a TAP block in addition to continuous wound infiltration, there was no difference in pain scores and morbidity between treatment groups, however there was a significant reduction in time to medically fit for discharge in the wound catheter infiltration group.
ERAS guidelines for open gynae-oncological surgery have also trended away from recommending thoracic epidural analgesia as the gold standard for pain management. On the current balance of available data, they support the use of incisional injection of local anaesthetic over both thoracic epidural analgesia and TAP blocks (39). Whether one technique can be truly superior to another is continuing to be evaluated and also whether this can affect primary outcomes and not just pain scores. A recent randomized controlled trial compared the use of intrathecal morphine versus epidural analgesia in patients undergoing laparotomy for gynae-oncological malignancy and found significantly reduced length of stays and opioid consumption in the intrathecal morphine group (13). There was no observable difference identified between groups in pain scores and health-related quality of life.
In contrast, for ERAS pathways for pancreaticoduodenectomy, open colorectal and open gastrointestinal surgery, thoracic epidural analgesia is in general favoured over other techniques (Figure 1).
Overall, in the context of open abdominal surgery, the available evidence is mixed and limited in enhanced recovery programmes. Thoracic epidural analgesia generally shows better pain scores when compared with other techniques. However, there is a less obvious advantage in morbidity reduction and length of stay. Wound catheter and TAP block techniques show promise but larger, more robust studies are needed to further evaluate the potential benefits. The traditional viewpoint that the best technique for patients undergoing open abdominal surgery remains a thoracic epidural as part of a multi-modal regimen will continue to be challenged as the ERAS evidence base grows. Whilst there is no definite answer to the optimal analgesic regimen for open surgery at the moment, the key is to minimise the side effects of the method chosen and to meet the ERAS nutritional goals and early mobilisation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Enhanced Recovery After Surgery (ERAS) Program in General Surgery”. The article has undergone external peer review.
Conflicts of Interest: CN Jones is an appointed committee officer (Expert Reviews) for the ERAS Society but has no other conflict of interest to declare. F Wilson has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pöpping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg 2014;259:1056-67. [Crossref] [PubMed]
- Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326-41; discussion: 341-3. [PubMed]
- Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postop- erative pain treatment. Anesth Analg 1993;77:1048-56. [PubMed]
- Rigg JRA, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002;359:1276-82. [Crossref] [PubMed]
- Guay J, Kopp S. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev 2016;CD005059. [PubMed]
- Hughes MJ, Ventham NT, McNally S, et al. Analgesia After Open Abdominal Surgery in the Setting of Enhanced Recovery Surgery. A Systematic Review and Meta-analysis. JAMA Surg 2014;149:1224-30. [Crossref] [PubMed]
- Hermanides J, Hollmann MW, Stevens MF, et al. Failed epidural: causes and management. Br J Anaesth 2012;109:144-54. [Crossref] [PubMed]
- Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retro- spective analysis of 19,259 deliveries. Int J Obstet Anesth 2004;13:227-33. [Crossref] [PubMed]
- Cook TM, Counsell D, Wildsmith JA, et al. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009;102:179-90. [Crossref] [PubMed]
- Levy BF, Scott MJ, Fawcett W, et al. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 2011;98:1068-78. [Crossref] [PubMed]
- Wongyingsinn M, Baldini G, Stein B, et al. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth 2012;108:850-6. [Crossref] [PubMed]
- Virlos I, Clements D, Beynon J, et al. Short term outcomes with intrathecal versus epidural analgesia in laparoscopic colorectal surgery. Br J Surg 2010;97:1401-6. [Crossref] [PubMed]
- Kjølhede P, Bergdahl O, Borendal Wodlin N, et al. Effect of intrathecal morphine and epidural analgesia on postoperative recovery after abdominal surgery for gynecologic malignancy: an open-label randomised trial. BMJ Open 2019;9:e024484. [PubMed]
- Thurm M, Kröger Dahlin BI, Winsö O, et al. Spinal analgesia improves surgical outcome after open nephrectomy for renal cell carcinoma: a randomized controlled study. Scand J Urol 2017;51:277-81. [Crossref] [PubMed]
- Donohoe CL, Phillips AW, Flynn E, et al. Multimodal analgesia using intrathecal diamorphine, and paravertebral and rectus sheath catheters are as effective as thoracic epidural for analgesia post-open two-phase esophagectomy within an enhanced recovery program. Dis Esophagus 2018; [Crossref] [PubMed]
- Charlton S, Cyna AM, Middleton P, et al. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev 2010;CD007705. [PubMed]
- Favuzza J, Delaney CP. Outcomes of discharge after elective laparoscopic colo- rectal surgery with transversus abdominis plane blocks and enhanced recovery pathway. J Am Coll Surg 2013;217:503-6. [Crossref] [PubMed]
- Cowlishaw PJ, Kotze PJ, Gleeson L, et al. Randomised comparison of three types of continuous anterior abdominal wall block after midline laparotomy for gynaecological oncology surgery. Anaesth Intensive Care 2017;45:453-8. [Crossref] [PubMed]
- Yassin HM, Abd Elmoneim AT, El Moutz H. The Analgesic Efficiency of Ultrasound Guided Rectus Sheath Analgesia Compared with Low Thoracic Epidural Analgesia After Elective Abdominal Surgery with a Midline Incision: A Prospective Randomized Controlled Trial. Anesth Pain Med 2017;7:e14244. [PubMed]
- Tudor EC, Yang W, Brown R, et al. Rectus sheath catheters provide equivalent analgesia to epidurals following laparotomy for colorectal surgery. Ann R Coll Surg Engl 2015;97:530-3. [Crossref] [PubMed]
- Kahokehr A, Sammour T, Soop M, et al. Intraperitoneal local anaesthetic in abdominal surgery - a systematic review. ANZ J Surg 2011;81:237-45. [Crossref] [PubMed]
- Ventham NT, Hughes M, O’Neill S, et al. Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg 2013;100:1280-9. [Crossref] [PubMed]
- Gupta A, Favaios S, Perniola A, et al. A meta-analysis of the efficacy of wound catheters for post-operative pain management. Acta Anaesthesiol Scand 2011;55:785-96. [Crossref] [PubMed]
- Bakker N, Deelder JD, Richir MC, et al. Risk of anastomotic leakage with nonsteroidal anti-inflammatory drugs within an enhanced recovery program. J Gastrointest Surg 2016;20:776-82. [Crossref] [PubMed]
- Marret E, Rolin M, Beaussier M, et al. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg 2008;95:1331-8. [Crossref] [PubMed]
- Vigneault L, Turgeon AF, Cote D, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth 2011;58:22-37. [Crossref] [PubMed]
- Khan JS, Yousuf M, Victor JC, et al. An estimation for an appropriate end time for an intraoperative intravenous lidocaine infusion in bowel surgery: a comparative meta-analysis. J Clin Anesth 2016;28:95-104. [Crossref] [PubMed]
- McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010;70:1149-63. [Crossref] [PubMed]
- Zakine J, Samarcq D, Lorne E, et al. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: a prospective, randomized, double-blind, controlled study. Anesth Analg 2008;106:1856-61. [Crossref] [PubMed]
- Laskowski K, Stirling A, McKay WP, et al. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth 2011;58:911-23. [Crossref] [PubMed]
- De Oliveira GS Jr, Castro-Alves LJ, Khan JH, et al. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2013;119:178-90. [Crossref] [PubMed]
- Murphy JD, Paskaradevan J, Eisler LL, et al. Analgesic efficacy of continuous intravenous magnesium infusion as an adjuvant to morphine for postoperative analgesia: a systematic review and meta-analysis. Middle East J Anaesthesiol 2013;22:11-20. [PubMed]
- Clarke H, Bonin RP, Orser BA, et al. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012;115:428-42. [Crossref] [PubMed]
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014;370:1504-13. [Crossref] [PubMed]
- Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: a non-opioid adjuvant analgesic. J Pain Res 2017;10:1899-904. [Crossref] [PubMed]
- Melloul E, Hübner M, Scott M, et al. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg 2016;40:2425. [Crossref] [PubMed]
- Revie EJ, McKeown DW, Wilson JA, et al. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB (Oxford) 2012;14:611-8. [Crossref] [PubMed]
- Hughes MJ, Harrison EM, Peel NJ, et al. Randomized clinical trial of perioperative nerve block and continuous local anaesthetic infiltration via wound catheter versus epidural analgesia in open liver resection (LIVER 2 trial). Br J Surg 2015;102:1619-28. [Crossref] [PubMed]
- Nelson G, Bakkum-Gamez J, Kalogera E, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int J Gynecol Cancer 2019;29:651-68. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019;43:659. [Crossref] [PubMed]
- Thorell A, MacCormick AD, Awad S, et al. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg 2016;40:2065-83. [Crossref] [PubMed]
- Lassen K, Coolsen MM, Slim K, et al. ERAS® Society; European Society for Clinical Nutrition and Metabolism; International Association for Surgical Metabolism and Nutrition. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:817-30. [Crossref] [PubMed]
- Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations. Clin Nutr 2013;32:879-87. [Crossref] [PubMed]
Cite this article as: Wilson F, Jones CN. Analgesia for open abdominal surgery. Dig Med Res 2019;2:23.