Efficacy of repeat doses of avatrombopag: a case series
Introduction
Thrombocytopenia is an important common hematologic complication of cirrhosis with approximately three-quarters of patients having some degree of thrombocytopenia; its values inversely relate to the severity of liver disease (1-3). Not only is the platelet count employed for laboratory estimates of fibrosis, low platelet count is associated with risks of bleeding (4-6). The degree of thrombocytopenia is stratified to mild, moderate and severe if the platelet count is: >75,000–<150,000/µL; 50,000–75,000/µL, and <50,000/µL, respectively (7).
Medical societies recommend a threshold platelet value of least 50,000 µL before most invasive procedures (1,8-11). Until recently, the only reliable treatment for increasing platelet count was a platelet transfusion. However, the use of transfusions is limited by administration logistics, short half-life, and potential adverse reactions (10,12-15). Two thrombopoietin (TPO) receptor agonists, avatrombopag (AVA) and lusutrombopag, are currently approved for adult patients with chronic liver disease who are scheduled to undergo a procedure (6,16-18). Typically, these TPO receptor agonists increase the platelet count and significantly reduce the need for platelet transfusion.
Patients with cirrhosis often require repeat invasive procedures (19,20). However, we are not aware of studies assessing efficacy of AVA with repeated use. The aim of this study is to describe the efficacy of TPO receptor agonists in increasing platelet count with recurrent uses.
Methods
We identified all patients who underwent repeat AVA dosing at the University of California Los Angeles Pfleger Liver Institute, Los Angeles, California (Table 1). Patients were prescribed AVA according to recommended dosing (17). Procedures were performed between 9 and 13 days after starting AVA, and the dose of AVA was dependent on baseline platelet count. Repeat dosing was performed no sooner than 30 days from completing the initial AVA dose. Three of the four patients had baseline platelet drawn before the repeat dosing. His repeat baseline platelet count was assumed to be the same as his original for analysis (Patient R).
Table 1
| Patient | Age (years) | Gender | Cause of cirrhosis | Platelet (first) baseline/post AVA | Indication for AVA | Platelet (repeat) baseline/post AVA | Indication for AVA |
|---|---|---|---|---|---|---|---|
| R | 71 | Male | Autoimmune cholangiopathy | 42,000/131,000 | EGD with banding | N/A/105,000 | EGD with banding |
| H | 59 | Male | Alcohol | 44,000/68,000 | EGD with banding | 47,000/67,000 | EGD/dental extractions |
| C | 69 | Female | HCV | 32,000/75,000 | EGD with banding | 36,000/80,000 | EGD with banding |
| D | 60 | Female | NASH | 35,000/87,000 | EGD with banding | 35,000/111,000 | Radiofrequency ablation |
AVA, avatrombopag; EGD, esophagogastroduodenoscopy; N/A, not available; HCV, hepatitis C; NASH, nonalcoholic steatohepatitis.
Results
Patient 1
Mr. R is a 71-year-old gentleman with well compensated cirrhosis from autoimmune cholangiopathy. He was found to have non-bleeding esophageal varices on screening esophagogastroduodenoscopy (EGD). Banding was deferred because his platelet count was below 50,000/µL. Mr. R underwent a repeat EGD with banding after being treated with a course of AVA. AVA 40 mg was administered by mouth daily for 5 days and an EGD was performed 10 days after starting AVA. His platelet count increased from 42,000/µL at baseline to 131,000/µL the day of EGD. He received a second course of AVA for a repeat EGD with banding 2 months later. He received 40 mg of AVA for 5 days. His platelet count increased to 105,000/µL at the time of his second procedure. Mr. R had no adverse effects from AVA, and did not require platelet transfusions or rescue therapy after the endoscopies.
Patient 2
Mr. H is a 59-year-old gentleman with cirrhosis from alcohol consumption. He required hospitalization and treatment for esophageal variceal bleeding. Follow up outpatient EGD was recommended. Several weeks after discharge, he was treated with a course of AVA 40 mg by mouth daily for 5 days prior to his repeat EGD because of severe thrombocytopenia. His platelet count increased from 44,000/µL before AVA to 68,000/µL the day of EGD. A month later he required dental extraction and a repeat EGD. He received a second course of AVA for another EGD and dental extraction procedures. His platelet count increased from 47,000/µL before his second dose of AVA to 67,000/µL. He underwent Argon plasma coagulation on the second outpatient EGD for gastric antral vascular ectasia. He had no adverse effects from AVA and did not require platelet transfusions or rescue therapy after the endoscopies and dental extraction.
Patient 3
Mrs. C is a 69-year-old-woman with decompensated liver cirrhosis from hepatitis C (HCV). She was found to have large non-bleeding esophageal varices on follow-up EGD for previous esophageal varices bleeding in 2017. She received a course of AVA 60 mg by mouth daily for 5 days for an EGD procedure. Her EGD was performed 10 days after starting AVA. Her platelet count increased from 32,000/µL before AVA to 75,000/µL the day of EGD. She underwent banding ligation. She then received a second course of AVA for another EGD (When/what was the time frame?). Her platelet count increased from 36,000/µL to 80,000/µL the day of EGD. She also required band ligation on the follow up EGD. She had no adverse effects on AVA and did not require platelet transfusions or rescue therapy after the endoscopies.
Patient 4
Mrs. D is a 60-year-old-woman with well-compensated liver cirrhosis from cryptogenic cirrhosis. She received a course of AVA 60 mg by mouth daily for 5 days prior to a screening EGD. Her platelet count increased from 35,000/µL before AVA to 87,000/µL 4 days prior to EGD. Banding was not required. A diagnosis of a 2.6 cm hepatocellular carcinoma (HCC) was subsequently made during surveillance. She received second course of AVA prior to radiofrequency ablation of HCC (when/what was the time frame?). Her platelet count increased from 35,000/µL to 111,000/µL the day of ablation. She had no adverse effects on AVA. She did not require platelet transfusion or rescue therapy on either procedure.
Discussion
The mechanism of action of TPO receptor agonists is to stimulate megakaryocytes in the bone marrow, which will lead to the production of platelets. AVA does not have homology to native TPO and although AVA increases platelet count, it is not believed to cause platelet activation (21). There are now several TPO receptor agonists available but only 2 are specifically approved by the FDA for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure (6,16-18).
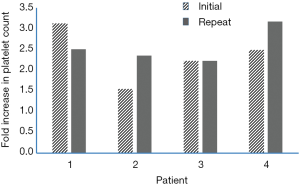
The greater the severity of liver disease, the more likely the degree of thrombocytopenia and likelihood of repeated invasive procedures. AVA has been shown to be effective—irrespective of disease severity and etiology (22). The results of our case series demonstrate not only the continual safety and tolerability of AVA with repeated use, but its continual efficacy. We found no evidence of tachyphylaxis with the second administration of AVA. The mean increase in platelet count was 2.3 folds after the first administration, and 2.6 folds after the second administration (Figure 1).
A limitation of our study is the few patients followed. However, our proof of concept is consistent with observations from other commonly used growth factors such as filgrastim and erythropoietin where no loss of efficacy is demonstrated after repeated uses (23,24). What is unclear is the optimal timing of repeated dosing of AVA. The results of a pharmacokinetic modeling study suggested that AVA can be redosed as early as 12 days after completion of the first dosing regimen without excessively increasing platelet counts (25). We chose to dose after 35 days when the platelet count returned to baseline to avoid excessive platelet elevation that may increase the risk of thrombosis.
Repeat use of AVA continues to be an effective tool to minimize the use of platelet transfusions in adult patients scheduled to undergo a procedure who have chronic liver disease. The effect of repeat dosing dose does not appear to reduce its efficacy. Patients with cirrhosis and thrombocytopenia can be treated with repeated dosing of AVA.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Author S Saab has received a speaker honorarium from Dova, Inc., and also a consultant for Dova, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was not obtained because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maan R, de Knegt RJ, Veldt BJ. Management of Thrombocytopenia in Chronic Liver Disease: Focus on Pharmacotherapeutic Strategies. Drugs 2015;75:1981-92. [Crossref] [PubMed]
- Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int 2017;37:778-93. [Crossref] [PubMed]
- Koruk M, Onuk MD, Akçay F, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis, and its relationship with circulating thrombocyte counts. Hepatogastroenterology 2002;49:1645-8. [PubMed]
- Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104-12. [Crossref] [PubMed]
- Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726-36. [Crossref] [PubMed]
- Terrault N, Chen YC, Izumi N, et al. avatrombopag Before Procedures Reduces Need for Platelet Transfusion in Patients With Chronic Liver Disease and Thrombocytopenia. Gastroenterology 2018;155:705-18. [Crossref] [PubMed]
- Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 2008;48:1000-7. [Crossref] [PubMed]
- Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009;49:1017-44. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707-18. [Crossref] [PubMed]
- Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol 2012;23:727-36. [Crossref] [PubMed]
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205-13. [Crossref] [PubMed]
- DeAngelis GA, Khot R, Haskal ZJ, et al. Bleeding Risk and Management in Interventional Procedures in Chronic Liver Disease. J Vasc Interv Radiol 2016;27:1665-74. [Crossref] [PubMed]
- Tripodi A, Primignani M, Chantarangkul V, et al. Global hemostasis tests in patients with cirrhosis before and after prophylactic platelet transfusion. Liver Int 2013;33:362-7. [Crossref] [PubMed]
- Hayashi H, Beppu T, Shirabe K, et al. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol 2014;20:2595-605. [Crossref] [PubMed]
- Stroncek DF, Rebulla P. Platelet transfusions. Lancet 2007;370:427-38. [Crossref] [PubMed]
- Peck-Radosavljevic M, Simon K, Iacobellis A, et al. Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures (L-PLUS 2). Hepatology 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Doptelet (avatrombopag) [package insert]. Durham, North Carolina: Dova Pharmaceuticals, Inc. 2018.
- Mulpleta (Lusutrombopag) [package insert]. Florham Park, NJ: Shionogi Inc. 2018.
- Szczepiorkowski ZM, Dunbar NM. Transfusion guidelines: when to transfuse. Hematology Am Soc Hematol Educ Program 2013;2013:638-44. [Crossref] [PubMed]
- Lin Y, Foltz LM. Proposed guidelines for platelet transfusion. BCMJ 2005;47:245-8.
- Michelson AD, Smolensky Koganov E, Forde EE, et al. avatrombopag increases platelet count but not platelet activation in patients with thrombocytopenia resulting from liver disease. J Thromb Haemost 2018;16:2515-9. [Crossref] [PubMed]
- Saab S, Allen LF, Aggarwal K, et al. Consistent Efficacy of avatrombopag Compared to Placebo in Patients with Thrombocytopenia and Chronic Liver Disease Undergoing Procedures Across Various Liver Disease Severities and Etiologies. J Hepatol 2018;68:S752. [Crossref]
- Muirhead N, Cattran DC, Zaltzman J, et al. Safety and efficacy of recombinant human erythropoietin in correcting the anemia of patients with chronic renal allograft dysfunction. J Am Soc Nephrol 1994;5:1216-22. [PubMed]
- Dale D. Current management of chemotherapy-induced neutropenia: the role of colony-stimulating factors. Semin Oncol 2003;30:3-9. [Crossref] [PubMed]
- Hayes S, Farrell C, Aggarwal K, et al. PK Simulation of avatrombopag-Induced Increases in Platelet Counts with Redosing in Patients with Thrombocytopenia and Chronic Liver Disease. Res Pract Thromb Haemost 2018;2:Abstract PB019.
Cite this article as: Saab S, McDaniel TK, Bau SN, Patel RP. Efficacy of repeat doses of avatrombopag: a case series. Dig Med Res 2019;2:9.