
Gastroesophageal reflux disease after sleeve gastrectomy
Introduction
Background
Gastroesophageal reflux disease (GERD) is a common complication of laparoscopic sleeve gastrectomy (SG) (1). According to the Seventh International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) (2) Global Registry Report 2022, around 280,000 bariatric surgeries were performed worldwide in 2022, with SG being the most standard procedure, accounting for 67% of all operations. Despite concerns about increased reflux after SG (3-6), several known mechanisms identified that contribute to a higher incidence of postoperative reflux, including a hypotonic lower esophageal sphincter (LES), division of the sling fibers, and the creation of a high-pressure cavity (4). In addition, persistent GERD can have long-term consequences, such as an increased risk of Barrett’s esophagus (BE) (7).
Rationale and knowledge gap
Although based on limited data, there is reasonable agreement on screening patients before and after SG using endoscopy (8). However, no consensus exists on when to employ other diagnostic options during postoperative follow-up.
Objective
This article explores the current understanding of post-SG GERD, including its causes, consequences, strategies for follow-up, available treatment options, and areas of controversy.
Anatomy of the gastroesophageal junction
The gastroesophageal junction includes the hiatus or diaphragmatic crura, a muscular structure that serves as a barrier between the abdomen and the thorax, contributing to 85% of reflux control. The right crus is the main contributor to the formation of the esophageal hiatus, forming a “noose” around the esophagus. There has been a debate on whether the LES is a structural or functional entity, and its major components are also debated (9).
The LES can divide into three parts, with circular fibers at the lower end of the esophagus that cross posteriorly, oblique muscle fibers near His angle, and circular muscle fibers on the anterior and posterior stomach surfaces. These three parts correlate with how the fibers are arranged, with the circular muscle placed in a spiral fashion in the esophagus and the sling fibers crossing the midline at the LES. The clasp fibers merge into the sling/oblique muscle fibers descending from the angle of His towards the greater curvature of the stomach, forming a valve at the entrance of the esophagus. The pressure over the gastric fundus closes the abdominal esophagus, developing anti-reflux mechanisms (10).
In addition, anatomical fixation elements stabilize the stomach to the different pressures of the thorax and abdomen. These elements include the gastrosplenic and phrenoesophageal ligaments and the more significant and minor omentum. It is important to note that the LES is a high-pressure zone due to the different gradients acting over the gastroesophageal junction, including the intrathoracic negative pressure and the positive intraabdominal pressure (11).
When the gastric fundus dilates after food passage, it applies pressure over the abdominal esophagus, compressing it and serving as an anti-reflux mechanism. The sling and clasp fibers then contract, promoting the closure of the cardia as a valve. The impairment or damage of some of those elements could generate reflux (4).
Causes (Figures 1,2)
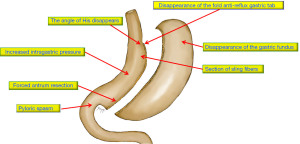
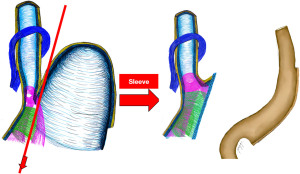
- Sectioning the gastric fundus leaves the upper esophagus open when the food passes and changes the angle of His from 36° to 51° (12,13).
- Damaging the sling fibers, cutting the noose that forms the LES, and promoting GERD (14).
- Augmented intragastric pressure, confirmed by manometry, increases the gastroesophageal pressure gradient and reflux (15).
- Herniation of the gastric tube into the thoracic cavity, disarming the associated gastro and esophagus-phrenic ligaments, leaving the pouch with a greater capacity to suffer from the pressures applied by the stomach and thorax, which may produce a hiatal hernia (HH) (5).
- Vagal nerve injury (16).
- Poor surgical technique leads to twisting, kinking, or strictures of the gastric tube.
- A dynamic pylorus (5).
- Ineffective esophageal clearing. However, the debate is around if impairment of the esophageal peristalsis promotes GERD or if GERD, resulting from other deficient anti-reflux mechanisms, may translate into esophageal dysmotility. The result is a vicious circle leading to an ineffective clearing and, therefore, to prolonged acid exposure (17).
Incidence
There is an ongoing debate about the impact of SG on GERD. However, the literature needs to have more precise conclusions. Thus, we included the best evidence regarding the causal relationship between SG and GERD.
SG does not promote GERD
Rebecchi et al. (18) reported patients who underwent SG for up to 2 years and divided them into group A (preoperative 24-hour pH monitoring with pathologic esophageal acid exposure) and group B (normal pH monitoring). Symptoms improved in group A, and “de novo” GERD occurred in 5.4% of group B. In addition, the authors describe two critical technical aspects, like a technically correct gastric resection without creating mid-stomach stenosis and a careful dissection of the angle of His, keeping a safe distance from the gastroesophageal junction. However, Patti et al. (19) questioned the validity of the conclusions due to the high rate of loss to follow-up of patients and the arbitrary exclusion of some patients with abnormal pH results.
Daes et al. (20) reported a significant reduction in GERD after SG. Out of 66 patients who had GERD before surgery, only 2 (1.5%) experienced GERD symptoms 6–12 months after surgery. The authors identified technical issues that may explain post-SG GERD, including narrowing at the incisura angularis, dilation of the fundus, and persistent HH. By performing a fundus resection, routine correction of HH, and avoiding relative narrowing or torsion of the sleeve, the need for postoperative endoscopy to investigate food intolerance or GERD symptoms decreased sharply. However, you should note that the authors lost 50% of the follow-up, which may affect the reliability of their conclusions.
SG does promote GERD
According to Qumseya et al. (7), a meta-analysis that included four papers that analyzed “de novo reflux” found that 40% of patients experienced “de novo” GERD and an 87% relative increase in EE in short-term studies. Additionally, a meta-regression analysis showed a 13% increase in the risk of esophagitis every year after surgery. Nevertheless, this does not necessarily mean that 100% of patients would experience GERD symptoms after eight years postoperatively. For example, if the risk of esophagitis is 10% in the first year after surgery, it would increase to 11.3% (10% + 10% × 13%) in the second year and to 12.7% (11.3% + 11.3% × 13%) in the third year.
Borbély et al. (3) investigated the occurrence of preoperative silent GERD in patients undergoing SG. They found that in patients who had silent GERD (indicated by grade B esophagitis or more or abnormal pH monitoring in the absence of GERD symptoms) before surgery, 66% developed symptomatic GERD (37 patients), 21% remained asymptomatic, and 7% had no GERD (no signs, experienced up to 2 GERD episodes per week, esophagitis Los Angeles (LA) grade A or lower, and no pathological esophageal acid exposure).
In a systematic review and meta-analysis, Yeung et al. (4) found a 19% increase in GERD after surgery and a 23% occurrence of new reflux cases. Interestingly, these findings did not align with a 28% incidence of esophagitis, which suggests that the actual number of post-sleeve GERD cases may be higher and that relying solely on symptom-based diagnosis may be unreliable.
Csendes et al. (5), in a 10-year follow-up study, divided patients before surgery into two groups: (I) without reflux; and (II) with reflux before SG. They concluded that in group 1, 58.8% of patients developed GERD, while GERD disappeared in 13.6% of patients in group 2.
In conclusion, even with adequate operative technique, GERD could still be a significant drawback after SG, even in asymptomatic individuals who may or may not have silent GERD. Given the ongoing debate surrounding the impact of SG on GERD, Table 1 shows a comprehensive summary of the latest literature on the incidence and limitations of the best available evidence regarding GERD after SG.
Table 1
| Primary author & year of publication | Number of patients | Conclusions | Limitations |
|---|---|---|---|
| Rebecchi et al. 2014 (18) | 71 (30 w/GERD, 41 normal) (18) | GSAS score improved in the GERD group (P<0.001) (18); decrease DMS 39.5 pre-op 10.6 postop (P<0.001) (18); decrease TAE 10.2 pre-op 4.2 postop (P<0.001) (18); “De novo”† GERD occurred in 5.4% of no GERD group (18) | Loss to follow-up of 40% of patients (18); short follow-up (2 years) (18); low volume of patients (18) |
| Qumseya et al. 2021 (7) | 680 (not all used for GERD study) (7) | 40% “de novo” GERD (7) | Primary outcome BE (7); only four papers talk of de novo GERD (7) |
| Borbély et al. 2018 (3) | 222 (3) | Patients w/silent GERD‡, 66% turned symptomatic (3); Patients w/non-GERD§, 42% turned symptomatic (3); Patients w/pre-op GERD, 37% turned to non-GERD & 4% to silent GERD (3) | Short follow-up (2 years) (3) |
| Csendes et al. 2019 (5) | 104 (5) | 58.5% de novo GERD (5); 13.6% disappearance of GERD (5) | Neither 24 h-pHmetry nor esophageal manometry was performed (5); low number of patients (5) |
| Yeung et al. 2020 (4) | 10,718 (4) | 19% increase in reflux symptoms (4); 23% de novo reflux (4) | Only a handful of studies of the highest quality (4); high heterogeneity (4) |
†, “de novo”: patients with silent or no GERD that became symptomatic; ‡, silent GERD: objective evidence of GERD (esophagitis LA grade ≥ B and/or pathological esophageal acid exposure) in the absence of symptoms; §, no GERD: patients had no symptoms (up to two GERD episodes per week), esophagitis LA grade A or less, and no pathological esophageal acid exposure. GERD, gastroesophageal reflux disease; SG, sleeve gastrectomy; w, with; GSAS score, Gastroesophageal Reflux Disease Symptom Assessment Score; DMS, DeMeester score; pre-op, preoperative; postop, postoperative; TAE, total acid exposure; BE, Barrett’s esophagus; LA, Los Angeles.
Preoperative and postoperative evaluation and GERD screening
Preoperative endoscopy
Before undergoing SG, a preoperative workup is recommended, including an upper gastrointestinal endoscopy (UGE). Bellorin et al. (21) identified preoperative endoscopic factors that may predict the development of GERD after SG. In this study, all 217 patients scheduled for SG underwent UGE before and after the procedure. The strongest predictors of GERD development were endoscopically diagnosed (P=0.02) and biopsy-proven esophagitis (P=0.04). Therefore, preoperative endoscopy highlights critical clinical and endoscopic criteria that should prompt consideration of alternatives to SG for weight loss. An international consensus (1) concluded that UGE should be mandatory before surgery (92% agreement).
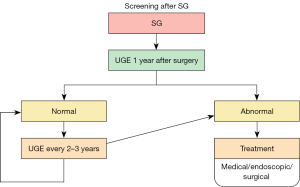
Postoperative endoscopy
After all bariatric/metabolic interventions, including SG, patients will need postoperative follow-up, including endoscopy, to monitor potential complications (Figure 3). A systematic review and meta-analysis of SG (7) reported that BE appeared around three years after SG and continued to be detected ten years after the procedure, with a prevalence of 11%. Therefore, the American Society for Gastrointestinal Endoscopy (ASGE) guidelines on screening and surveillance of BE for the general population recommends screening when the prevalence of BE is higher than 10% (22). In addition, the IFSO position statement (8) on the role of esophageal-gastro-duodenal endoscopy before and after bariatric and metabolic surgery procedures recommends that UGE should be mandatory after surgery (78% agreement among experts). First, however, there needs to be a consensus on when it should be performed after surgery.
Preoperative/postoperative pH and manometry
So far, no evidence supports the routine use of pHmetry before or after bariatric/metabolic surgery (1,23). However, in the latest expert consensus, some aspects of the preoperative workup regarding pHmetry and manometry were discussed, despite the need for more solid evidence. Experts suggest 24-hour pH/manometry studies are not mandatory in bariatric patients with GERD symptoms, mild or even severe esophagitis discovered during gastroscopy. However, the evidence supporting this needs a higher scientific level. Soliman et al. (24) found that preoperative manometry does not predict postoperative GERD, but ambulatory 24-hour esophageal pH monitoring may have a role. According to Patti et al. (19), pH monitoring should be performed in patients with foregut symptoms but no esophagitis on endoscopy. Furthermore, Ashrafi et al. argue that a thorough investigation, including a 24-hour pH study and high-resolution manometry, should be conducted to select the most suitable bariatric procedure for long-term success, even though no quality evidence supports this (25).
Interestingly, Emile et al. (26) developed an artificial intelligence-based model to predict the onset of GERD after SG, with moderate sensitivity (79.2%) and specificity (86.1%). The top 5 predictors were age, weight, preoperative GERD, size of the bougie, and distance of the first stapler firing from the pylorus.
Consequences of reflux (BE/cancer)
BE & SG
Yeung et al. (4), in a meta-analysis, found that studies with one or more years of follow-up after SG reported an 8% prevalence of BE. The authors recommend routine postoperative screening with UGE as cancer cases after SG may be diagnosed later due to patients’ common (or lack of) upper GI symptoms, leading to a delayed diagnosis. In another systematic review and meta-analysis, the pooled prevalence of BE was 11.4%. It appeared around three years after SG and continued to be detected up to 10 years after the procedure (7).
GERD is the primary risk factor for BE, and although patients understand the risk of GERD, the same cannot be said of esophagitis and BE. In addition, patients who undergo bariatric/metabolic surgery tend to be between 30 and 50 years old, and worsening GERD after SG may have severe unintended consequences for patient outcomes and long-term GERD-related complications (27).
A case series of seven patients with post-SG esophageal adenocarcinoma (28) raises concern about upper gastrointestinal symptoms, such as heartburn, dysphagia, and substantial weight loss are usually attributed to the operation itself and often overlooked. Therefore, the authors recommend following the IFSO position statement (7) with a postoperative UGE one year after any bariatric surgery and then every 2–3 years after SG to enable early detection of BE or upper GI malignancy.
Diagnosis and treatment algorithm
Diagnosis (Figure 4)
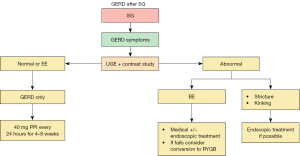
Patients with symptoms of GERD after SG should undergo an endoscopy and an upper gastrointestinal contrast, especially if there are symptoms before surgery (8,29). IFSO (8) recommends UGE as a routine; at least one UGE three years later. However, UGE may not be as accurate in detecting abnormalities such as twisting, kinking, esophageal function, or motility disorders and may bring attention to BE or erosive esophagitis (EE).
On the other hand, an upper gastrointestinal (GI) contrast series can help diagnose HHs, strictures, kinking, twisting, and stenosis. In addition, endoscopic balloon dilation may be proposed for strictures or kinking (30).
Treatment (Figure 5)
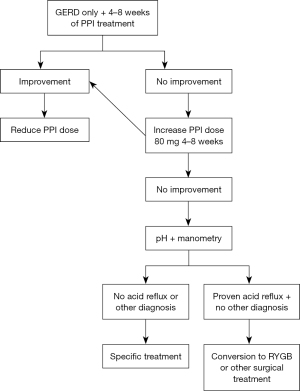
For patients with GERD or EE, treatment starts with proton pump inhibitor (PPI) once daily for at least 4 weeks (31). If symptoms persist, the PPI dosage should increase while optimizing lifestyle changes and weight loss. Conversely, if GERD symptoms improve with either dosage, the PPI dose should be reduced to the lowest effective dosage. However, if symptoms persist, further tests such as pHmetry and manometry are necessary.
Once reflux is diagnosed, there are various treatment options available (Figure 6)

Roux-en-Y gastric bypass
Involves creating a small gastric pouch that is separate from the rest of the stomach (known as the “remnant”), connecting the pouch to the distal part of the transected bowel loop (known as the Roux limb), and connecting the proximal portion of the transected small bowel loop (known as the biliopancreatic limb) to the Roux limb at a distance from its connection with the gastric pouch (32). The American Society for Metabolic and Bariatric Surgery recommends receiving PPI therapy for patients with new-onset GERD and severe symptoms after SG. Those who do not respond to medical treatment should be considered for conversion to RYGB (with grade C evidence) (33).
Two large randomized controlled trials with 5- and 10-year follow-ups found that the conversion rate of SG to RYGB due to severe GERD was 9% (34) and 13%, respectively (35). However, while some studies suggest that conversion to RYGB improves all symptoms, other studies report that approximately 28% of patients still experience GERD-related symptoms despite endoscopic improvement or even the disappearance of esophagitis (36). Furthermore, the short and long-term complication rates of laparoscopic conversion of failed SG to RYGB are low. This study claims to have the lowest short-term complication rate (3.3% vs. 4.5–22%) compared to similar studies comparing SG to RYGB (31).
The Stretta procedure
The Stretta procedure is an endoscopic method for treating GERD that uses temperature-controlled radiofrequency energy to stimulate the muscle at the GE junction, which can reduce esophageal acid exposure. During the procedure, a balloon assembly with needle electrodes is positioned just above the GE junction, delivering radiofrequency energy to the submucosa (37). The procedure has shown to be a safe and effective treatment for GERD, with a morbidity rate of less than 0.6%, and can be performed on an outpatient basis (38). Complications include mucosal injury, bleeding, and perforation of the esophagus. A single study evaluated the safety and efficacy of Stretta treatment for post-SG GERD and found that, at 6 months, 66.7% of patients were unsatisfied, only 20% stopped taking PPI medications, and 13.3% required RYGB conversion 8 months after Stretta. However, the study also reported a complication rate of 6.7% (39). There is no long-term data on outcomes.
LINX system
This is a magnetic sphincter augmentation device that can also be used to treat GERD (40). The device involves placing a ring of titanium beads with a magnetic core around the esophagus at the GE junction, which creates a physiological LES that resists abnormal opening and relaxation of the sphincter due to transient relaxation or a hypotonic sphincter. The device can be implanted laparoscopically and done on an outpatient basis. Although the device can improve regurgitation, DeMeester score, and dissatisfaction with GERD symptoms, pH normalization was only achieved in six out of 27 subjects. Additionally, the study that evaluated the device reported a complication rate of 50%, with 6.7% of complications being severe and requiring conversion to RYGB. Finally, it is worth noting that this study was conducted on patients with HHs of less than 3 cm (41).
Transoral incisionless fundoplication (TIF)
This minimally invasive procedure uses implantable fasteners to create a fold of tissue at the esophagus’s base to help prevent stomach acid from flowing back into the esophagus (42). Although there is no evidence in the literature supporting the use of the TIF procedure for treating GERD in patients who have undergone SG, it is a procedure that needs further studies.
Strengths and limitations
Strengths: this literature review covers a range of articles from 2017 to 2023 and a few earlier ones deemed necessary. The information will guide clinicians in evaluating and following patients after SG.
Limitations: the scientific evidence analyzed in this review needs to be higher. However, it is the best currently available.
Conclusions
GERD is a significant concern after SG and may lead to EE, BE, and increased use of proton-pump inhibitors. Therefore, it is crucial to adhere to the postoperative screening guidelines recommended by IFSO. Patients presenting with GERD symptoms or UGE should undergo an upper contrast study to check for correctible anatomical issues, and if any are found, they should be fixed. Otherwise, medical treatment should be started. If the patient is unresponsive to medical therapy, pH and manometry studies may be conducted, and RYGB conversion may be recommended.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-23-7/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-23-7/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Assalia A, Gagner M, Nedelcu M, et al. Gastroesophageal Reflux and Laparoscopic Sleeve Gastrectomy: Results of the First International Consensus Conference. Obes Surg 2020;30:3695-705. [Crossref] [PubMed]
- Brown WA, Shikora S, Liem R, et al. IFSO 7th Registry Report 2022. Scribd. August 1, 2022. Retrieved February 4, 2023. Available online: https://www.scribd.com/document/600382297/ifso-7th-registry-report-2022
- Borbély Y, Schaffner E, Zimmermann L, et al. De novo gastroesophageal reflux disease after sleeve gastrectomy: role of preoperative silent reflux. Surg Endosc 2019;33:789-93. [Crossref] [PubMed]
- Yeung KTD, Penney N, Ashrafian L, et al. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: A systematic review and meta-analysis. Ann Surg 2020;271:257-65. [Crossref] [PubMed]
- Csendes A, Orellana O, Martínez G, et al. Clinical, Endoscopic, and Histologic Findings at the Distal Esophagus and Stomach Before and Late (10.5 Years) After Laparoscopic Sleeve Gastrectomy: Results of a Prospective Study with 93% Follow-Up. Obes Surg 2019;29:3809-17. [Crossref] [PubMed]
- Guzman-Pruneda FA, Brethauer SA. Gastroesophageal Reflux After Sleeve Gastrectomy. J Gastrointest Surg 2021;25:542-50. [Crossref] [PubMed]
- Qumseya BJ, Qumsiyeh Y, Ponniah SA, et al. Barrett's esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc 2021;93:343-352.e2. [Crossref] [PubMed]
- Brown WA, Johari Halim Shah Y, Balalis G, et al. IFSO Position Statement on the Role of Esophago-Gastro-Duodenal Endoscopy Prior to and after Bariatric and Metabolic Surgery Procedures. Obes Surg 2020;30:3135-53. [Crossref] [PubMed]
- Zifan A, Kumar D, Cheng LK, et al. Three-Dimensional Myoarchitecture of the Lower Esophageal Sphincter and Esophageal Hiatus Using Optical Sectioning Microscopy. Sci Rep 2017;7:13188. [Crossref] [PubMed]
- Jobe BA, Kahrilas PJ, Vernon AH, et al. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. Am J Gastroenterol 2004;99:233-43. [Crossref] [PubMed]
- Paterson WG. The normal anti-reflux mechanism. Chest Surg Clin N Am 2001;11:473-83. [PubMed]
- Fiorillo C, Quero G, Dallemagne B, et al. Effects of Laparoscopic Sleeve Gastrectomy on Gastric Structure and Function Documented by Magnetic Resonance Imaging Are Strongly Associated with Post-operative Weight Loss and Quality of Life: a Prospective Study. Obes Surg 2020;30:4741-50. [Crossref] [PubMed]
- Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett's esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis 2017;13:568-74. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Shiraga S, et al. Intraoperative assessment of the effects of laparoscopic sleeve gastrectomy on the distensibility of the lower esophageal sphincter using impedance planimetry. Surg Endosc 2016;30:4904-9. [Crossref] [PubMed]
- Mion F, Tolone S, Garros A, et al. High-resolution Impedance Manometry after Sleeve Gastrectomy: Increased Intragastric Pressure and Reflux are Frequent Events. Obes Surg 2016;26:2449-56. [Crossref] [PubMed]
- van Rijn S, Rinsma NF, van Herwaarden-Lindeboom MY, et al. Effect of Vagus Nerve Integrity on Short and Long-Term Efficacy of Antireflux Surgery. Am J Gastroenterol 2016;111:508-15. [Crossref] [PubMed]
- Balla A, Meoli F, Palmieri L, et al. Manometric and pH-monitoring changes after laparoscopic sleeve gastrectomy: a systematic review. Langenbecks Arch Surg 2021;406:2591-609. [Crossref] [PubMed]
- Rebecchi F, Allaix ME, Giaccone C, et al. Gastroesophageal reflux disease and laparoscopic sleeve gastrectomy: a physiopathologic evaluation. Ann Surg 2014;260:909-15. [Crossref] [PubMed]
- Patti MG, Schlottmann F. Gastroesophageal Reflux After Sleeve Gastrectomy. JAMA Surg 2018;153:1147-8. [Crossref] [PubMed]
- Daes J, Jimenez ME, Said N, et al. Laparoscopic sleeve gastrectomy: symptoms of gastroesophageal reflux can be reduced by changes in surgical technique. Obes Surg 2012;22:1874-9. [Crossref] [PubMed]
- Bellorin O, Senturk JC, Cruz MV, et al. Predictive Factors for Developing GERD After Sleeve Gastrectomy: Is Preoperative Endoscopy Necessary? J Gastrointest Surg 2022;26:1015-20. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90:335-59.e2. [Crossref] [PubMed]
- Gagner M, Hutchinson C, Rosenthal R. Fifth International Consensus Conference: current status of sleeve gastrectomy. Surg Obes Relat Dis 2016;12:750-6. [Crossref] [PubMed]
- Soliman H, Coupaye M, Cohen-Sors B, et al. Do preoperative esophageal pH monitoring and high-resolution manometry predict symptoms of GERD after sleeve gastrectomy? Obes Surg 2021;31:3490-7. [Crossref] [PubMed]
- Ashrafi D, Osland E, Memon MA. Bariatric surgery and gastroesophageal reflux disease. Ann Transl Med 2020;8:S11. [Crossref] [PubMed]
- Emile SH, Ghareeb W, Elfeki H, et al. Development and Validation of an Artificial Intelligence-Based Model to Predict Gastroesophageal Reflux Disease After Sleeve Gastrectomy. Obes Surg 2022;32:2537-47. [Crossref] [PubMed]
- Sohn S, Fischer J, Booth M. Adenocarcinoma of the gastro-oesophageal junction after sleeve gastrectomy: a case report. ANZ J Surg 2017;87:E163-4. [Crossref] [PubMed]
- Genco A, Castagneto-Gissey L, Lorenzo M, et al. Esophageal adenocarcinoma after sleeve gastrectomy: actual or potential threat? Italian series and literature review. Surg Obes Relat Dis 2021;17:848-54. [Crossref] [PubMed]
- Felinska E, Billeter A, Nickel F, et al. Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann N Y Acad Sci 2020;1482:26-35. [Crossref] [PubMed]
- D'Urso A, Vix M, Perretta S, et al. Indications and Long-Term Outcomes of Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass. Obes Surg 2021;31:3410-8. [Crossref] [PubMed]
- Yadlapati R, Gyawali CP, Pandolfino JE, et al. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin Gastroenterol Hepatol 2022;20:984-994.e1. [Crossref] [PubMed]
- Magema JMMK, Himpens J. "What Really Matters When Performing a Laparoscopic Roux-en Y Gastric Bypass?" Literature-Based Key Steps Towards Success and Standardization of the Procedure. Obes Surg 2021;31:5441-5. [Crossref] [PubMed]
- Mechanick JI, Apovian C, Brethauer S, et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures - 2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists - Executive Summary. Endocr Pract 2019;25:1346-59. [Crossref] [PubMed]
- Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA 2018;319:255-65. [Crossref] [PubMed]
- Salminen P, Grönroos S, Helmiö M, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss, Comorbidities, and Reflux at 10 Years in Adult Patients With Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg 2022;157:656-66. [Crossref] [PubMed]
- Carandina S, Soprani A, Montana L, et al. Conversion of sleeve gastrectomy to Roux-en-Y gastric bypass in patients with gastroesophageal reflux disease: results of a multicenter study. Surg Obes Relat Dis 2020;16:732-7. [Crossref] [PubMed]
- Crawford C, Gibbens K, Lomelin D, et al. Sleeve gastrectomy and anti-reflux procedures. Surg Endosc 2017;31:1012-21. [Crossref] [PubMed]
- Wolfsen HC, Richards WO. The Stretta procedure for the treatment of GERD: a registry of 558 patients. J Laparoendosc Adv Surg Tech A 2002;12:395-402. [Crossref] [PubMed]
- Khidir N, Angrisani L, Al-Qahtani J, et al. Initial Experience of Endoscopic Radiofrequency Waves Delivery to the Lower Esophageal Sphincter (Stretta Procedure) on Symptomatic Gastroesophageal Reflux Disease Post-Sleeve Gastrectomy. Obes Surg 2018;28:3125-30. [Crossref] [PubMed]
- Zhuang QJ, Tan ND, Chen SF, et al. Magnetic sphincter augmentation in treating refractory gastroesophageal reflux disease: A systematic review and meta-analysis. J Dig Dis 2021;22:695-705. [Crossref] [PubMed]
- Khaitan L, Hill M, Michel M, et al. Feasibility and efficacy of magnetic sphincter augmentation for the management of gastroesophageal reflux disease post-sleeve gastrectomy for obesity. Obes Surg 2023;33:387-96. [Crossref] [PubMed]
- Ajmera K, Thaimuriyil N, Shah N. Recent advances in the endoscopic management of gastro-esophageal reflux disorder: a review of literature. Cureus 2022;14:e26218. [Crossref] [PubMed]
Cite this article as: Serra FE, Cohen RV. Gastroesophageal reflux disease after sleeve gastrectomy. Dig Med Res 2024;7:5.


