
Borderline resectable pancreatic cancer: a review of recent radiation therapy literature and clinical practice
Introduction
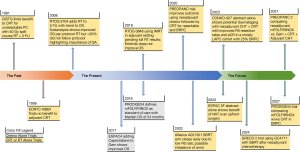
Borderline resectable pancreatic cancer (BRPC) is defined as pancreatic ductal adenocarcinoma (PDAC) in contact with vascular structures, unfavorable tumor biology including subclinical metastatic disease, or in a patient with poor performance status (1). The role of radiation therapy (RT) for BRPC continues to evolve. As advances in chemotherapy have improved control of systemic disease in BRPC, there is an increased need to control local disease through the use of RT in conjunction with surgery. Further, a proportion of patients die of local disease recurrence, which can be mitigated by incorporating radiation into the treatment regimen. Surgery remains the only cure for pancreatic cancer. Thus, in BRPC patients, every effort should be made to achieve a margin negative resection. Neoadjuvant therapy (NAT) has the advantage of treating micrometastatic disease upfront to allow for better patient selection prior to radical surgery, as well as to downstage patients and improve margin-negative resections, which are associated with superior outcomes for patients. In this paper, we explore the evolution of treatment approaches for BRPC (Figure 1).
Adjuvant radiation for pancreatic cancer
Since surgery is considered the only cure for PDAC, early investigations into pancreatic cancer treatments focused on the delivery of adjuvant therapy after upfront surgical resection for all patients with PDAC. Several trials clearly established a benefit to adjuvant chemotherapy over surgery alone for these patients, while the role of chemoradiotherapy (CRT) is less clear (2-6). It should be noted, however, that the early studies of CRT for pancreatic cancer used outdated techniques, varying dose schemas, and failed to employ strict quality assurance, which has been shown to impact patient outcomes (5,7).
Studies have shown that adequate systemic therapy to control metastatic disease is critical to improving survival of patients with pancreatic cancer and recent trials have clearly demonstrated the superiority of multi-agent systemic therapy for patients over single-agent chemotherapy alone, as was used in early trials of CRT (4,5). The GI-PRODIGE trial established mFOLFIRINOX as the standard of care chemotherapy regimen given a superior median overall survival (OS) of 53.5 vs. 35.5 months and 5-year OS of 43.2% vs. 31.4% when compared to gemcitabine plus capecitabine (8,9). However, up to one-third of patients develop local recurrence (LR) following adjuvant chemotherapy alone and thus there may also be a role for RT in the adjuvant setting with modern radiation therapy techniques and adequate systemic therapy (8). Once published, the two-step randomization trial NRG/RTOG 0848 may shed further light on the question of adjuvant RT using modern techniques with intensity-modulated radiation therapy (IMRT), although the trial notably does not employ mFOLFIRINOX (10). In step 1 of NRG/RTOG 0848 randomization, the addition of erlotinib to gemcitabine did not provide an OS benefit with a median survival of 29.9 months using gemcitabine and 28.1 months with the addition of erlotinib (P=0.62) (10).
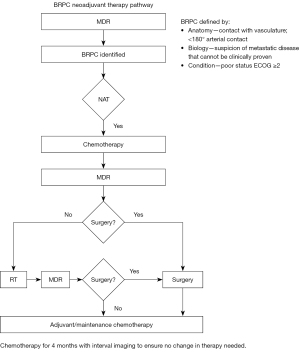
Studies of upfront surgery alone for pancreatic cancer demonstrate relatively high rates of margin positive resections, mostly due to the retroperitoneal margin, which is challenging to clear surgically due to the presence of vasculature in this area (11,12). Margin-positive resections have been associated with poorer outcomes for patients, even when adjuvant treatments such as those discussed above are delivered. Gnerlich et al. reported median OS of 16.4 months for margin positive resections vs. 21.7 months for margin negative (P=0.01) and Fatima et al. reported similar findings with median OS of 19 vs. 15 vs. 10 months for margin negative (R0), margin positive (R1), and gross tumor left (R2) resections (11,12). In an effort to improve resectability for patients, there has been an emphasis on increased scrutiny in determining which patients are at elevated risk of having incomplete resections, so that this risk can be addressed preoperatively. In 2006, a distinct subgroup of patients with BRPC was identified by the National Comprehensive Cancer Network (NCCN) Guidelines as a distinct entity and a consensus was reached on its definition in 2017 (1). The definition of BRPC is predicated on three distinct entities: (I) anatomy (tumors in contact with vascular structures including arterial and venous specific definitions: celiac axis, superior mesenteric artery less than 180 degrees and common hepatic artery contact allowing for safe resection and reconstruction; superior mesenteric vein and portal vein >180 degrees); (II) tumor biology (suspicion of metastatic disease that cannot be clinically proven); or (III) patient condition (those with poor functional status) (1).
Since the identification of this subgroup of patients, there has been a shift from adjuvant to neoadjuvant therapies for BRPC patients.
Neoadjuvant radiation for BRPC
There are a number of advantages to NAT over adjuvant therapy. These include treatment of micrometastatic disease, potential tumor downstaging, use of smaller radiation field size, clearance of surgical margins, and adherence/maximization of chemotherapy (13-16). Neoadjuvant treatment provides a window for occult metastases to declare themselves, sparing patients with unfavorable disease from high-risk surgery. Further, only 40–60% of patients who undergo resection ultimately receive needed adjuvant therapy, which can be improved if treatment is delivered prior to surgery (17-20). Additionally, at least one quarter of patients undergoing upfront resection have positive margins (11,12). Neoadjuvant treatment can help decrease the rate of margin-positive resections, which are known to be associated with inferior outcomes. Taken together, the benefits of NAT may potentially improve OS for BRPC patients (15,21-25). Of note, identification of which patients would benefit most from neoadjuvant approaches is best determined in a multi-disciplinary setting given the challenges of adequately identifying BRPC patients using imaging alone. The optimal neoadjuvant regimen remains under active investigation and thus should also be discussed in a multi-disciplinary setting (Figure 2) (19,23,24,26,27).
Early data to support neoadjuvant approaches for patients with BRPC were largely retrospective (28-30) and included a mixture of patients with resectable, borderline, and unresectable disease. A meta-analysis of trials comparing upfront surgery with neoadjuvant treatment for resectable and BRPC showed that NAT improved R0 resection, decreased the pathological nodal stage, and potentially improved OS (24). The only randomized controlled trials included in this meta-analysis were conducted in patients with resectable pancreatic cancer and failed to accrue (26,27).
More recently, prospective investigations into the role of NAT for patients with BRPC have shown better outcomes with careful patient selection and more intensive systemic regimens. A phase II prospective trial of 48 patients with BRPC underwent tailored RT following an initial course of FOLFIRINOX. Patients with complete response at the vasculature received short course RT (25 Gy in 5 fractions) and those with persistent vascular involvement underwent long course RT (50.4 Gy in 28 fractions with the vascular margin given 58.8 Gy in 28 fractions), both with concurrent chemotherapy (31). The R0 resection rate was high (97%) among patients undergoing resection. The 2-year PFS was also excellent at 43%, suggesting a benefit to neoadjuvant chemotherapy followed by CRT in patients with BRPC.
A multi-institutional phase II/III South Korean study compared outcomes for BRPC patients given neoadjuvant CRT followed by surgery to surgery with adjuvant CRT (32). The trial aimed to enroll 110 patients but was terminated early. Of the 58 patients enrolled, 27 were included in the neoadjuvant CRT arm treated to 54 Gy with concurrent Gemcitabine followed by surgery, and 23 patients were in the upfront resection followed by adjuvant CRT arm. The trial was terminated early due to improved efficacy of the neoadjuvant approach compared to the adjuvant approach on interim analysis, in terms of rate of R0 resection 51.8% vs. 26.1% (P=0.004), 2-year OS 40.7% vs. 26.1% (P=0.028) and median survival 21 vs. 12 months (P=0.028) (32). Additionally, the trial documents very poor adherence to post operative therapy with 13 out of 18 patients resected starting adjuvant therapy and only 6 patients completing adjuvant chemotherapy compared to 26 out of 27 completing neoadjuvant CRT prior to surgery (32).
The PREOPANC-1 trial randomized 246 patients with resectable or BRPC to three cycles of neoadjuvant gemcitabine CRT and four cycles adjuvant gemcitabine vs. surgery followed by six cycles of adjuvant gemcitabine (13). RT was delivered to 36 Gy in 15 fx with concurrent gemcitabine. This trial reported significantly improved R0 rate (71% vs. 40%) using the neoadjuvant approach and the updated report revealed a statistically significant improvement in OS in patients treated with NAT (13,14). In the predefined subgroup of patients with BRPC, OS, DFS, and locoregional failure-free survival, were all improved with neoadjuvant CRT with the subset of BRPC showing a hazard ratio (HR) of 0.67 (95% CI: 0.45–0.99, P=0.045) in favor of CRT.
The PREOPANC-2 trial continues to explore neoadjuvant approaches for BRPC, using intensified chemotherapy. This trial compares neoadjuvant chemotherapy alone using FOLFIRINOX vs. neoadjuvant CRT and adjuvant chemotherapy (with single agent gemcitabine) (33). Unfortunately, since the systemic therapy used in the radiation arm is single-agent gemcitabine alone and not multi-agent chemotherapy, which is known to improve outcomes, the question regarding the optimal neoadjuvant approach may remain unanswered. The four-arm prospective multicenter phase II ESPAC-5F trial has also attempted to address the question of the optimal neoadjuvant approach for patients with BRPC. The trial compared upfront surgery to one of three neoadjuvant treatment arms (gemcitabine plus capecitabine vs. FOLFIRINOX vs. CRT to 50.4 Gy with concurrent capecitabine) (15). Again, given the lack of multi-agent neoadjuvant systemic therapy in the CRT arm, the final results of this study when reported are unlikely to answer the question as to the optimal neoadjuvant approach. On interim analysis, the results overall favor a neoadjuvant approach over immediate surgery with 1-year OS 77% with NAT vs. 40% with immediate surgery (P<0.001).
Stereotactic body radiation therapy (SBRT) is a safe alternative to conventionally fractionated RT for patients with pancreatic cancer and offers advantages including short treatment times, a higher biological effective dose (BED), reduced risk of lymphopenia, and minimal delays to starting chemotherapy (34). Early studies on SBRT were primarily for patients with locally advanced pancreatic cancer (LAPC) (35-39). Results from some of these early studies exploring the role of SBRT for LAPC showed that a subset of patients were ultimately able to undergo surgery following SBRT leading to investigations on the role of SBRT for patients with BRPC (40,41). In a study by Chuong et al., 56% of BRPC ultimately were able to undergo resection and 96.9% of these were R0 resections (41). Patients who underwent surgery and were able to achieve an R0 resection had improved OS as compared to the patients who did not get surgery. A study from the group at Moffitt delivered SBRT to patients with LAPC and BRPC following systemic chemotherapy (40). Fifty-one percent of patients with BRPC who completed NAT were able to undergo surgery and of these, 96% achieved an R0 resection. Median OS was 34.2 months for surgically resected patients vs. 14 months for unresected patients. Results from these early studies of SBRT for BRPC showed that about half of the patients who completed NAT without distant disease progression were able to ultimately undergo surgery and most of these resections were to negative margins. Survival was improved in patients undergoing surgery vs. those who did not have their cancers removed.
The promising results of early work on SBRT led to the multicenter Alliance A021501 phase II clinical trial exploring the role of SBRT for BRPC. This study randomized BRPC patients to eight cycles of mFOLFIRINOX or seven cycles of mFOLFIRINOX followed by five fractions of SBRT. Of note, the goal of the study was to define the standard of care regimen for future phase III trials, and outcomes were compared to historical controls of OS of 50% at 18 months. Unfortunately, the SBRT arm was terminated at interim analysis after not having met the goal of 12 or more of the first 30 resections being R0 resection with R0 rate noted 57% for chemotherapy alone vs. 33% for the arm including SBRT. The final results showed 18-month OS was 66.7% with chemotherapy alone and 47.3% with chemotherapy and SBRT with median OS was 29.8 and 17.1 months respectively (42). Notably, the SBRT arm had higher Cancer Antigen 19-9 (CA 19-9) levels at baseline, and these patients were randomized before starting induction chemo, which could account for the higher levels of progression and metastasis in the SBRT arm. Of note, in patients who underwent surgery, 18-month OS was more similar (87.5%; 95% CI: 70.0–95.1%) in the chemotherapy alone arm and 78.9% (95% CI: 53.2–91.5%) in the SBRT arm. Also of note, surgical pathology from this trial reported pathological complete response (pCR) and near pCR rates favoring neoadjuvant SBRT (42). Given conflicting results of the PREOPANC, South Korean study, and ALLIANCE trial, further and better timed (43) studies are needed to elucidate how to best select which patients are likely to benefit from neoadjuvant RT and subsequent surgery.
Radiation techniques
Advances in radiation techniques have allowed the delivery of ablative radiation doses with increased safety and provide hopes of improving future outcomes. These advances, however, can lead to variable patient outcomes across trials employing different RT techniques, resulting in considerable debate on best practices given the lack of randomized data on the optimal mode of RT delivery. The increasing number of treatment modalities including IMRT, SBRT, stereotactic magnetic resonance (MR)-guided adaptive radiation therapy (SMART), and intraoperative radiation therapy (IORT) have added to the controversy. Improvements in target accuracy, motion-mitigation techniques, and faster treatment delivery have increased the safety and efficacy of RT. Randomized trials have not uniformly mandated the use of many of these delivery techniques, but single institutional data and dosimetric studies provide early insights.
Motion mitigation is critical for the delivery of high dose treatment to the abdomen, where organs are in constant motion. Respiratory gating is frequently used to decrease setup margins and reduce dose to surrounding tissues such as the bowel (44). One of the drawbacks of respiratory gating is the decreased duty cycle of delivery. Because the beam is on for a fraction of the respiratory cycle (typically near end-expiration), treatment time is often increased by a factor of three. Intensity-modulated flattening filter free beams can offset this increase in treatment time by utilizing the higher dose rates possible when the attenuating flattening filter is removed from the beam (45). In a Varian linear accelerator, dose rates more than double from 600 to 1,400 MU/min for a 6 MV beam and quadruple from 600 to 2,400 MU/min for a 10 MV beam. This combination of technologies allows for better motion mitigation without long treatment times. The GRECO-2 trial (NCT04698915) for unresectable or borderline resectable nonmetastatic pancreatic cancer, for example, utilizes SBRT and requires all patients to have some form of respiratory motion mitigation.
Meanwhile, MR-guided radiation therapy utilizes on-board MR imaging to provide excellent soft tissue contrast in imaging used for initial patient setup, real-time target tracking and respiratory gating (46), and monitoring of changes due to treatment which can be used to estimate tumor response or adapt the treatment to target malignant tissue more accurately. SMART uses technological advancements in MR-guided radiation therapy for improved soft tissue contrast compared to CT-guided therapy. SMART further allows for real time intrafraction assessment of tumor and organ at risk (OAR) structures with on-table re-planning and automatic beam gating (47). Early data has demonstrated the safety of SMART with doses up to 50 Gy. Hassanzadeh et al. reported a 4.6% grade 3 GI toxicity (2 duodenal ulcers) with 1-year local control of 84.3% with 93% of radiation plans reoptimized during the course of treatment (48). Chuong et al. showed 2.6% late grade 3 toxicity (one bleed—not requiring transfusions) with 1-year local control of 87.8%. Of note, elective nodal irradiation was also completed to 57.1% of patients, highlighting the safety of MR guidance for both gross and regional nodal disease outside of OARs (49). Though the majority of patients were unresectable in early reports on the use of SMART, a multi-institutional abstract reported the safety of SMART in a cohort of almost 30% BRPC (50). The use of MR-guided radiation therapy brings promise for the future of radiation therapy including bringing daily adaptive therapy to the forefront.
Though advancements in MR guidance have captured the headlines, improvements in conventional CT-based image guidance hold significant promise as well. Iterative cone-beam CT reconstruction, for example, has substantially improved low contrast resolution compared to traditional filtered backprojection (51) and several studies are utilizing CT to identify radiomic biomarkers for local control and survival (52,53). Additionally, one should not ignore the significant difference in cost between a dedicated MR-LINAC and the traditional C-Arm LINAC with kilovoltage on-board imaging. For many clinics, MR-LINACS are simply unaffordable and improvements in CT guidance provide hope for improving pancreatic cancer outcomes.
An area of debate in treating patients with pancreatic cancer, including those with borderline resectable disease, has been treatment volumes. Historically, with conventional CRT, treatment volumes have included large margins usually incorporating nodal stations which provided greater coverage at the expense of potential toxicity. Although historically SBRT or ablative dose volumes were limited to gross tumor, the early closure of the Alliance trial with the success of PREOPANC with larger target volumes have ignited controversy on best practice. Further, given data on the benefit of elective nodal irradiation, the ideal target volume remains to be determined (54). Miller et al. reported in a matched propensity study that SBRT to 40 Gy to gross tumor with elective nodal irradiation to 25 Gy improved locoregional progression with control rates of 22.6% vs. 44.6% (P=0.021) in favor of elective nodal irradiation vs. SBRT to tumor alone at the expense of increased (60% vs. 20%, P<0.001) acute grade 1–2 nausea (54). Meanwhile, Chuong et al. as discussed noted SMART safety in a cohort that included 20 elective nodal irradiation patients (49). Currently, guidelines advise against elective nodal irradiation when using SBRT until there is further prospective evidence to support expanding SBRT volumes (55).
There is considerable discussion on dose escalation in pancreatic cancer with goal of ablative radiation dose [biologically effective dose (BED10 ≥100 Gy)]. However, delivery of actual ablative doses is limited by nearby allowance of gastrointestinal OARs of duodenum, stomach, and small bowel. Dose escalated IMRT (DE-IMRT), CT-guided SBRT, SMART, and IORT all provide potential avenues for dose escalation to ablative doses. Dose escalation provides a potential avenue to downstage patients for resection, improve locoregional control, and offer complete pathological response. Ablative radiation therapy using dose painted approaches (56) could combine high dose treatment with coverage of elective nodal volumes, combining the potential benefits of both approaches in the event the patient is not surgically resected or explored. Additionally, the use of IORT after preoperative radiation therapy may be an attractive option for the delivery of ablative doses of radiation, though its use is limited by institutions that offer it as a therapeutic option. A recent series on IORT by Sekigami et al. reported on 201 (80 BRPC and 121 LAPC) patients treated with neoadjuvant FOLFIRINOX and chemoradiation (median dose: 50.4 Gy in 28 fractions) followed by surgical resection with or without IORT to 10 Gy in 1 fraction. No difference was seen in post operative toxicity, DFS or OS between patients who had R0 and R1 resections, suggesting that IORT may reduce the negative effect of an R1 resection (57).
Conclusions
BRPC is a distinct entity within the spectrum of patients with pancreatic cancer. As NAT for patients with pancreatic cancer becomes increasingly utilized, the role of radiation continues to evolve. Existing data suggest a benefit of neoadjuvant CRT in appropriately selected patients. Technological advances have created a number of treatment options for patients with pancreatic cancer, but future studies are needed to determine how to integrate these more novel approaches in a multidisciplinary setting. Ongoing trials such as PREOPANC2 (EudraCT: 2017–002036-17) and PRODIGE44 (NCT02676349) continue to incorporate radiation therapy in the treatment paradigm for these patients as the optimal role of radiation continues to be defined. Importantly, this is not an easy task, as clinical resectability does not always correlate with imaging, and therefore due to this imaging bias, a multi-disciplinary approach is vital in defining which patients have BRPC and in selection for neoadjuvant treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michael D. Chuong) for the series “Novel Therapies for Pancreas Cancer” published in Digestive Medicine Research. The article has undergone external peer review.
Peer Review File: available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-69/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-69/coif). The series “Novel Therapies for Pancreas Cancer” was commissioned by the editorial office without any funding or sponsorship. JMH reports personal consulting for Boston Scientific, personal consulting, and stock ownership at Histosonics and 1440 Foundation, Canopy Cancer Collective grant with funding to institution. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981;48:1705-10. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 2011;18:1319-26. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2012;82:809-16. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Conroy T, Castan F, Lopez A, et al. Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol 2022;8:1571-8. [Crossref] [PubMed]
- Abrams RA, Winter KA, Safran H, et al. Results of the NRG Oncology/RTOG 0848 Adjuvant Chemotherapy Question-Erlotinib+Gemcitabine for Resected Cancer of the Pancreatic Head: A Phase II Randomized Clinical Trial. Am J Clin Oncol 2020;43:173-9. [Crossref] [PubMed]
- Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 2012;147:753-60. [Crossref] [PubMed]
- Fatima J, Schnelldorfer T, Barton J, et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg 2010;145:167-72. [Crossref] [PubMed]
- Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol 2020;38:1763-73. [Crossref] [PubMed]
- Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 2022;40:1220-30. [Crossref] [PubMed]
- Ghaneh P, Palmer D, Cicconi S, et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2023;8:157-68. [Crossref] [PubMed]
- Fietkau R, Grützmann R, Wittel UA, et al. R0 resection following chemo (radio)therapy improves survival of primary inoperable pancreatic cancer patients. Interim results of the German randomized CONKO-007± trial. Strahlenther Onkol 2021;197:8-18. [Crossref] [PubMed]
- Sohal DPS, Duong M, Ahmad SA, et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2021;7:421-7. [Crossref] [PubMed]
- Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 2014;21:2873-81. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372-7. [Crossref] [PubMed]
- Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma - A population-based cohort study. Acta Oncol 2016;55:265-77. [Crossref] [PubMed]
- Hall WA, Dawson LA, Hong TS, et al. Value of Neoadjuvant Radiation Therapy in the Management of Pancreatic Adenocarcinoma. J Clin Oncol 2021;39:3773-7. [Crossref] [PubMed]
- Van Eijck CHJ, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: Long-term results of the multicenter randomized phase III PREOPANC trial. J Clin Oncol 2021;39:4016. [Crossref]
- Bakens MJ, van der Geest LG, van Putten M, et al. The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: a nationwide population-based analysis. Cancer Med 2016;5:2825-31. [Crossref] [PubMed]
- Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 2018;105:946-58. [Crossref] [PubMed]
- Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol 2017;35:515-22. [Crossref] [PubMed]
- Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 2012;214:33-45. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Cho IR, Chung MJ, Bang S, et al. Gemcitabine based neoadjuvant chemoradiotherapy therapy in patients with borderline resectable pancreatic cancer. Pancreatology 2013;13:539-43. [Crossref] [PubMed]
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619-27. [Crossref] [PubMed]
- Takai S, Satoi S, Yanagimoto H, et al. Neoadjuvant chemoradiation in patients with potentially resectable pancreatic cancer. Pancreas 2008;36:e26-32. [Crossref] [PubMed]
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:963-9. [Crossref] [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- Janssen QP, van Dam JL, Bonsing BA, et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 2021;21:300. [Crossref] [PubMed]
- Tchelebi LT, Lehrer EJ, Trifiletti DM, et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): An international systematic review and meta-analysis. Cancer 2020;126:2120-31. [Crossref] [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005;76:48-53. [Crossref] [PubMed]
- Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2010;78:735-42. [Crossref] [PubMed]
- Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011;81:e615-22. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [Crossref] [PubMed]
- Katz MHG, Shi Q, Meyers J, et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol 2022;8:1263-70. [Crossref] [PubMed]
- Ryckman JM, Reames BN, Klute KA, et al. The timing and design of stereotactic radiotherapy approaches as a part of neoadjuvant therapy in pancreatic cancer: Is it time for change? Clin Transl Radiat Oncol 2021;28:124-8. [Crossref] [PubMed]
- Jung J, Yoon SM, Park JH, et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS One 2019;14:e0214970. [Crossref] [PubMed]
- Xiao Y, Kry SF, Popple R, et al. Flattening filter-free accelerators: a report from the AAPM Therapy Emerging Technology Assessment Work Group. J Appl Clin Med Phys 2015;16:5219. [Crossref] [PubMed]
- van Sörnsen de Koste JR, Palacios MA, Bruynzeel AME, et al. MR-guided Gated Stereotactic Radiation Therapy Delivery for Lung, Adrenal, and Pancreatic Tumors: A Geometric Analysis. Int J Radiat Oncol Biol Phys 2018;102:858-66. [Crossref] [PubMed]
- Boldrini L, Cusumano D, Cellini F, et al. Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol 2019;14:71. [Crossref] [PubMed]
- Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv Radiat Oncol 2020;6:100506. [Crossref] [PubMed]
- Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract Radiat Oncol 2021;11:134-47. [Crossref] [PubMed]
- Chuong MD, Kirsch C, Herrera R, et al. Long-Term Multi-Institutional Outcomes of 5-Fraction Ablative Stereotactic MR-Guided Adaptive Radiation Therapy (SMART) for Inoperable Pancreas Cancer With Median Prescribed Biologically Effective Dose of 100 Gy10. Int J Radiat Oncol Biol Phys 2021;111:S147-8. [Crossref]
- Washio H, Ohira S, Funama Y, et al. Dose Reduction and Low-Contrast Detectability Using Iterative CBCT Reconstruction Algorithm for Radiotherapy. Technol Cancer Res Treat 2022;21:15330338211067312. [Crossref] [PubMed]
- Gregucci F, Fiorentino A, Mazzola R, et al. Radiomic analysis to predict local response in locally advanced pancreatic cancer treated with stereotactic body radiation therapy. Radiol Med 2022;127:100-7. [Crossref] [PubMed]
- Cozzi L, Comito T, Fogliata A, et al. Computed tomography based radiomic signature as predictive of survival and local control after stereotactic body radiation therapy in pancreatic carcinoma. PLoS One 2019;14:e0210758. [Crossref] [PubMed]
- Miller JA, Toesca DAS, Baclay JRM, et al. Pancreatic Stereotactic Body Radiation Therapy With or Without Hypofractionated Elective Nodal Irradiation. Int J Radiat Oncol Biol Phys 2022;112:131-42. [Crossref] [PubMed]
- Brunner TB, Haustermans K, Huguet F, et al. ESTRO ACROP guidelines for target volume definition in pancreatic cancer. Radiother Oncol 2021;154:60-9. [Crossref] [PubMed]
- Koay EJ, Hanania AN, Hall WA, et al. Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract Radiat Oncol 2020;10:e495-507. [Crossref] [PubMed]
- Sekigami Y, Michelakos T, Fernandez-Del Castillo C, et al. Intraoperative Radiation Mitigates the Effect of Microscopically Positive Tumor Margins on Survival Among Pancreatic Adenocarcinoma Patients Treated with Neoadjuvant FOLFIRINOX and Chemoradiation. Ann Surg Oncol 2021;28:4592-601. [Crossref] [PubMed]
Cite this article as: Nosrati JD, Sidiqi BU, Chitti B, Riegel AC, Herman JM, Tchelebi LT. Borderline resectable pancreatic cancer: a review of recent radiation therapy literature and clinical practice. Dig Med Res 2023;6:10.


