Histopathologic diagnosis of gastritis and gastropathy: a narrative review
Introduction
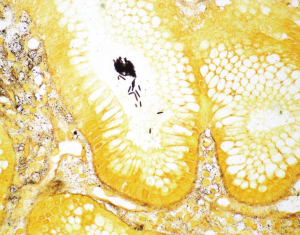
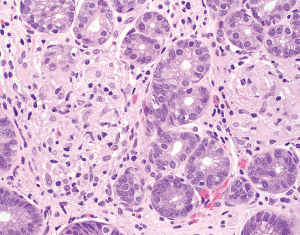
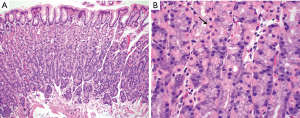
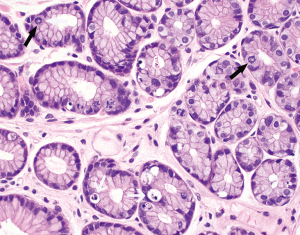
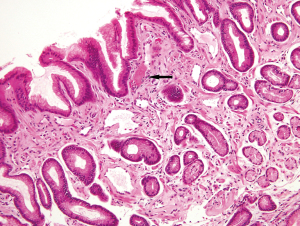
The normal gastric mucosa varies histologically in different regions but can be divided into antral and oxyntic types in general. Antral-type mucosa lines the antrum and pylorus where the deeper glands are loosely packed and mucin-producing. The ratio of deeper glands to overlying foveolae is roughly 50:50 (Figure 1A). A unique type of cells present in antral mucosa is gastrin-producing cells (G cells), which are more concentrated in the neck region of mucus glands. These cells can be recognized on routine hematoxylin-eosin (H&E) stain with a fried egg appearance characterized by round, dark and centrally located nuclei and pale or clear cytoplasm (Figure 1B). The oxyntic-type mucosa is seen in the body and fundus and consists of tightly packed oxyntic glands with a ratio to overlying foveolae of roughly 80:20 (Figure 2A). The oxyntic glands contain acid-secreting parietal cells, which have abundant pink cytoplasm on H&E stain, and zymogenic cells (chief cells), which are more numerous in the lower portion of the glands and stain purplish (Figure 2B). Transitional mucosa with mixed mucus and oxyntic glands is seen at the antral-body junction including the incisura angularis. Isolated parietal cells can be seen in antral-type mucosa, but chief cells are essentially absent outside the oxyntic and transitional mucosae. On the other hand, rare G cells may be detected in oxyntic mucosa by immunohistochemistry (1). The mucosa lining the gastric cardia is somewhat similar to antral mucosa but does not contain G cells. The glands in cardiac mucosa can be entirely mucinous or composed of a mixture of mucinous and oxyntic glands (cardio-oxyntic mucosa). Mild cystic dilation of the mucinous glands is a common finding in cardiac mucosa.
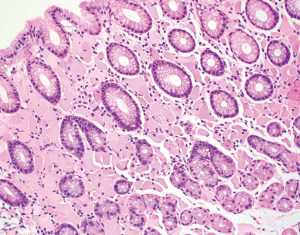
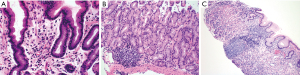
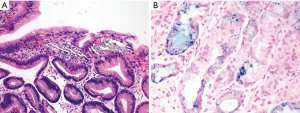
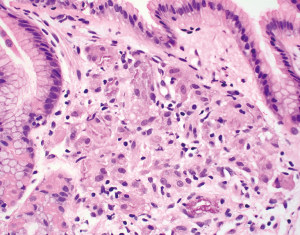
The lamina propria of the gastric mucosa contains only a minimal number of inflammatory cells including lymphocytes, plasma cells, eosinophils, mast cells and histiocytes. When the numbers of lamina propria inflammatory cells (mainly lymphocytes and plasma cells) exceed the normal limit, a diagnosis of “chronic gastritis” may be rendered. However, the definition of “normal limit” remains controversial. In the Updated Sydney System published in 1996, the normal limit was viewed as a maximum of 2–5 lymphocytes, plasma cells and histiocytes per high power (40× objective) microscopic field, or 2–3 lymphocytes or plasma cells between foveolae (2). This threshold is apparently too low to many gastrointestinal (GI) pathologists and gastroenterologists. In fact, a histopathologic diagnosis of “mild chronic gastritis” in the absence of Helicobacter pylori (H. pylori) or other identifiable etiologies (Figure 3A) is often considered nonspecific or even normal by experienced GI pathologists and gastroenterologists. The normal gastric mucosa may also contain occasional small lymphoid aggregates without well-formed germinal centers, typically located in the deep portion of the mucosa just above the muscularis mucosae (Figure 3B). Their presence should not be viewed as evidence of chronic gastritis. However, the presence of lymphoid follicles with well-formed germinal centers is usually an indication of ongoing or past H. pylori infection (Figure 3C), and a careful search for H. pylori organisms, including using special stains or immunohistochemistry, should be performed. The presence of large or irregularly shaped lymphoid follicles in a background of dense lymphocytic infiltrates should also raise the concern for extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT).
The normal gastric mucosa is typically devoid of neutrophils outside the vascular spaces. When neutrophils are present in the lamina propria, within the epithelium, or within the lumen of gastric glands, the inflammation is termed “active”. Active gastritis or activity is a histologic hallmark of H. pylori infection but can also be seen in other infectious and inflammatory conditions. It is usually seen in the background of chronic gastritis and should not be confused with acute gastritis that is only rarely encountered in clinical practice. Acute gastritis may result from ischemic injury secondary to debilitating illness, hypotension, or embolization. Ingestion of caustic agents, industrial chemicals, large doses of nonsteroidal anti-inflammatory drugs (NSAIDs) or large quantities of alcohol may cause acute gastritis. Pyogenic bacterial infections from Streptococcus, Staphylococcus, Enterobacter, Escherichia coli, and Pneumococcus (amongst others) can also induce acute gastritis. The histopathologic findings associated with these etiologies may include hemorrhagic gastritis or phlegmonous gastritis with varying degrees of edema, necrosis, and ulceration, which usually does not require endoscopic biopsies for the diagnosis. Neutrophils may be sparse or absent in acute gastritis but can be numerous in the setting of phlegmonous gastritis with mural abscess formation.
Gastritis and gastropathy differ mainly based on the presence or absence of inflammation associated with mucosal injury. In contrast to the presence of inflammatory cell infiltrates seen in gastritis, gastropathy is defined by mucosal damage with minimal or no inflammation. The correct diagnosis of various types of gastritis and gastropathy rests primarily on histopathologic evaluation of endoscopic biopsies of the gastric mucosa, but these findings should always be taken in the context of clinical presentations, endoscopic findings, and laboratory results. Relevant clinical information, such as knowing whether the patient has been treated for H. pylori or has taken specific medications (i.e., NSAIDs, iron pills, immunotherapy, or antibiotics, etc.), provides invaluable information for the histopathologic diagnosis. The endoscopic impression and labeled biopsy location of the specimen are also crucial items that should always be evaluated concurrently with histology. An accurate histopathologic diagnosis can only be obtained with a good understanding of the clinical and endoscopic picture.
Satisfactory mucosal sampling and proper specimen orientation are also of critical importance to optimal histologic evaluation. In general, two biopsies from the antrum and two from the body, which should be separately designated when submitted to the pathology laboratory, will suffice, although one additional biopsy from the incisura angularis is also recommended by the Updated Sydney System (2). Tissue orientation can be difficult for small-sized specimens and is best achieved in the pathology laboratory at the time of tissue embedding.
In this article, we focus on the histopathologic features that are essential to the recognition of most common types of chronic gastritis. A few uncommon types of non-Helicobacter infectious gastritis are also discussed. In addition, histopathologic features of reactive gastropathy, which usually shows no or minimal inflammation but are frequently encountered in daily practice, are described. We present this article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-42/rc).
Method
The content in this article is based on comprehensive literature review conducted on PubMed (Table 1) and authors’ practice experience.
Table 1
| Items | Specification |
|---|---|
| Date of search | 01/12/2021–21/04/2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Free text searches: H. pylori, H. heilmannii, infectious gastritis, chronic gastritis, Sarcina ventriculi, lymphocytic gastritis, collagenous gastritis, eosinophilic gastritis, atrophic gastritis, autoimmune gastritis, reactive gastropathy, drugs and stomach, medical resins, iron and stomach, calcium and stomach, radiation and stomach, portal hypertensive gastropathy, gastric antral vascular ectasia, granulomatous gastritis, Russell body gastritis, etc. |
| Timeframe | 1980–2022 |
| Inclusion and exclusion criteria | Inclusion: English research and review articles relevant to the content of this review article |
| Exclusion: non-English or articles irrelevant to the content of this review article | |
| Selection process | Selection was made independently by individual authors |
Helicobacter gastritis
H. pylori is a Gram-negative, spiral shaped rod with flagella. The major virulence factors include cytotoxin-associated gene A (CagA), cag pathogenicity island (Cag PAI), and vacuolating cytotoxin A (VacA). Studies have shown that over half of the world’s population has been infected by the organism through contaminated food or water (3,4). Mother-to-child transmission is believed to be a main route of infection (5,6), which explains why infection is typically first acquired during early childhood. The majority of infected patients are asymptomatic or have mild dyspeptic symptoms. Under the endoscope, the gastric mucosa may appear normal, erythematous, nodular, erosive, or ulcerated. Persistent H. pylori infection may lead to gastroduodenal ulceration, mucosal atrophy, intestinal metaplasia, dysplasia, gastric adenocarcinoma, and lymphoma, particularly the MALT type (4,7,8). There are several laboratory tests that can be used to help the diagnosis and management of H. pylori infection (9). The most used are the serological test for H. pylori antibodies, urease breath test, rapid urease test (also known as the Campylobacter-like organism or CLO test), and enzyme-linked immunosorbent assay for bacterial antigens in stool.
Diffuse lymphoplasmacytic inflammation with variable numbers of neutrophils is the histopathologic hallmark of H. pylori gastritis. The inflammation tends to be heavier and band-like in the superficial portion of the mucosa (Figure 4A). Lymphoid follicles with germinal centers are frequently present (Figure 3C). In most cases, H. pylori organisms can be visualized on routine H&E stain as slender, slightly curved or curvilinear rods in the mucin on the mucosal surface and in the gastric pits (Figure 4B). The organisms are typically absent or sparse in areas with intestinal metaplasia. Special stains like Giemsa, Diff-Quik and Warthin-Starry, and immunohistochemistry can help highlight the bacteria in biopsies where histologic findings are suspicious but have inconspicuous organisms not readily identifiable on H&E stain (Figure 4C). There is no consensus on the minimal histologic findings that should prompt the use of special stain or immunohistochemistry. Some authors want to see the presence of activity (i.e., neutrophils) or a moderate degree of chronic inflammation before ordering a stain while others think that any degree of chronic gastritis, even without activity, should trigger the use of an ancillary stain (10). Upfront or reflex use of ancillary stains on every gastric biopsy is not recommended in current practice (11).
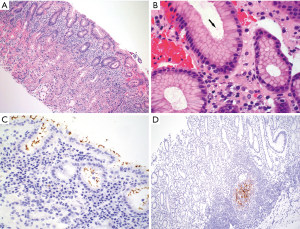
Immunohistochemistry is more sensitive than special stains in detecting H. pylori and is useful when the organisms are sparse following antibiotic and/or proton pump inhibitor (PPI) therapies that may substantially suppress but do not entirely eliminate the organisms (12). Immunohistochemistry is also superior to special stains in detecting deformed (coccoid) organisms in patients who have been treated with antibiotics before gastric biopsy (13). While untreated H. pylori gastritis is typically an antral-predominant disease, patients on PPIs may show more significant inflammation and more numerous organisms in the gastric body (14). For those cases, immunohistochemistry may need to be performed on both antral and body biopsies. Following successful H. pylori eradication, neutrophils disappear rapidly. Lymphoplasmacytic infiltrates may show reduced intensity but can persist for years. Lymphoid follicles may also show reduced size and number, but some may stay indefinitely. H. pylori antigen, but not viable organisms, can be detected in the germinal centers in a reticular pattern (15) (Figure 4D).
Polyclonal and some monoclonal antibodies for H. pylori cross-react with other Helicobacter species such as Helicobacter heilmannii (16). H. heilmannii is corkscrew-shaped organism with 5–7 distinct spirals. It is twice as long and thicker than H. pylori (Figure 5). Gastritides caused by H. heilmannii and H. pylori appear similar, but H. heilmannii gastritis is usually milder and more patchy. The clinical treatment options for H. heilmannii are identical to those for H. pylori gastritis. H. heilmannii infection is usually acquired from exposure to domestic pets or farm animals, and carries a limited risk of gastric adenocarcinoma and lymphoma (17).
Non-Helicobacter infectious gastritis
In addition to pyogenic bacteria causing acute phlegmonous gastritis (as mentioned above), a variety of infectious organisms can infrequently cause damage to the stomach. Etiologic bacteria include mycobacteria and Treponema pallidum. Viruses such as cytomegalovirus (CMV), herpes simplex virus, and Epstein-Barr virus (EBV) can also induce gastritis. Inciting fungi include Candida species, Histoplasma capsulatum, and Mucoraceae. Parasites such as Anisakis and Strongyloides can cause gastric injury.
CMV gastritis occurs in both immunocompromised and immunocompetent patients and carries a high mortality rate (18,19). Biopsies may show a spectrum of histologic findings ranging from minimal inflammation to deep ulceration with necrosis and granulation tissue (Figure 6A). Lamina propria edema and glandular destruction with increased apoptotic activity can be prominent. The virus is capable of infecting endothelial, epithelial, and stromal cells as well as histiocytes. The diagnosis rests on the identification of infected cells with characteristic virus-induced cytopathic changes. On H&E stain, the infected cells are distinctively enlarged and display amphophilic owl’s eye intranuclear inclusions and/or basophilic and granular cytoplasmic inclusions. Immunohistochemistry can be very helpful in equivocal cases (Figure 6B).
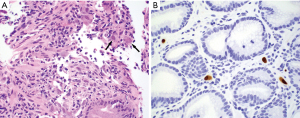
Syphilitic gastritis is a rare presentation of secondary syphilis. Histologically, syphilitic gastritis is characterized by dense and diffuse lamina propria lymphoplasmacytic infiltrates with gland destruction (20,21), mimicking H. pylori gastritis. Features of erosion, ulceration, vasculitis, and ill-formed granulomas may be seen. The diagnosis requires a high index of suspicion particularly in patients with a high risk of sexually transmitted diseases. The presence of prominent plasma cells in the inflammatory infiltrates in the absence of H. pylori or other identifiable etiologies should alert the pathologist to consider the diagnosis. Treponema pallidum can be visualized using the Warthin-Starry stain or immunohistochemistry for spirochetes.
EBV gastritis also features dense and diffuse lamina propria lymphocytic infiltrates with varying numbers of plasma cells (22-24). Erosions and gland destruction can be prominent, but neutrophils may be inconspicuous. Large, atypical lymphocytes and lymphoepithelial lesions may be present, which should not be confused with lymphoma (22). Features that may help distinguish EBV gastritis from MALT lymphoma include predominance of mature T-lymphocytes in the infiltrates including those involved in lymphoepithelial lesions, lack of clonality in B-lymphocytes and plasma cells, and lack of aberrant coexpression of T-cell markers (such as CD43) in B-lymphocytes. EBV gastritis can be seen in immunocompetent patients in the setting of infectious mononucleosis and may show diffuse thickening of the gastric wall with numerous ulcerations on endoscopy that are concerning for malignancy. In situ hybridization for EBV-encoded RNA (EBER) is diagnostic and a high index of suspicion is the key to the correct diagnosis.
Sarcina ventriculi is a Gram-positive anaerobic coccus that can be easily recognized on routine H&E stain as distinctive tetrads in the mucin on the gastric surface. While its pathogenesis remains debated, it has been associated with abdominal pain, dyspepsia, delayed gastric emptying, gastric ulcer, emphysematous gastritis, and gastric perforation (25,26). The histopathologic findings in endoscopic biopsies can vary from normal gastric mucosa to chronic gastritis, ulceration, and acute hemorrhagic gastritis. Transmural necrosis can be seen in gastrectomy specimens, but the presence of organisms in this setting is likely a secondary and incidental finding.
Lymphocytic gastritis (LG)
LG represents 1–5% of chronic gastritis cases (27-29). By itself, LG is not a specific diagnosis or etiology but rather a pattern of injury associated with various conditions. Thus, its presence should prompt a search for the underlying condition as treatment can lead to LG resolution. LG is primarily characterized by increased intraepithelial lymphocytes (IELs) within the surface and foveolar epithelium (Figure 7). In some studies, LG is arbitrarily defined by the presence of >25 IELs per 100 epithelial cells (28,30-33), but notably, the first authors to formally describe LG used a threshold of >30 IELs per 100 epithelial cells (27). Regardless of the exact quantitative definition, IELs are typically prominent enough to reach the diagnostic threshold at low scanning magnification, obviating the need for a formal IEL counting. IELs stand out from the background epithelium by their dark nuclei rimmed by clear, likely artifactual retraction halos. IELs are usually found at the base of the epithelium and are more concentrated within the surface epithelium rather than the deeper glands. LG can affect the entire stomach but predominantly involves the gastric body (27,34). However, IELs in LG associated with celiac disease are usually antral predominant (32,35).
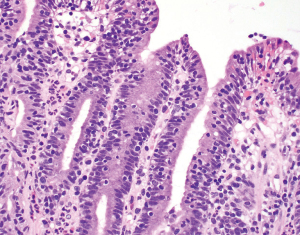
LG also demonstrates other histopathologic features in addition to increased IELs. The lamina propria is usually expanded by lymphoplasmacytic infiltrates, which can be minimal or marked. The degree of IELs is not associated with the severity of the lamina propria inflammation (27). The surface epithelium may show features of damage with regenerative and hyperplastic foveolar changes. The pits and foveolar epithelium may become elongated and corrugated.
Celiac disease is one of the most common associations with LG (35). About 30% of patients with LG have celiac disease (35,36). IELs in LG are predominantly CD3-positive T-cells with a CD8-positive cytotoxic phenotype (30,34). While immunophenotyping of the IELs is not required for the diagnosis of LG, it is mentioned here to highlight that both LG and celiac disease are postulated to have the same immunologic process with gluten-free diets resulting in clinical and histologic improvement (34,37,38). Studies have shown that the pattern of LG involvement (i.e., antral-predominant IELs) is predictive of the presence of duodenal pathology (32). Therefore, the presence of antral-predominant LG may suggest the need for additional serologic testing for celiac disease and duodenal biopsy to exclude histologic features of celiac disease.
Helicobacter infection is the other most frequently associated entity with LG (35,39). As described above, superficial band-like inflammation with or without activity should lead to a careful search for Helicobacter organisms. However, the absence of organisms does not entirely exclude Helicobacter infection as Helicobacter serology is frequently positive when histology is negative. It has been shown that ~80% of patients with LG demonstrate positive H. pylori serology and ~30% of these patients show organisms on histology (28,33). Helicobacter eradication leads to clinical and histologic improvement, regardless of the histologic status (33,40-42).
Other associations with LG include human immunodeficiency virus (HIV) infection, common variable immunodeficiency (CVID), Crohn disease, lymphocytic enterocolitis, medications (including immune checkpoint inhibitors), and gastric lymphoma (35,43-45). LG with CVID would show a lack or paucity of plasma cells in the lamina propria. LG with lymphocytic enterocolitis would have similar findings of increased IELs in the lower GI biopsies. Cessation of implicated medications, such as olmesartan and ticlopidine, results in clinical and histologic resolution (46,47). Patients on olmesartan can show celiac disease-like enteropathy with villous blunting and prominent intraepithelial lymphocytosis with a subset showing a lymphocytic and/or collagenous pattern of gastritis (46). LG with gastric lymphomas would show architectural disruption by an atypical lymphoid population. Most gastric lymphomas are B-cell processes whereas LG IELs are mostly T-cells with mixed lamina propria inflammatory cell infiltrates. IELs in LG are smaller in size and are singly and evenly distributed compared to the cells in lymphomatous lymphoepithelial lesions, which are typically clustered (44). LG was originally described as the histologic counterpart to the endoscopic entity varioliform gastritis (i.e., enlarged and thickened rugal folds with nodules and erosions) (27), but subsequent studies have shown that patients can also have normal endoscopic findings (48). Despite these associations, up to 20% of LG cases have an unknown etiology (35).
Collagenous gastritis (CG)
CG is a rare pattern of mucosal injury defined by the presence of subepithelial collagen deposition thicker than 10 µm associated with lamina propria inflammation (Figure 8). The thickened subepithelial collagen layer may have a patchy distribution, which correlates to the macroscopic nodularity sometimes seen on endoscopy (49). The collagen band typically averages 30–40 µm in thickness (50) and can measure over 100 µm (49-54). The subepithelial collagen band stains blue with Masson trichrome, which can be used to help confirm the diagnosis. It can also be highlighted by sirius red stain (50) and tenascin immunohistochemical stain (55), but these stains are seldom used in daily practice. The deep border of the collagen band is typically irregular with collagen fibers seeping in between the glands, a feature likened to candle wax drippings. The subepithelial collagen band can contain entrapped inflammatory cells, extravasated red blood cells, and capillaries. The inflammatory infiltrates of the lamina propria are predominantly lymphocytes and plasma cells, although some cases can have prominent lamina propria eosinophilia (52,55-57) with one study even designating an “eosinophil-rich” pattern (55). Other histologic features include varying degrees of surface epithelial detachment and reactive changes, intraepithelial lymphocytosis, and glandular atrophy (38,50,55,58). Subepithelial collagen deposition preferentially affects the gastric body and fundus in pediatric patients and the antrum in adults (55), but this finding can be seen throughout the stomach. Collagen deposition in CG is limited to the subepithelial region and should be differentiated from diffuse lamina propria fibrosis seen in other conditions such as autoimmune (atrophic) gastritis, radiation gastritis, scleroderma, healing ulcer, and NSAIDs injury (50,54).
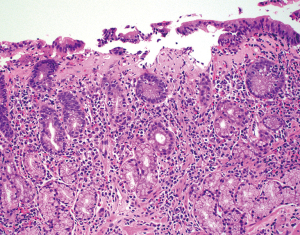
Patients with CG are seldom asymptomatic and frequently present with anemia, abdominal pain, nausea, vomiting, diarrhea (59), and rarely profound weight loss (54). Initial reports have shown that children and young adults usually present with iron deficiency anemia and have a nodular pattern on endoscopy limited to the stomach, whereas adults commonly present with chronic diarrhea and have associated collagenous colitis and duodenitis (50-52,60-64). However, there have been multiple reports showing extragastric manifestations in children (52,59,60,65-67). Patients with CG range in age from 9 months to 89 years (38,55,59,65). Some studies have shown a slight female predominance (55,59), while others have shown equal sex distribution (50,52).
CG is commonly associated with celiac disease (38,52,55,68), collagenous enteritis/colitis (50,52,53,55,69-72), and lymphocytic gastritis (38,50,52,55,68,73). Other clinical associations include Hashimoto thyroiditis (52), Sjögren syndrome (59), CVID, and polymyositis (52). Medications such as olmesartan and antidepressants have also been implicated (67). While H. pylori infection has been found in some patients with CG (50,55,60,62), there is no clear association between the two entities. The pathogenesis of CG is unknown, although some studies have suggested that the subepithelial collagen deposition is a reparative response to injury from toxic or infectious agents (50,74-76). Others have suggested that abnormal pericryptal fibroblasts cause collagen replacement of plasma protein exudate within the superficial lamina propria (75,77). The associated iron deficiency anemia has been theorized to arise from bleeding due to superficial capillary entrapment within the collagen (61,76). The presence of eosinophilic infiltrate is hypothesized to result from cytokine release. Tissue damage caused by eosinophil degranulation with resultant fibroblast proliferation may be involved in the pathogenesis of subepithelial collagen deposition (53,56,57).
Treatment strategies for CG are poorly defined and have yielded variable results. Management predominantly involves gluten-free diets, steroids, iron therapy, H. pylori eradication and acid suppression, with some patients showing clinical improvement (38,51,52,54,55,60,64,66,68,69,71-73). There can be histologic improvement with a decrease in thickness of the subepithelial collagen band (38,50,52,64), but most patients show unchanged or increased thickness on subsequent biopsies up to 10 or more years after diagnosis (38,49,51-55,57,58,60,62,64,65,69,72,76,78). Lamina propria cellularity before and after treatment can be similar (50,53). Untreated cases of CG have demonstrated increased collagen band thickness over time (52,53). Long-term follow-up on the first reported case of CG showed development of endocrine cell hyperplasia, intestinal metaplasia, moderate glandular atrophy, and epithelial changes that were indeterminate for dysplasia 13 years after initial diagnosis (58,78).
Eosinophilic gastritis
Eosinophilic gastritis is defined by eosinophilic infiltration of various layers of the stomach. It has an estimated prevalence of 6.3/100,000 with a female predilection and can occur in all age groups (79). Patients with eosinophilic gastritis may present with nonspecific GI symptoms such as abdominal/chest/throat pain, dyspepsia, nausea/vomiting, gas/bloating, diarrhea, GI bleeding, heartburn, and failure to thrive (79,80). Possible endoscopic findings may include erythema, erosion, and ulceration, but the mucosa can also appear entirely normal (81). Additionally, patients with eosinophilic gastritis may also have eosinophilic involvement in other parts of the GI tract such as eosinophilic esophagitis, enteritis and/or colitis.
The diagnosis of eosinophilic gastritis can be challenging due to the lack of specific clinical, endoscopic and laboratory findings (as mentioned above). Therefore, the diagnosis relies mainly on histologic examination of mucosal biopsies. A small number of eosinophils may be normally present in the lamina propria although the exact normal range of intramucosal eosinophils has not been well defined. In addition, the patchy nature of the disease adds to the diagnostic challenge and the diagnosis may thus require multiple biopsies from both normal- and abnormal-appearing mucosae (82). Histologically, eosinophilic gastritis is primarily characterized by an overall increase in the number of lamina propria eosinophils (Figure 9). Eosinophil infiltration of the muscularis mucosae and submucosa is another useful feature. Other features that may be helpful but are not essential to the diagnosis result from altered eosinophil behavior and distribution, and include the following: intraepithelial eosinophils (eosinophilic cryptitis), eosinophilic crypt abscesses, degranulation, concentration of eosinophils in the superficial (subepithelial) region, and formation of eosinophil aggregates (81). There can be substantial chronic or active inflammation, edema, and mucosal changes such as foveolar hyperplasia (83). Although there are no consensus diagnostic criteria, ≥30 eosinophils per high power field (HPF) in at least 5 different but most concentrated HPFs have been suggested (84) and accepted by the Food and Drug Administration as the diagnostic criteria in certain clinical trials (85). Importantly, other causes of eosinophilia must be excluded, which may include parasitic and Helicobacter infections, food allergy, drug reaction, inflammatory bowel disease, mastocytosis, Langerhans cell histiocytosis, and connective tissue diseases (84).
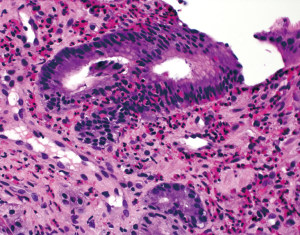
The pathogenesis of idiopathic eosinophilic gastritis is not well understood. Studies have shown that in patients with eosinophilic gastritis, T-cells release higher levels of IL-4 and IL-5, but less interferon-γ (83). IL-5 and the chemokine eotaxin have been implicated in eosinophil aggregation (82). Additionally, IL-1β, IL-4, IL-13, TNFα and leukotrienes have been postulated to play a role in eosinophilic recruitment (82). In patients with allergic eosinophilic gastritis, IgE has been implicated in mast cell degranulation. The relationship between mast cells and eosinophils is complex as they have been shown to activate each other and are important in potentiating an allergic response (82). Treatment is difficult due to the multifactorial etiologies and poorly understood pathogenesis. Interventions may include dietary modification, mast cell stabilizers, corticosteroids, leukotriene inhibitors, and antihistamine agents (82).
Atrophic gastritis
Atrophic gastritis predisposes patients to gastric adenocarcinoma, neuroendocrine tumor (NET) and lymphoma, necessitating its early recognition and appropriate surveillance to prevent disease progression (86-88). Serological testing for markers such as gastrin-17, pepsinogen I/II, anti-parietal cell antibodies, anti-intrinsic factor antibodies, and anti-H. pylori antibodies can help screen patients at risk for atrophic gastritis, but histologic evaluation of gastric antral and body mucosal biopsies remains the gold standard for diagnosis (88-90). Suspicious endoscopic findings may include mucosal nodularity, swelling, loss of gastric folds, and prominent vascular patterns. Electronic chromoendoscopy with narrow band imaging or blue light imaging allows enhanced recognition of metaplastic atrophic gastritis for targeted biopsies (88,91).
There are two common causes of atrophic gastritis: autoimmune and environmental. Recognizing the compartmental pattern of injury in atrophic gastritis can help elucidate the underlying etiologies and provide actionable information for patient management. Environmental atrophic gastritis exhibits an antral-predominant pattern of mucosal injury and may show low serum gastrin levels due to destruction of antral glands rich in G cells (88,89). Etiologies may include Helicobacter infection, chemical/bile injury, NSAIDs, dietary factors (i.e., nitrates, high salt smoked food, etc.), and other direct toxins (i.e., alcohol, smoking, etc.). Among these, H. pylori gastritis is the most common cause and is characterized by top-heavy, band-like lymphoplasmacytic inflammation as described above. Active inflammation, lymphoid follicles, antral gland atrophy and intestinal metaplasia are commonly seen. Intestinal metaplasia and gland atrophy of the oxyntic mucosa can occur late in the disease, resulting in atrophic changes in both antrum and body, hence its former name of multifocal atrophic gastritis.
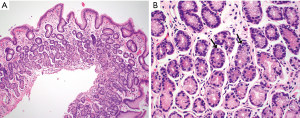
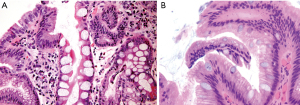
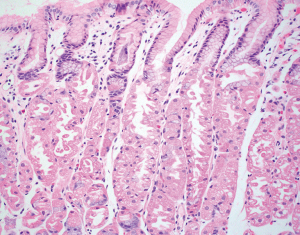
While not widely accepted to report for clinical care, it may be useful to be aware of the difference between complete and incomplete intestinal metaplasia. Complete intestinal metaplasia shows interspersed goblet cells in a background of absorptive-type cells resembling enterocytes with well-formed brushed borders and eosinophilic cytoplasm (Figure 10A). Paneth cells may be present. In contrast, incomplete intestinal metaplasia shows interspersed goblet cells in a background of mucin-producing foveolar-type cells that lack well-defined brush borders (Figure 10B). Subtyping can be made on routine H&E stain, however having a mixture of complete and incomplete intestinal metaplasia is not uncommon. Some earlier studies suggest that incomplete intestinal metaplasia may carry a higher risk of progression to dysplasia, but there has been no agreement on its impact on surveillance intervals or the risk of cancer progression (92).
Autoimmune gastritis exhibits a body-predominant pattern of mucosal injury due to immune-mediated destruction of parietal cells, resulting in decreased acid and intrinsic factor production. Reduced acid secretion leads to loss of G cell feedback inhibition, which consequently leads to hypergastrinemia and enterochromaffin-like (ECL) endocrine cell hyperplasia in the body. Serological tests may show anti-parietal cell and anti-intrinsic factor antibodies, anti-H+/K+ ATPase antibodies, high gastrin and chromogranin levels, low level of pepsinogen I, and low pepsinogen I/II ratio (88,89). There is a higher prevalence of autoimmune gastritis in patients with other autoimmune conditions such as Hashimoto thyroiditis, type 1 diabetes mellitus, rheumatoid arthritis, and Sjögren syndrome. Autoimmune gastritis is most common in women over the age of 55, but it can occur in any age group or ethnicity (93). Patients with autoimmune gastritis may present with pernicious anemia due to decreased intrinsic factor secretion and consequently decreased vitamin B12 absorption. There is an increased risk of type 1 well-differentiated NET due to ECL cell hyperplasia, which is typically indolent and carries a much better prognosis than type 3 (sporadic) NET of the stomach (94). The risk for pyloric gland adenoma and gastric adenocarcinoma is also increased (87). Surveillance is recommended every 3 years or every 1–2 years if there is a first-degree family history of gastric cancer (88).
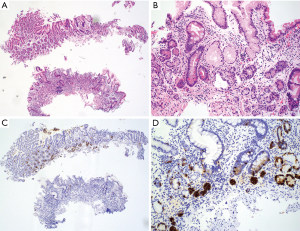
Antralization of the oxyntic mucosa can be misinterpreted as chronic antral gastritis if the location of the biopsy is not specified, especially when multiple biopsies are submitted in one container. Fortunately, there are some histopathologic features and immunohistochemical markers that can help avoid this pitfall. The main histopathologic features of autoimmune gastritis are diffuse oxyntic atrophy with extensive loss of oxyntic glands (Figure 11A,11B), lamina propria lymphoplasmacytic infiltrates, intestinal metaplasia, pseudopyloric metaplasia, pancreatic acinar metaplasia, and ECL cell hyperplasia (95,96). Activity is usually absent or focal, but concurrent H. pylori infection can happen, which may require immunohistochemistry to evaluate for Helicobacter organisms (95). On H&E stain, there should be no recognizable G cells in atrophic oxyntic mucosa, which have a fried egg appearance with round, dark and centrally located nuclei and pale or clear cytoplasm as described above. The antral mucosa is typically spared and devoid of significant inflammation but usually display G cell hyperplasia. In difficult cases, immunostaining for gastrin can help highlight G cells. Complete lack of G cells or presence of only rare G cells confirms that the biopsy is from atrophic oxyntic mucosa (Figure 11C). ECL cell hyperplasia in atrophic oxyntic mucosa can be better illustrated by immunostaining for a neuroendocrine marker such as chromogranin, which is defined as at least 5 adjacent ECL cells forming a linear or micronodular configuration (Figure 11D). Expansile or infiltrative nodules of ECL proliferation ≥0.5 cm or any size nodules within the submucosa are categorized as well-differentiated NET.
Reactive gastropathy
Reactive gastropathy, also termed chemical gastropathy, is a commonly reported pattern of gastric injury thought to arise from chemically induced mucosal damage (97,98). The clinical symptoms and endoscopic findings are often mild and nonspecific with poor histopathologic correlation. This lack of clinicopathologic correlation along with unclear clinical significance of the diagnosis may contribute to overdiagnosis of histologically normal or near-normal gastric biopsies by pathologists. It is also unclear whether this diagnosis should prompt changes or cessation in medications such as NSAIDs or PPIs. Therefore, the diagnosis of reactive gastropathy should not be used loosely (99).
Reactive gastropathy encompasses a constellation of histologic features including foveolar hyperplasia with a corkscrew-like appearance of the gastric pits (Figure 12), surface mucin depletion, lamina propria edema, and prominent lamina propria smooth muscle fibers extending towards the surface with little to no inflammation. Superficial erosion, ulceration and focal intestinal metaplasia can be present (99). Mucosal injury is generally restricted to the antrum but can extend into the body in severe cases. Overinterpretation of foveolar hyperplasia can be avoided by recognizing elongated pits greater than the 50:50 foveolae-to-gland ratio normally seen in the antrum. Occasional wisps of smooth muscle in the lamina propria can be seen in the normal antrum, in contrast to more prominent hyperplastic smooth muscle fibers in reactive gastropathy.

There are several chemical agents that can cause a reactive gastropathy pattern of injury. Acid, alkali, and large quantities of alcohol can cause severe and extensive damage that can heal over time after exposure removal. Binge alcohol consumption may cause subepithelial petechiae due to localized mucosal hemorrhages and associated edema (100). Patients who have undergone Billroth II partial gastrectomy may suffer from bile reflux and can develop polypoid lesions at the anastomotic site consisting of hyperplastic and irregularly dilated cystic foveolae. They are sometimes called gastritis cystica polyposa or gastritis cystica profunda depending on whether the lesion is polypoid or inverted, respectively (101).
Cases of medication-related reactive gastropathy are escalating due to an increasing number of available medications and an aging population. Reviewing the clinical history, medication list, and laboratory data can be helpful to identify the offending agent. A summary of the most common medications associated with reactive gastropathy are discussed below.
NSAIDs are the most common culprit of medication-induced reactive gastropathy. Their mechanism of action of nonselective cyclooxygenase inhibition results in decreased production of mucosa-protective products (i.e., prostaglandins, mucins, bicarbonate) and decreased circulation. Features of erosion and ulceration are frequently found. Concurrent corticosteroids administration may exacerbate the effects of NSAIDs-induced injury. Conversely, selective cyclooxygenase inhibitors, prostaglandin analogues and PPIs can reduce the likelihood of NSAIDs-induced ulceration, but their effects on the development of reactive gastropathy are unclear (102-104).
PPIs are another commonly used hospital administrated and over-the-counter medication class that can cause reactive gastropathy. These drugs are used to treat peptic ulcer disease and reflux esophagitis by inhibiting parietal cell acid secretion, which inadvertently leads to increased gastrin secretion that results in parietal cell hypertrophy and endocrine cell hyperplasia. Characteristic histologic features of PPI effects include dilated oxyntic glands lined by hypertrophic parietal cells with cytoplasmic snouting (Figure 13). Parietal cells may become flattened or have vacuolated cytoplasm after a long-term use. Fundic gland polyp can develop (105).
Gastropathies associated with tissue deposition
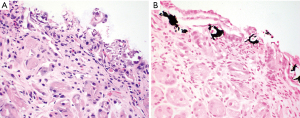
Iron deposition in the gastric mucosa can be classified into three patterns. Pattern A (nonspecific gastric siderosis) is most common and is associated with prior mucosal damage and microhemorrhages. Subtle iron deposition is found in scattered macrophages and stromal cells. Pattern B (iron-pill gastritis) is the most recognized subtype and is associated with ferrous sulfate therapy. Characteristic extracellular coarse brown crystalline iron deposits are seen in the mucosa and luminal debris (Figure 14A). Associated erosion, ulceration and fibrinoinflammatory exudate should prompt careful evaluation by endoscopists. Pattern C (gastric glandular siderosis) is least commonly encountered and is usually seen in the setting of iron overload (i.e., multiple blood transfusions, hereditary hemochromatosis, etc.). Iron deposits are subtle and present as uniform fine golden granules in epithelial cells of deep gastric glands (Figure 14B). Early diagnosis can help prevent further exacerbation of iron overload. Prussian blue stain is a useful tool to help highlight iron deposits in all three patterns (106).
Mucosal calcium deposition, or mucosal calcinosis, is classified as metastatic, dystrophic, or idiopathic. Metastatic calcinosis is the most common subtype and is due to calcium and phosphate imbalance that causes calcium deposition in normal tissue, typically seen in patients with end-stage renal disease. Other etiologies may include tumor lysis syndrome, hypervitaminosis A, atrophic gastritis, organ transplantation, drug/medication injury (i.e., isotretinoin, sucralfate, aluminum-containing antacids), and citrate-containing blood products. Recognition is important as patients are at risk for cardiac calcium deposition, which can be prevented with early intervention. Dystrophic calcification refers to calcium deposition in damaged tissue in the setting of normal laboratory values. On endoscopy, small white flecks, plaques, or nodules may be seen. Histologically, the deposits are greyish-black, coarse, and irregular, and are typically found in the superficial portion of the lamina propria just beneath the surface epithelium (Figure 15A). A von Kossa stain can be helpful, which imparts a black color to the deposits (107) (Figure 15B).
Nonabsorbable medication resins used for ion exchange can be identified on H&E stain and confirmed by review of patient’s medication list. The most encountered resins are kayexalate, sevelamer and bile acid sequestrants (108,109). Kayexalate (sodium polystyrene sulfonate) is a potassium-binding agent used to treat hyperkalemia in renal failure. It has a rectangular, purple “fish-scale” or “mosaic-like” appearance with regular narrow crackling lines (Figure 16). The surrounding mucosa may show ischemic and ulcerative changes. Identification should prompt clinician notification as it has been linked to GI perforation and fatalities. Sevelamer (Renagel/Renvela) is used to treat hyperphosphatemia in renal failure and is also associated with mucosal injury. Sevelamer also has “fish-scales” and may display a two-toned (pink and yellow) appearance (Figure 16). These resins may be embedded in ischemic or ulcerated tissues or in necrotic debris. Lastly, bile acid sequestrants (cholestyramine, colestipol, colesevelam) used to treat hypercholesterolemia, pruritis, and bile acid-mediated diarrhea are not associated with mucosal injury and thus medication adjustment is not necessary. These resins have a smooth glassy texture and can be bright pink or orange on H&E stain.

Lanthanum carbonate is another oral phosphate binder used to treat hyperphosphatemia in renal failure. The medication can deposit in the mucosa throughout the GI tract, including the stomach. Endoscopic findings may include nonspecific gastritis, erosion, ulceration, and gastric polyps. Biopsies show lamina propria accumulation of histiocytes filled with brown-purple granular materials within the cytoplasm (110,111) (Figure 17).
Gastropathies associated with chemotherapeutic and radiation therapies
Chemotherapeutic agents can cause distinctive histopathologic features. Colchicine and taxanes both inhibit microtubule polarization and result in mitotic arrest with characteristic ring-form mitoses (Figure 18), increased apoptotic activity, and epithelial reactive changes (112,113). In patients treated with colchicine, these findings indicate toxicity and should prompt adjustment of colchicine dosage. In contrast, these histologic findings are “normally” seen in taxane administration and dosage adjustment is not necessary. Immune checkpoint inhibitors (i.e., anti-PD1, anti-PD-L1, and anti-CTLA therapies) are increasingly being used for a growing list of malignancies. Histologic findings of immune-related adverse events in the stomach are nonspecific but most commonly show chronic gastritis with or without activity followed by granulomatous gastritis, focally enhanced gastritis, and lymphocytic gastritis (114,115).
Radiation therapy can also cause a reactive gastropathy pattern of injury. Histologic features that would indicate prior radiation treatment include dilated capillaries, lamina propria fibrosis and hyalinization, and atypical cytology in stromal, endothelial, and epithelial cells. Yttrium-90 microspheres administered for unresectable primary and metastatic hepatic malignancies can be inadvertently circulated to the stomach and cause unintendedly radiation injury. These microspheres are 30–40 µm in diameter and appear as uniform, perfectly round opaque purple spheres. Their half-life is about 2.5 days with emissions occurring out to 14 days post-delivery (116).
Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE)
Portal hypertensive gastropathy occurs in patients with cirrhosis. In addition to the general histologic features of reactive gastropathy, portal hypertensive gastropathy characteristically shows dilated and congested capillaries in the lamina propria primarily seen in the gastric body. The diagnosis requires correlation with a clinical history of portal hypertension and endoscopic features of “snake-skin mosaic-like” pattern or bulging red/brown marks. GAVE also shows ectatic lamina propria capillaries that may be similar in diameter to adjacent antral glands but may additionally contain fibrin thrombi (Figure 19). “Watermelon stomach” is the characteristic endoscopic finding in GAVE (117).
Granulomatous gastritis
Granulomatous gastritis is a nonspecific diagnosis describing histologic findings of multiple granulomas in the gastric mucosa and occasionally in the submucosa (Figure 20). The finding of a single granuloma does not qualify for the diagnosis. The prevalence has been reported to be between 0.08% and 0.35% (118). Etiologically, granulomatous gastritis can be infectious, noninfectious, or idiopathic. Infectious etiologies mainly include mycobacteria, fungi, and parasites, but bacteria such as Treponema pallidum and possibly H. pylori are also potential etiologies. Mycobacterial granulomas may show central necrosis and the organisms may be visualized by special stains such as acid-fast bacteria (AFB) stain and immunohistochemistry. Negative AFB stain should not exclude the possibility because of low sensitivity of the stain on tissue sections. Fungal granulomas may also show necrosis and the organisms can be easily visualized on routine H&E stain or on special stains such as Grocott methenamine silver (GMS) and periodic acid-Schiff with diastase (PASD) stains. Parasitic granulomas may be associated with prominent eosinophilic infiltrates.
Noninfectious etiologies mainly include Crohn disease, sarcoidosis, drugs, foreign bodies, and neoplasms. Gastric granulomas are seen in 5–30% of Crohn patients and are more prevalent in children and young patients (118). Crohn granulomas are typically non-necrotizing and may be poorly formed with a small size. The background gastric mucosa may show features of nonspecific chronic gastritis or focally enhanced gastritis. The latter is characterized by localized lamina propria periglandular inflammatory cell infiltrates composed of lymphocytes and plasma cells (with or without neutrophils) with intervening uninvolved mucosa. Though commonly described in Crohn patients, particularly in pediatric patients, focally enhanced gastritis has been observed in other conditions such as ulcerative colitis (119,120), hematopoietic stem cell transplantation suggestive of late-onset graft-versus-host disease (121), and immune checkpoint inhibitor-related injury (114,115). Granulomas seen in the setting of sarcoidosis are typically numerous, large, well-formed and non-necrotizing. Langhans giant cells with or without cytoplasmic Schaumann and asteroid bodies may be present. While clinical and radiographic correlation is important for the diagnosis of sarcoidosis, gastric involvement can be the first sign of the disease in some cases (122).
Russell body gastritis
Russell body gastritis is characterized by lamina propria accumulation of plasma cells with prominent eosinophilic cytoplasmic inclusions of immunoglobulin termed “Russell bodies” (Figure 21). The etiology and pathogenesis are unclear, although some reported cases are associated with H. pylori infection. Immunophenotypically, the Russell bodies are either polyclonal or κ light chain restricted. There are no reported cases that have progressed to lymphoma. The current belief is that Russell body gastritis is a reactive process (123-125).
Conclusions
Gastric biopsy is frequently performed to rule out H. pylori during upper GI endoscopy performed on patients with GI symptoms. The diagnosis of H. pylori gastritis and various types of non-Helicobacter gastritis or gastropathy rests primarily on histopathologic evaluation (Table 2). Knowledge of normal histology of the gastric mucosa, recognition of characteristic histopathologic features of different types of gastritis and gastropathy, and correlation with clinical and endoscopic findings are essential to accurate diagnosis in order to achieve the best patient care. Adequate sampling of the gastric mucosa by endoscopists and appropriate handling of tissue samples by laboratory technicians are also critically important.
Table 2
| Type of gastritis and gastropathy | Key histologic features | Helpful stains |
|---|---|---|
| Helicobacter gastritis | • Superficial band-like lymphoplasmacytic inflammation with variable activity (top heavy) | Giemsa, Diff-Quik, Steiner, Warthin-Starry, IHC |
| • Lymphoid follicles with germinal centers | ||
| • H. pylori: slender and curved/curvilinear rods in mucin on surface and in gastric pits | ||
| • H. heilmannii: corkscrew-shaped, 5–7 spirals, longer and thicker than H. pylori | ||
| • Treated disease may show chronic gastritis without activity, and coccoid organisms | ||
| Non-Helicobacter infectious gastritis | Pyogenic bacteria/phlegmonous gastritis | |
| • Numerous neutrophils, mural abscess formation | ||
| Cytomegalovirus | IHC | |
| • Viral cytopathic changes: large cells with amphophilic “owl’s eye” intranuclear inclusions and/or basophilic and granular cytoplasmic inclusions | ||
| Syphilis | Warthin-Starry, IHC | |
| • Prominent plasma cell infiltrates with gland destruction | ||
| Epstein-Barr virus | EBER in situ hybridization | |
| • Large, atypical lymphocytes and lymphoepithelial lesions | ||
| Sarcina ventriculi | ||
| • Organisms arranged in tetrads on mucosal surface | ||
| Lymphocytic gastritis | • >25 IELs per 100 epithelial cells in surface epithelium | |
| • Lamina propria lymphoplasmacytic inflammation | ||
| Collagenous gastritis | • >10 µm subepithelial collagen band | Masson trichrome |
| • Lamina propria lymphoplasmacytic inflammation | ||
| • Glandular atrophy | ||
| Eosinophilic gastritis | • Increased eosinophils in lamina propria: ≥30 eosinophils per HPF in at least 5 different but most concentrated HPFs | |
| • Varying degrees of eosinophilic cryptitis, eosinophilic crypt abscess, and eosinophilic infiltration of the muscularis mucosae may be seen | ||
| Autoimmune gastritis | • Antralization of oxyntic mucosa with diffuse loss of oxyntic glands | IHC for gastrin and chromogranin |
| • Lamina propria lymphoplasmacytic inflammation | ||
| • Pseudopyloric, pancreatic acinar and intestinal metaplasia | ||
| • ECL cell hyperplasia | ||
| • Relatively normal antral mucosa | ||
| Environmental atrophic gastritis | • Antral-predominant inflammation and atrophy | IHC for H. pylori |
| • Varying degrees of oxyntic gland atrophy | ||
| • Intestinal metaplasia | ||
| Reactive gastropathy | • Foveolar hyperplasia with corkscrew-like elongation (>50:50 foveolae-to-gland ratio) | |
| • Lamina propria edema and/or smooth muscle proliferation | ||
| • Little or no inflammation, but erosion or ulceration may be seen | ||
| Therapy-associated mucosal injury | Colchicine and Taxanes | |
| • Ring-form mitoses, increased apoptosis, and epithelial reactive changes | ||
| Immune checkpoint inhibitors | ||
| • Nonspecific changes (chronic gastritis +/– activity, granulomatous gastritis, focally enhanced gastritis, lymphocytic gastritis) | ||
| PPIs | ||
| • Dilated oxyntic glands lined by hypertrophic parietal cells with cytoplasmic snouting (can be flattened or have vacuolated cytoplasm) | ||
| • Fundic gland polyps may occur | ||
| Radiation | ||
| • Atypical cytology in stromal, endothelial, and epithelial cells | ||
| • Dilated capillaries | ||
| • Lamina propria fibrosis and hyalinization | ||
| Tissue deposits | Iron | Prussian blue |
| • Extracellular coarse brown crystalline iron deposits in mucosa and luminal debris (iron-pill gastritis) | ||
| • Iron deposits in macrophages and stromal cells (nonspecific gastric siderosis) | ||
| • Iron deposits in epithelial cells of deep glands (gastric glandular siderosis) | ||
| Calcium | von Kossa | |
| • Grey-black, coarse and irregular deposits in superficial lamina propria | ||
| Nonabsorbable medication resins | ||
| • Kayexalate: rectangular, purple, “fish-scale” deposits | ||
| • Sevelamer: two-toned (pink-yellow), “fish-scale” deposits | ||
| • Bile acid sequestrants: bright pink or orange, smooth and glassy deposits | ||
| Lanthanum carbonate | ||
| • Accumulation of lamina propria histiocytes containing cytoplasmic brown-purple granular material | ||
| Portal hypertensive gastropathy | • Ectatic lamina propria capillaries | |
| • Predominantly seen in gastric body | ||
| Gastric antral vascular ectasia | • Ectatic lamina propria capillaries +/– fibrin thrombi | |
| • Primarily seen in gastric antrum | ||
| Granulomatous gastritis | Infectious | Gram, GMS, PASD, AFB, IHC for mycobacteria and spirochetes |
| • Mycobacteria, fungi, parasites, Treponema pallidum, etc. | ||
| • May show central necrosis and/or prominent eosinophils | ||
| Crohn disease | ||
| • Small, poorly formed, non-necrotizing granulomas | ||
| • Nonspecific chronic gastritis or focally enhanced gastritis | ||
| Sarcoidosis | ||
| • Large, well-formed, non-necrotizing granulomas +/– Langhans giant cells with cytoplasmic Schaumann and/or asteroid bodies | ||
| Russell body gastritis | • Lamina propria accumulation of plasma cells with prominent Russell bodies | IHC for plasma cell markers (CD79a, CD138), kappa and lambda light chains |
IHC, immunohistochemistry; EBER, Epstein-Barr virus-encoded RNA; IELs, intraepithelial lymphocytes; HPF, high power field; ECL, enterochromaffin-like; PPIs, proton pump inhibitors; GMS, Grocott methenamine silver stain; PASD, periodic acid-Schiff stain with diastase; AFB, acid-fast bacteria stain.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-42/rc
Peer Review File: Available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-42/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.org/article/view/10.21037/dmr-22-42/coif). HLW serves as an unpaid editorial board member of Digestive Medicine Research from July 2021 to June 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cockburn AN, Morgan CJ, Genta RM. Neuroendocrine proliferations of the stomach: a pragmatic approach for the perplexed pathologist. Adv Anat Pathol 2013;20:148-57. [Crossref] [PubMed]
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-81. [Crossref] [PubMed]
- Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420-9. [Crossref] [PubMed]
- Robinson K, Atherton JC. The Spectrum of Helicobacter-Mediated Diseases. Annu Rev Pathol 2021;16:123-44. [Crossref] [PubMed]
- Konno M, Yokota S, Suga T, et al. Predominance of mother-to-child transmission of Helicobacter pylori infection detected by random amplified polymorphic DNA fingerprinting analysis in Japanese families. Pediatr Infect Dis J 2008;27:999-1003. [Crossref] [PubMed]
- Mamishi S, Eshaghi H, Mahmoudi S, et al. Intrafamilial transmission of Helicobacter pylori: genotyping of faecal samples. Br J Biomed Sci 2016;73:38-43. [Crossref] [PubMed]
- Jessurun J. Helicobacter pylori: an evolutionary perspective. Histopathology 2021;78:39-47. [Crossref] [PubMed]
- Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212-39. [Crossref] [PubMed]
- Nakamura RM. Laboratory tests for the evaluation of Helicobacter pylori infections. J Clin Lab Anal 2001;15:301-7. [Crossref] [PubMed]
- Yantiss RK, Lamps LW. To stain or not to stain...That remains the question. Am J Clin Pathol 2012;137:343-5. [Crossref] [PubMed]
- Batts KP, Ketover S, Kakar S, et al. Appropriate use of special stains for identifying Helicobacter pylori: Recommendations from the Rodger C. Haggitt Gastrointestinal Pathology Society. Am J Surg Pathol 2013;37:e12-22. [Crossref] [PubMed]
- Graham DY, Genta R, Evans DG, et al. Helicobacter pylori does not migrate from the antrum to the corpus in response to omeprazole. Am J Gastroenterol 1996;91:2120-4. [PubMed]
- Goldstein NS. Chronic inactive gastritis and coccoid Helicobacter pylori in patients treated for gastroesophageal reflux disease or with H pylori eradication therapy. Am J Clin Pathol 2002;118:719-26. [Crossref] [PubMed]
- Schenk BE, Kuipers EJ, Nelis GF, et al. Effect of Helicobacter pylori eradication on chronic gastritis during omeprazole therapy. Gut 2000;46:615-21. [Crossref] [PubMed]
- Nelson ND, Tondon R, Fortuna D, et al. Helicobacter pylori Antigen But Not the Organism Is Occasionally Present Within Germinal Centers: Implications for Patient Management and Biology. Am J Surg Pathol 2020;44:1528-34. [Crossref] [PubMed]
- Makristathis A, Hirschl AM, Mégraud F, et al. Review: Diagnosis of Helicobacter pylori infection. Helicobacter 2019;24:e12641. [Crossref] [PubMed]
- Singhal AV, Sepulveda AR. Helicobacter heilmannii gastritis: a case study with review of literature. Am J Surg Pathol 2005;29:1537-9. [Crossref] [PubMed]
- Yeh PJ, Chiu CT, Lai MW, et al. Cytomegalovirus gastritis: Clinicopathological profile. Dig Liver Dis 2021;53:722-8. [Crossref] [PubMed]
- Chaemsupaphan T, Limsrivilai J, Thongdee C, et al. Patient characteristics, clinical manifestations, prognosis, and factors associated with gastrointestinal cytomegalovirus infection in immunocompetent patients. BMC Gastroenterol 2020;20:22. [Crossref] [PubMed]
- Kim K, Kim EJ, Kim MJ, et al. Clinicopathological features of syphilitic gastritis in Korean patients. Pathol Int 2009;59:884-9. [Crossref] [PubMed]
- Mylona EE, Baraboutis IG, Papastamopoulos V, et al. Gastric syphilis: a systematic review of published cases of the last 50 years. Sex Transm Dis 2010;37:177-83. [Crossref] [PubMed]
- Chen ZM, Shah R, Zuckerman GR, et al. Epstein-Barr virus gastritis: an underrecognized form of severe gastritis simulating gastric lymphoma. Am J Surg Pathol 2007;31:1446-51. [Crossref] [PubMed]
- Kim JM, Song CW, Song KS, et al. Acute gastritis associated with Epstein-Barr virus infection in a child. Korean J Pediatr 2016;59:S68-71. [Crossref] [PubMed]
- Dursun N, Hacıhasanoğlu E, Okçu O, et al. Epstein-Barr virus infection in patients with chronic gastritis without Helicobacter pylori infection. Turk J Gastroenterol 2020;31:205-10. [Crossref] [PubMed]
- Tartaglia D, Coccolini F, Mazzoni A, et al. Sarcina Ventriculi infection: a rare but fearsome event. A Systematic Review of the Literature. Int J Infect Dis 2022;115:48-61. [Crossref] [PubMed]
- Al Rasheed MR, Senseng CG. Sarcina ventriculi: Review of the Literature. Arch Pathol Lab Med 2016;140:1441-5. [Crossref] [PubMed]
- Haot J, Hamichi L, Wallez L, et al. Lymphocytic gastritis: a newly described entity: a retrospective endoscopic and histological study. Gut 1988;29:1258-64. [Crossref] [PubMed]
- Dixon MF, Wyatt JI, Burke DA, et al. Lymphocytic gastritis: Relationship to Campylobacter pylori infection. J Pathol 1988;154:125-32. [Crossref] [PubMed]
- Jaskiewicz K, Price SK, Zak J, et al. Lymphocytic gastritis in nonulcer dyspepsia. Dig Dis Sci 1991;36:1079-83. [Crossref] [PubMed]
- Feeley KM, Heneghan MA, Stevens FM, et al. Lymphocytic gastritis and coeliac disease: evidence of a positive association. J Clin Pathol 1998;51:207-10. [Crossref] [PubMed]
- Jones EA, Flejou JF, Potet F, et al. Lymphocytic gastritis: A clinicopathological study of 32 patients. Eur J Gastroenterol Hepatol 1990;2:367-72.
- Hayat M, Arora DS, Wyatt JI, et al. The pattern of involvement of the gastric mucosa in lymphocytic gastritis is predictive of the presence of duodenal pathology. J Clin Pathol 1999;52:815-9. [Crossref] [PubMed]
- Hayat M, Arora DS, Dixon MF, et al. Effects of Helicobacter pylori eradication on the natural history of lymphocytic gastritis. Gut 1999;45:495-8. [Crossref] [PubMed]
- Lynch DA, Sobala GM, Dixon MF, et al. Lymphocytic gastritis and associated small bowel disease: a diffuse lymphocytic gastroenteropathy? J Clin Pathol 1995;48:939-45. [Crossref] [PubMed]
- Wu TT, Hamilton SR. Lymphocytic gastritis: association with etiology and topology. Am J Surg Pathol 1999;23:153-8. [Crossref] [PubMed]
- Brown IS, Smith J, Rosty C. Gastrointestinal pathology in celiac disease: a case series of 150 consecutive newly diagnosed patients. Am J Clin Pathol 2012;138:42-9. [Crossref] [PubMed]
- Gabrieli D, Ciccone F, Capannolo A, et al. Subtypes of chronic gastritis in patients with celiac disease before and after gluten-free diet. United European Gastroenterol J 2017;5:805-10. [Crossref] [PubMed]
- Stancu M, De Petris G, Palumbo TP, et al. Collagenous gastritis associated with lymphocytic gastritis and celiac disease. Arch Pathol Lab Med 2001;125:1579-84. [Crossref] [PubMed]
- Niemelä S, Karttunen T, Kerola T, et al. Ten year follow up study of lymphocytic gastritis: further evidence on Helicobacter pylori as a cause of lymphocytic gastritis and corpus gastritis. J Clin Pathol 1995;48:1111-6. [Crossref] [PubMed]
- Crampton JR, Hunter JO, Neale G, et al. Chronic lymphocytic gastritis and protein losing gastropathy. Gut 1989;30:71-4. [Crossref] [PubMed]
- Müller H, Volkholz H, Stolte M. Healing of lymphocytic gastritis by eradication of Helicobacter pylori. Digestion 2001;63:14-9. [Crossref] [PubMed]
- Madisch A, Miehlke S, Neuber F, et al. Healing of lymphocytic gastritis after Helicobacter pylori eradication therapy: A randomized, double-blind, placebo-controlled multicentre trial. Aliment Pharmacol Ther 2006;23:473-9. [Crossref] [PubMed]
- Daniels JA, Lederman HM, Maitra A, et al. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol 2007;31:1800-12. [Crossref] [PubMed]
- Miettinen A, Karttunen TJ, Alavaikko M. Lymphocytic gastritis and Helicobacter pylori infection in gastric lymphoma. Gut 1995;37:471-6. [Crossref] [PubMed]
- Griffiths AP, Wyatt J, Jack AS, et al. Lymphocytic gastritis, gastric adenocarcinoma, and primary gastric lymphoma. J Clin Pathol 1994;47:1123-4. [Crossref] [PubMed]
- Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc 2012;87:732-8. [Crossref] [PubMed]
- Ruget O, Burtin P, Cérez H, et al. Chronic diarrhea associated with villous atrophy and lymphocytic gastritis, caused by ticlopidine. Gastroenterol Clin Biol 1992;16:290. [PubMed]
- Haot J, Jouret A, Willette M, et al. Lymphocytic gastritis: Prospective study of its relationship with varioliform gastritis. Gut 1990;31:282-5. [Crossref] [PubMed]
- Kamimura K, Kobayashi M, Narisawa R, et al. Collagenous gastritis: endoscopic and pathologic evaluation of the nodularity of gastric mucosa. Dig Dis Sci 2007;52:995-1000. [Crossref] [PubMed]
- Lagorce-Pages C, Fabiani B, Bouvier R, et al. Collagenous gastritis: a report of six cases. Am J Surg Pathol 2001;25:1174-9. [Crossref] [PubMed]
- Dray X, Reignier S, Vahedi K, et al. Collagenous gastritis. Endoscopy 2007;39:E292-3. [Crossref] [PubMed]
- Leung ST, Chandan VS, Murray JA, et al. Collagenous gastritis: histopathologic features and association with other gastrointestinal diseases. Am J Surg Pathol 2009;33:788-98. [Crossref] [PubMed]
- Pulimood AB, Ramakrishna BS, Mathan MM. Collagenous gastritis and collagenous colitis: a report with sequential histological and ultrastructural findings. Gut 1999;44:881-5. [Crossref] [PubMed]
- Wang HL, Shah AG, Yerian LM, et al. Collagenous gastritis: an unusual association with profound weight loss. Arch Pathol Lab Med 2004;128:229-32. [Crossref] [PubMed]
- Arnason T, Brown IS, Goldsmith JD, et al. Collagenous gastritis: a morphologic and immunohistochemical study of 40 patients. Mod Pathol 2015;28:533-44. [Crossref] [PubMed]
- Park S, Kim DH, Choe YH, et al. Collagenous gastritis in a Korean child: a case report. J Korean Med Sci 2005;20:146-9. [Crossref] [PubMed]
- Kajino Y, Kushima R, Koyama S, et al. Collagenous gastritis in a young Japanese woman. Pathol Int 2003;53:174-8. [Crossref] [PubMed]
- Colletti RB, Trainer TD. Collagenous gastritis. Gastroenterology 1989;97:1552-5. [Crossref] [PubMed]
- Kamimura K, Kobayashi M, Sato Y, et al. Collagenous gastritis World J Gastrointest Endosc 2015;7:265-73. Review. [Crossref] [PubMed]
- Kori M, Cohen S, Levine A, et al. Collagenous gastritis: a rare cause of abdominal pain and iron-deficiency anemia. J Pediatr Gastroenterol Nutr 2007;45:603-6. [Crossref] [PubMed]
- Wilson C, Thompson K, Hunter C. Nodular collagenous gastritis. J Pediatr Gastroenterol Nutr 2009;49:157. [Crossref] [PubMed]
- Jain R, Chetty R. Collagenous gastritis. Int J Surg Pathol 2010;18:534-6. [Crossref] [PubMed]
- Gopal P, McKenna BJ. The collagenous gastroenteritides: similarities and differences. Arch Pathol Lab Med 2010;134:1485-9. [Crossref] [PubMed]
- Hijaz NM, Septer SS, Degaetano J, et al. Clinical outcome of pediatric collagenous gastritis: case series and review of literature. World J Gastroenterol 2013;19:1478-84. [Crossref] [PubMed]
- Billiémaz K, Robles-Medranda C, Le Gall C, et al. A first report of collagenous gastritis, sprue, and colitis in a 9-month-old infant: 14 years of clinical, endoscopic, and histologic follow-up. Endoscopy 2009;41:E233-4. [Crossref] [PubMed]
- Suskind D, Wahbeh G, Murray K, et al. Collagenous gastritis, a new spectrum of disease in pediatric patients: two case reports. Cases J 2009;2:7511. [Crossref] [PubMed]
- Ma C, Park JY, Montgomery EA, et al. A Comparative Clinicopathologic Study of Collagenous Gastritis in Children and Adults: The Same Disorder With Associated Immune-mediated Diseases. Am J Surg Pathol 2015;39:802-12. [Crossref] [PubMed]
- Vesoulis Z, Lozanski G, Ravichandran P, et al. Collagenous gastritis: a case report, morphologic evaluation, and review. Mod Pathol 2000;13:591-6. [Crossref] [PubMed]
- Castellano VM, Muñoz MT, Colina F, et al. Collagenous gastrobulbitis and collagenous colitis: Case report and review of the literature. Scand J Gastroenterol 1999;34:632-8. [Crossref] [PubMed]
- Stolte M, Ritter M, Borchard F, et al. Collagenous gastroduodenitis on collagenous colitis. Endoscopy 1990;22:186-7. [Crossref] [PubMed]
- Rustagi T, Rai M, Scholes JV. Collagenous gastroduodenitis. J Clin Gastroenterol 2011;45:794-9. [Crossref] [PubMed]
- Soeda A, Mamiya T, Hiroshima Y, et al. Collagenous gastroduodenitis coexisting repeated Dieulafoy ulcer: A case report and review of collagenous gastritis and gastroduodenitis without colonic involvement. Clin J Gastroenterol 2014;7:402-9. [Crossref] [PubMed]
- Groisman GM, Meyers S, Harpaz N. Collagenous gastritis associated with lymphocytic colitis. J Clin Gastroenterol 1996;22:134-7. [Crossref] [PubMed]
- Jawhari A, Talbot IC. Microscopic, lymphocytic and collagenous colitis. Histopathology 1996;29:101-10. [Crossref] [PubMed]
- Flejou JF, Grimaud JA, Molas G, et al. Collagenous colitis: Ultrastructural study and collagen immunotyping of four cases. Arch Pathol Lab Med 1984;108:977-82. [PubMed]
- Côté JF, Hankard GF, Faure C, et al. Collagenous gastritis revealed by severe anemia in a child. Hum Pathol 1998;29:883-6. [Crossref] [PubMed]
- Kingham JG, Levison DA, Morson BC, et al. Collagenous colitis. Gut 1986;27:570-7. [Crossref] [PubMed]
- Winslow JL, Trainer TD, Colletti RB. Collagenous gastritis: a long-term follow-up with the development of endocrine cell hyperplasia, intestinal metaplasia, and epithelial changes indeterminate for dysplasia. Am J Clin Pathol 2001;116:753-8. [Crossref] [PubMed]
- Jensen ET, Martin CF, Kappelman MD, et al. Prevalence of Eosinophilic Gastritis, Gastroenteritis, and Colitis: Estimates From a National Administrative Database. J Pediatr Gastroenterol Nutr 2016;62:36-42. [Crossref] [PubMed]
- Abou Rached A, El Hajj W. Eosinophilic gastroenteritis: Approach to diagnosis and management. World J Gastrointest Pharmacol Ther 2016;7:513-23. [Crossref] [PubMed]
- Collins MH, Capocelli K, Yang GY. Eosinophilic Gastrointestinal Disorders Pathology. Front Med (Lausanne) 2018;4:261. [Crossref] [PubMed]
- Sunkara T, Rawla P, Yarlagadda KS, et al. Eosinophilic gastroenteritis: diagnosis and clinical perspectives. Clin Exp Gastroenterol 2019;12:239-53. [Crossref] [PubMed]
- Caldwell JM, Collins MH, Stucke EM, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol 2014;134:1114-24. [Crossref] [PubMed]
- Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol 2011;24:556-63. [Crossref] [PubMed]
- Turner KO, Collins MH, Walker MM, et al. Quantification of Mucosal Eosinophils for the Histopathologic Diagnosis of Eosinophilic Gastritis and Duodenitis: A Primer for Practicing Pathologists. Am J Surg Pathol 2022;46:557-66. [Crossref] [PubMed]
- Lahner E, Conti L, Annibale B, et al. Current Perspectives in Atrophic Gastritis. Curr Gastroenterol Rep 2020;22:38. [Crossref] [PubMed]
- Coati I, Fassan M, Farinati F, et al. Autoimmune gastritis: Pathologist's viewpoint. World J Gastroenterol 2015;21:12179-89. [Crossref] [PubMed]
- Annibale B, Esposito G, Lahner E. A current clinical overview of atrophic gastritis. Expert Rev Gastroenterol Hepatol 2020;14:93-102. [Crossref] [PubMed]
- Agréus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol 2012;47:136-47. [Crossref] [PubMed]
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353-67. [Crossref] [PubMed]
- Okamura T, Iwaya Y, Kitahara K, et al. Accuracy of Endoscopic Diagnosis for Mild Atrophic Gastritis Infected with Helicobacter pylori. Clin Endosc 2018;51:362-7. [Crossref] [PubMed]
- Pittayanon R, Rerknimitr R, Klaikaew N, et al. The risk of gastric cancer in patients with gastric intestinal metaplasia in 5-year follow-up. Aliment Pharmacol Ther 2017;46:40-5. [Crossref] [PubMed]
- Rodriguez-Castro KI, Franceschi M, Noto A, et al. Clinical manifestations of chronic atrophic gastritis. Acta Biomed 2018;89:88-92. [PubMed]
- Borch K, Ahrén B, Ahlman H, et al. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg 2005;242:64-73. [Crossref] [PubMed]
- Torbenson M, Abraham SC, Boitnott J, et al. Autoimmune gastritis: distinct histological and immunohistochemical findings before complete loss of oxyntic glands. Mod Pathol 2002;15:102-9. [Crossref] [PubMed]
- Jhala NC, Montemor M, Jhala D, et al. Pancreatic acinar cell metaplasia in autoimmune gastritis. Arch Pathol Lab Med 2003;127:854-7. [Crossref] [PubMed]
- Wolf EM, Plieschnegger W, Geppert M, et al. Changing prevalence patterns in endoscopic and histological diagnosis of gastritis? Data from a cross-sectional Central European multicentre study. Dig Liver Dis 2014;46:412-8. [Crossref] [PubMed]
- Maguilnik I, Neumann WL, Sonnenberg A, et al. Reactive gastropathy is associated with inflammatory conditions throughout the gastrointestinal tract. Aliment Pharmacol Ther 2012;36:736-43. [Crossref] [PubMed]
- Genta RM. Differential diagnosis of reactive gastropathy. Semin Diagn Pathol 2005;22:273-83. [Crossref] [PubMed]
- Franke A, Teyssen S, Singer MV. Alcohol-related diseases of the esophagus and stomach. Dig Dis 2005;23:204-13. [Crossref] [PubMed]
- Geboes K, Rutgeerts P, Broeckaert L, et al. Histologic appearances of endoscopic gastric mucosal biopsies 10-20 years after partial gastrectomy. Ann Surg 1980;192:179-82. [Crossref] [PubMed]
- Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology 1997;112:1000-16. [Crossref] [PubMed]
- Lanas A, Serrano P, Bajador E, et al. Risk of upper gastrointestinal bleeding associated with non-aspirin cardiovascular drugs, analgesics and nonsteroidal anti-inflammatory drugs. Eur J Gastroenterol Hepatol 2003;15:173-8. [Crossref] [PubMed]
- Lanas A. Are COX-2 selective inhibitors safer than NSAIDs for patients with osteoarthritis and rheumatoid arthritis? Nat Clin Pract Gastroenterol Hepatol 2007;4:648-9. [Crossref] [PubMed]
- Carmack SW, Genta RM, Graham DY, et al. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009;6:331-41. [Crossref] [PubMed]
- Marginean EC, Bennick M, Cyczk J, et al. Gastric siderosis: patterns and significance. Am J Surg Pathol 2006;30:514-20. [Crossref] [PubMed]
- Gorospe M, Fadare O. Gastric mucosal calcinosis: clinicopathologic considerations. Adv Anat Pathol 2007;14:224-8. [Crossref] [PubMed]
- Gonzalez RS, Lagana SM, Szeto O, et al. Challenges in Diagnosing Medication Resins in Surgical Pathology Specimens: A Crystal-Clear Review Guide. Arch Pathol Lab Med 2017;141:1276-82. [Crossref] [PubMed]
- Voltaggio L, Lam-Himlin D, Limketkai BN, et al. Message in a bottle: decoding medication injury patterns in the gastrointestinal tract. J Clin Pathol 2014;67:903-12. [Crossref] [PubMed]
- Hoda RS, Sanyal S, Abraham JL, et al. Lanthanum deposition from oral lanthanum carbonate in the upper gastrointestinal tract. Histopathology 2017;70:1072-8. [Crossref] [PubMed]
- Ban S, Suzuki S, Kubota K, et al. Gastric mucosal status susceptible to lanthanum deposition in patients treated with dialysis and lanthanum carbonate. Ann Diagn Pathol 2017;26:6-9. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Lee EL, Abraham SC, et al. Colchicine toxicity: distinct morphologic findings in gastrointestinal biopsies. Am J Surg Pathol 2001;25:1067-73. [Crossref] [PubMed]
- Daniels JA, Gibson MK, Xu L, et al. Gastrointestinal tract epithelial changes associated with taxanes: marker of drug toxicity versus effect. Am J Surg Pathol 2008;32:473-7. [Crossref] [PubMed]
- Patil PA, Zhang X. Pathologic Manifestations of Gastrointestinal and Hepatobiliary Injury in Immune Checkpoint Inhibitor Therapy. Arch Pathol Lab Med 2021;145:571-82. [Crossref] [PubMed]
- Zhang ML, Deshpande V. Histopathology of Gastrointestinal Immune-related Adverse Events: A Practical Review for the Practicing Pathologist. Am J Surg Pathol 2022;46:e15-26. [Crossref] [PubMed]
- Ogawa F, Mino-Kenudson M, Shimizu M, et al. Gastroduodenitis associated with yttrium 90-microsphere selective internal radiation: an iatrogenic complication in need of recognition. Arch Pathol Lab Med 2008;132:1734-8. [Crossref] [PubMed]
- Han S, Chaudhary N, Wassef W. Portal hypertensive gastropathy and gastric antral vascular ectasia. Curr Opin Gastroenterol 2015;31:506-12. [Crossref] [PubMed]
- Liang Y, Cui S, Polydorides AD. Clinicopathological characteristics and aetiological factors of granulomatous gastritis. Histopathology 2021;79:1040-50. [Crossref] [PubMed]
- Ushiku T, Moran CJ, Lauwers GY. Focally enhanced gastritis in newly diagnosed pediatric inflammatory bowel disease. Am J Surg Pathol 2013;37:1882-8. [Crossref] [PubMed]
- Roka K, Roma E, Stefanaki K, et al. The value of focally enhanced gastritis in the diagnosis of pediatric inflammatory bowel diseases. J Crohns Colitis 2013;7:797-802. [Crossref] [PubMed]
- Johncilla M, Elsoukkary S, Jessurun J. The significance of focally enhanced gastritis in haematopoietic stem cell transplant recipients. Histopathology 2021;79:599-606. [Crossref] [PubMed]
- Brito-Zerón P, Bari K, Baughman RP, et al. Sarcoidosis Involving the Gastrointestinal Tract: Diagnostic and Therapeutic Management. Am J Gastroenterol 2019;114:1238-47. [Crossref] [PubMed]
- Zhang H, Jin Z, Cui R. Russell body gastritis/duodenitis: a case series and description of immunoglobulin light chain restriction. Clin Res Hepatol Gastroenterol 2014;38:e89-97. [Crossref] [PubMed]
- Qiao J, Dudrey E, Gilani S. Russell body gastritis. Pathologica 2019;111:76-8. [Crossref] [PubMed]
- Altindag SD, Cakir E, Ekinci N, et al. Analysis of clinical and histopathological findings in Russell body gastritis and duodenitis. Ann Diagn Pathol 2019;40:66-71. [Crossref] [PubMed]
Cite this article as: Zhang SL, Lollie TK, Chen Z, Narasimhalu T, Wang HL. Histopathologic diagnosis of gastritis and gastropathy: a narrative review. Dig Med Res 2023;6:9.