Ovarian cancer peritoneal carcinomatosis: a narrative review
Introduction
Incidence, prevalence, and demographics of ovarian cancer peritoneal carcinomatosis
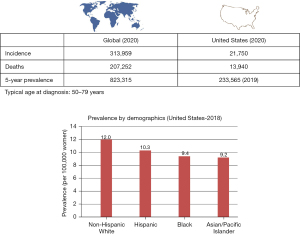
Ovarian cancer is the most lethal gynecologic cancer in the United States with over 19,800 new cases and 12,800 deaths estimated to occur in 2022 (1). Globally, ovarian cancer had an incidence of 313,000 cases in 2020 with 207,000 deaths resulting from the disease (Figure 1) (2-5). It also ranks among the most lethal cancers in Russia, Canada, northern Europe, Britain, and New Zealand. The estimated incidence in the United States is 1 in 70. Incidence rates are higher in North America and most industrialized European countries whereas the disease is less common in Asia and Africa. Japan for example, sees among the lowest recorded rates in ovarian cancer with an incidence of almost 1 in 1,000 (2). While no clear causative factors are identified in the majority of cases, factors such as infrequent ovulation, parity, breastfeeding, and use of combination oral contraception have been shown in epidemiological studies to reduce the risk of ovarian cancer. In addition, the discovery of hereditary causes among subsets of ovarian cancer patients, such as mutations in the BRCA1 and BRCA2 genes, has helped identify genetic causes in up to 25% (6) of all cases as well as elucidate that a significant portion of ovarian cancers originate in the fallopian tube.
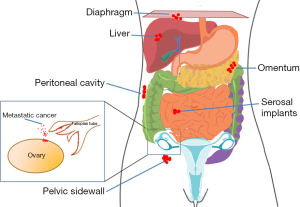
Epithelial ovarian cancers fall into four main histologic subtypes: serous (high grade and low grade), endometrioid, clear cell, and mucinous. Other subtypes, such as carcinosarcoma, malignant Brenner tumor, and mixed histologies, also exist and are less common. Advanced epithelial ovarian cancers follow a specific pattern of spread. Metastatic cancer cells can disseminate throughout the peritoneal cavity, attaching to the mesothelium and organ surfaces within the peritoneal cavity before specific symptoms arise (7). Of note, increasing evidence in recent years has demonstrated that many ovarian cancers, specifically the high-grade serous subtype, actually originate in the fimbria of the fallopian tube (8,9). Histologically however, ovarian, fallopian tube, and primary peritoneal cancers are equivalent and are treated the same; for the purposes of this review, we will refer to these collectively as ovarian cancer. At the time of diagnosis, patients often have omental and peritoneal carcinomatosis (Figure 2). The non-specific symptoms associated with this cancer, such as bloating, early satiety, and pelvic/abdominal discomfort, also contribute to the fact that the majority of ovarian cancers are discovered in advanced stages [51% at International Federation of Gynecology and Obstetrics (FIGO) stage III and 29% in FIGO stage IV] (4). Ultrasound and computed tomography (CT) imaging are used in combination with serum CA-125 (MUC16) level measurement to characterize a suspicious pelvic mass once discovered; however, no effective tools or methodologies exist to screen for ovarian cancer. While most patients will have a complete clinical response following initial treatment, unfortunately approximately 80% will experience recurrence of the disease, which ultimately proves to be fatal in most cases. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-13/rc).
Objectives
This review summarizes the historical context and current knowledge regarding the upfront treatment and management of peritoneal carcinomatosis arising from ovarian cancer. Peritoneal carcinomatosis arising from a rarer subtype, mucinous ovarian cancer, deserves special mention in this review given the response to treatment mimics that of gastrointestinal (GI) malignancies and will therefore be discussed separately.
Methods
Literature search of PubMed, MEDLINE, Embase and the Cochrane Library were performed in November 2021 on topics relating to diagnosis, surgical management of advanced ovarian cancer, hyperthermic intraperitoneal chemotherapy (HIPEC), intravenous and intraperitoneal chemotherapy treatment of ovarian cancers, mucinous and epithelial ovarian cancer. Furthermore, reference sections in selected studies were searched manually to locate any additional relevant papers. Literature search was limited to English-language publications. Case reports, meeting abstracts were excluded. See Table 1.
Table 1
| Items | Specifications |
|---|---|
| Date of search | November 15, 2021 |
| Databases and other sources searched | PubMed, MEDLINE, Embase and the Cochrane Library |
| Search terms used | Ovarian cancer, peritoneal, carcinomatosis, treatment, HIPEC |
| Timeframe | All articles up to November 15, 2021 |
| Inclusion and exclusion criteria | Language: English language articles were included |
| Study type: original research, reviews were included | |
| Case reports, meeting abstracts were excluded | |
| Selection process | All authors conducted selection |
HIPEC, hyperthermic intraperitoneal chemotherapy.
Discussion
Upfront treatment of ovarian cancer peritoneal carcinomatosis
The mainstay of treatment for peritoneal carcinomatosis arising from ovarian cancer is surgical resection of all macroscopic disease combined with platinum-based chemotherapy. The impact of chemotherapy on the survival of advanced stage ovarian cancer patients is significant; however, maximal effort with cytoreductive surgery to minimize residual disease has been shown to be similarly important.
History of surgical management in the primary setting
Primary cytoreductive surgery followed by adjuvant chemotherapy is the preferred initial approach for advanced-stage ovarian cancers when feasible. Several retrospective studies in the 1980’s established the role of primary cytoreductive surgery followed by platinum-based chemotherapy as standard of care for treatment of advanced ovarian cancer. Surgical resection typically consists of total abdominal hysterectomy, bilateral salpingo-oophorectomy, infracolic and infragastric omentectomy, and cytoreduction of any visible or palpable tumor deposits. Omental involvement is often substantial and can frequently approach the splenic hilum and distal pancreas however less commonly invades these sites. Similarly, pelvic involvement is frequently extensive with obliteration of the posterior cul-de-sac and vesicouterine space, however the pelvic tumor can almost always be cleared due to preservation of the retroperitoneal space. The entire abdominal cavity, including all organ and peritoneal surfaces, should be meticulously and systematically inspected. In clinically early-stage ovarian cancers, pelvic and para-aortic lymphadenectomy is routinely performed for staging purposes. Additionally, if any of these lymph nodes appear grossly enlarged intra-operatively or on imaging, lymph node sampling may be performed regardless of stage (10). A recent randomized controlled trial evaluating the role of routine lymphadenectomy in advanced ovarian cancer demonstrated that systematic lymphadenectomy does not confer a survival benefit and may increase postoperative complications (10). Given the importance of removing all gross disease, surgical cytoreduction may additionally necessitate small and/or large bowel resection, pelvic and abdominal peritonectomy, diaphragm peritonectomy or resection, liver resection, and/or splenectomy (11).
Cancer stage and the remaining volume of disease following cytoreductive surgery are the strongest predictive factors for progression-free survival and overall survival. The best outcomes are noted with maximal surgical effort when all macroscopic disease can be either completely resected or ‘optimal’ cytoreduction, where the maximal tumor dimension is less than one cm. The inverse relationship between the size of residual disease and overall survival in ovarian cancer was first described by Griffiths in 1975 (12) and then further demonstrated in several studies since then (13-16). The median survival in ovarian cancer studies more than doubled from 17 to 39 months following successful cytoreductive surgical efforts (17). The experience of the surgeon and volume of cases treated both play an important role in the success of cytoreductive efforts. In North America, patients treated by gynecologic oncologists at high volume surgical centers have been shown to have better survival outcomes than general gynecologists or general surgeons (18-20).
The Gynecologic Oncology Group (GOG) (21) demonstrated that survival is significantly longer when the maximal diameter of residual tumor is less than one cm, which has led to the current definition of 1 cm tumor width as ‘optimal’ cytoreduction. ‘Suboptimal’ cytoreductive surgery, defined as residual tumor greater than 1 cm in the upfront setting, does not confer any survival benefit to the patient relative to chemotherapy alone, but comes with added post-operative morbidity and delay of initiating adjuvant chemotherapy. For this reason, there has been a shift over the past decade to using neoadjuvant chemotherapy in patients for whom a primary optimal cytoreduction is not feasible, with consideration of an interval cytoreduction depending on response to chemotherapy. How much residual tumor following cytoreductive surgery is acceptable without compromising patient outcomes has been the subject of much research. The definition of ‘suboptimal’ has evolved over the past two decades. Historically, residual tumor size greater than 2 cm was used as the definition of suboptimal. Bristow published a meta-analysis in 2002 based on 81 studies, demonstrating that for every 10% increase in proportion of patients undergoing optimal cytoreduction, median survival improved by 5–6% (22). The best outcomes were reported in those with cytoreduction to no visible residual disease. There was no difference in 5-year survival for patients having unresectable tumor and surgery that left residual tumor with a maximal diameter of up to two cm. The current definition of ‘optimal’ arose following an intergroup study in which patients with a maximal tumor of 1 cm or less following cytoreduction and adjuvant platinum/taxane chemotherapy had a progression-free survival and overall survival of 22 and 52 months respectively. In contrast, for those with residual tumor greater than one cm, progression-free survival and overall survival were 14 and 26 months respectively (23). Many attempts have been made to develop predictive criteria and algorithms for the identification of patients for whom primary surgery is unlikely to result in an optimal cytoreduction though no universal guidelines exist in making this determination. Preoperative imaging and laparoscopic surgery have been utilized to identify patients for whom surgery is likely to succeed in removing all macroscopic disease. An initial diagnostic laparoscopy may be used to make this determination in surgically appropriate candidates with some trials demonstrating its utility in reducing futile laparotomy and attempted cytoreduction (24-26). Scoring systems have also been proposed that aim to predict suitability for optimal cytoreduction (27,28). The Fagotti score takes into account the presence of carcinomatosis involving the peritoneum, diaphragm, mesentery, bowel, stomach, and liver. Involvement of four or more of these targets was reported to have a positive predictive value of 100% in predicting unsuccessful optimal tumor resection (27). In addition, poor performance status can determine if a patient will tolerate surgery. A low serum albumin level is often predictive of nutrition status and ultimately, a patient’s risk of post-operative morbidity and mortality (29).
Advances in surgical management
Exploratory laparotomy via midline incision remains the standard of care for all cytoreductive procedures in ovarian cancer. The goal of cytoreductive surgery remains to leave no visible residual disease. If this is not feasible, the definition of an ‘optimal’ cytoreduction remains surgery with residual tumor of largest maximal dimension of 1 cm. This determination is done at the conclusion of the case and remains subjective in nature. The impact that degree of cytoreduction has on survival has been demonstrated in several studies. A 2011 Cochrane review looking at 11 retrospective studies of primary surgical management of advanced ovarian cancer showed that survival was notably improved with complete resection of all tumors (no visible residual disease) compared to optimal cytoreduction—[hazard ratio (HR), 2.20; 95% confidence interval (CI): 1.90–2.54]. When complete resection is not possible, optimal cytoreduction (≤1 cm residual tumor) still had favorable implications for prognosis compared to suboptimal cytoreduction (>1 cm)—(HR, 1.36; 95% CI: 1.10–1.68) (30).
Minimally invasive techniques, however, are under active investigation given the lower risk of perioperative morbidity, shorter hospital stays, and quicker recovery times seen in other cancer types (31,32). The diffuse pattern of peritoneal spread in ovarian cancer makes it particularly challenging for minimally invasive cytoreductive approaches. Minimally invasive approaches have not yet been prospectively validated in ovarian cancer. No randomized controlled trial data exists examining minimally invasive surgery in ovarian cancer. Observational studies have shown a high rate of optimal cytoreduction with excellent perioperative outcomes and progression-free survival among women undergoing minimally invasive interval cytoreduction after neoadjuvant chemotherapy (33-35). A randomized trial to assess a minimally invasive approach in this setting is ongoing with the Laparoscopic Cytoreduction After Neoadjuvant Chemotherapy trial, (LANCE trial, NCT04575935), investigating laparotomy vs. laparoscopy for interval cytoreduction in advanced ovarian cancer patients who have undergone neoadjuvant chemotherapy (36).
Another area of active investigation is the role of imaging-based methods to improve detection of tumors at the time of surgery. For example, one newer imaging technique studied in folate receptor positive ovarian cancer has recently been approved for use. In a randomized, multi-center study of women with ovarian cancer undergoing cytoreductive surgery, the use of pafolacianine sodium injection under near-infrared fluorescence imaging during surgery led to at least one additional cancerous lesion being detected intra-operatively that was not observed by standard visual or tactile inspection (37). This approach presents new possibilities in recognizing all gross disease, both with traditional laparotomy and minimally invasive approaches. The hope is that this may allow for less residual disease, which may ultimately improve survival, though these endpoints are still being investigated.
History of medical management in the primary setting
The use of chemotherapy in ovarian cancer has made significant advances in the last three decades. In the 1970’s, alkylating agents such as cyclophosphamide and melphalan were the primary chemotherapy drugs used with median survival rates of 10 to 18 months. Platinum chemotherapy agents beginning with cisplatin debuted in the late 1970’s (38) and exerted their effect via cross-linking with DNA. Platinum compounds increased response rates from 20–60% with previous regimens to 50% to 80% along with an increase in median survival rates.
Combination chemotherapy regimens were introduced in the 1980’s with the standard of care regimen initially evolving from CAP (cyclophosphamide, doxorubicin, and cisplatin) (39) to CP (cyclophosphamide and cisplatin) (40). The introduction of paclitaxel in 1993 would prove to be another major milestone in the treatment of ovarian cancer. Taxanes such as paclitaxel and docetaxel exert their effects via stabilization of microtubules thereby preventing disassembly. The GOG 111 trial and the Canadian-European trial OV-10 both showed paclitaxel-cisplatin had significantly improved response rates compared to cyclophosphamide-cisplatin which was the standard at the time (41,42). When used in combination with platinum chemotherapy agents as first line chemotherapy treatments, the doublet combination produced a response in over 80% of patients. Carboplatin was noted to be as effective as cisplatin in combination with paclitaxel but better tolerated, resulting in its modern use as a first line agent in ovarian cancer (43).
For the past two decades, the standard of care for chemotherapy treatment has been platinum and paclitaxel doublet therapy (43,44). The National Comprehensive Cancer Network (NCCN) guidelines recommend carboplatin and paclitaxel as first line treatment for ovarian cancer peritoneal carcinomatosis (44). The preferred selection, dosing, and timing of these cytotoxic agents have been extensively studied and have subsequently undergone several iterations. Furthermore, while doublet therapy offers significant improvements over monotherapy, the utilization of more than two drugs [carboplatin and paclitaxel with the addition of: (I) gemcitabine, (II) PEGylated liposomal doxorubicin, or (III) topotecan] was not shown to improve outcomes in the ICON5 trial (45). A weekly dose-dense chemotherapy regimen with carboplatin at a dose of area under the curve (AUC) 6 every 3 weeks with weekly paclitaxel 80 mg/m2 was explored in a Japanese GOG phase III trial (46) with improvement in overall and progression-free survival in the dose-dense paclitaxel arm. These findings were not replicated however in the subsequent ICON8 study in which weekly dose-dense carboplatin/paclitaxel treatment did not significantly improve progression-free survival compared with the standard regimen administered every 3 weeks. (47). For this reason, especially in light of the increased logistical complexity and quality-of-life implications associated with a weekly regimen, many providers currently administer carboplatin and paclitaxel every 3 weeks rather than in a dose-dense fashion.
Neoadjuvant chemotherapy
The timing of surgery and chemotherapy has undergone a significant evolution in the last decade. Since 2010, several prospective randomized controlled trials have explored neoadjuvant chemotherapy treatment followed by interval cytoreductive surgery and post-operative chemotherapy vs. primary cytoreductive surgery with adjuvant chemotherapy in patients with advanced stage disease. The EORTC 55971 trial (48) randomized patients with FIGO stage IIIC (defined as peritoneal metastases 2 cm or larger) or higher ovarian cancers to primary cytoreductive surgery followed by adjuvant platinum-based chemotherapy or to neoadjuvant platinum-based chemotherapy followed by interval cytoreduction. The study showed that neoadjuvant chemotherapy with interval cytoreduction was non-inferior to primary surgery followed by adjuvant chemotherapy for survival. It is worth noting, however, that postoperative complication rates were lower in the neoadjuvant group. In both groups, complete resection of all visible disease was the most important independent variable in predicting patient outcomes. Two critiques of the EORTC 55971 trial include the lower progression-free survival and overall survival (29–30 months) when compared to outcomes in trials in the United States during the same period (50 months) (49,50) Additionally, the trial had lower rates of optimal cytoreductive surgery rates.
Additional prospective randomized controlled trials have been published since evaluating patients with FIGO stage III or higher ovarian cancer randomized to neoadjuvant chemotherapy vs. primary cytoreductive surgery (51-53). In general, these studies have generated evidence supporting non-inferior survival outcomes for the neoadjuvant approach compared with primary surgery (48,53,54) as well as shorter operating times, shorter hospitalization lengths of stay, and reduced short-term post-operative complications. In addition, most of these trials showed a higher likelihood of removal of all macroscopic disease or optimal cytoreduction. Ideal patient selection for primary surgery has been evaluated and various algorithms have been proposed (27,28,55,56), however this decision-making remains a challenge. A meta-analysis pooling data from the EORTC 55971 and CHORUS trials found that patients with stage IIIC disease (defined as peritoneal metastases greater than 2 cm) and extrapelvic metastases less than 5 cm had improved progression-free survival with primary cytoreduction rather than neoadjuvant chemotherapy, whereas stage IV patients (those with positive pleural cytology or distant metastases outside the peritoneal cavity or involving splenic or liver parenchyma) showed improved survival with the neoadjuvant approach compared with primary surgery (57). The timing of primary vs. interval cytoreductive surgery in this population continues to be the subject of investigation (58). At this point, given the complexity of disease-related and patient-related factors in advanced ovarian cancer, the decision about surgical timing in patients with carcinomatosis due to ovarian cancer should be individualized and made in conjunction with a gynecologic oncologist.
Advances in medical management
Several exciting advances in recent years have resulted in a paradigm shift in initial and maintenance therapies for ovarian cancer outside the traditional model of surgery and cytotoxic chemotherapies. Historically, maintenance treatment was not used after initial chemotherapy. The GOG 178 trial investigated the role of continuing paclitaxel maintenance therapy in women with stage III and IV ovarian cancer patients following a complete response to initial carboplatin/paclitaxel therapy (59). Patients were randomized to 3 vs. 12 cycles of additional paclitaxel. Patients receiving 12 paclitaxel cycles had a significantly longer progression-free survival though an overall survival benefit was not observed. Additionally, increasing cycles of paclitaxel resulted in higher rates of grade 2 or 3 peripheral neuropathy. The standard of care did not change as a result of this trial due in part to limited generalizability.
More recent studies have evaluated non-cytotoxic therapies for maintenance. GOG 218 was a phase III trial which explored the use of carboplatin/paclitaxel with concurrent and maintenance bevacizumab (60); the study demonstrated a 4-month progression-free survival benefit compared to standard treatment (carboplatin and paclitaxel without maintenance) which led to its approval by the U.S. Food and Drug Administration (FDA) for upfront treatment in stage III and IV ovarian cancer. Several studies since then have demonstrated only a modest benefit in progression-free and no overall survival benefit except in an exploratory analysis among stage IV patients in one study (in the ICON7 trial) (61) with the use of bevacizumab (62-64). There has been a more consistent benefit seen in patients with stage IV disease, those with suboptimal cytoreductions, and patients with stage III disease that is considered to be inoperable.
In 2018, the FDA approved the use of olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, for frontline maintenance treatment in ovarian cancer patients with BRCA1/BRCA2 mutations after several approvals for PARP inhibitors in the recurrent setting (65). In 2020, this drug was also approved for use in combination with bevacizumab for front-line maintenance in patients with homologous recombination deficient tumors (66). Niraparib, another PARP inhibitor, is now also approved as front-line maintenance therapy in all patients with advanced epithelial ovarian cancer following a complete or partial response to platinum chemotherapy (67). Despite this broad approval, its most significant benefit remains in women with homologous recombination repair deficiency (68) and BRCA1 or BRCA2 mutations (67). Other PARP inhibitor-containing maintenance therapies currently under investigation in the front-line setting include combinations of bevacizumab with a PARP inhibitor and an immune checkpoint inhibitor (NCT03602859, NCT03737643) (69,70) and PARP inhibitors with immune checkpoint inhibitors (NCT03522246) (71).
Although immune therapies, such as those targeting PD-1, PD-L1, and CTLA-4 have shown benefit in other tumor types, such therapies have proven less effective in ovarian cancer in the recurrent setting (72-74). Trials are currently ongoing investigating immunotherapy options in the frontline setting (NCT03642132, NCT03740165).
Intraperitoneal chemotherapy in the upfront treatment of advanced ovarian cancer
The controversial role of intraperitoneal chemotherapy has been explored in advanced ovarian cancer. The theory behind intraperitoneal chemotherapy use is the potential benefit of prolonged exposure to high concentrations of cytotoxic agents at the primary site of disease with relative sparing of normal tissue. Two randomized phase III trials in the 1990’s explored intraperitoneal vs. intravenous chemotherapy in advanced stage ovarian cancer patients (23,75) who had undergone primary cytoreductive surgery. Both studies found a survival benefit among the intraperitoneal chemotherapy group: a 7-month overall survival advantage (75) and a 6-month progression-free survival improvement (23).
These studies resulted in the development of GOG 172, which subsequently established intraperitoneal chemotherapy as a standard of care option in treating advanced ovarian cancer. This landmark study demonstrated an overall survival benefit for patients treated with intraperitoneal cisplatin and paclitaxel. The trial enrolled patients who had optimal primary cytoreductive surgery and randomized them to receive either: (I) six cycles of intravenous paclitaxel and cisplatin or (II) intraperitoneal cisplatin with intraperitoneal and intravenous paclitaxel. Patients in the intraperitoneal treatment group experienced a 5-month longer progression-free survival and 16-month survival benefit compared to the intravenous treatment group. Interestingly, 48% of the patients in the intraperitoneal treatment group received three or fewer cycles due to treatment intolerance and toxic side effects. Patients who were unable to tolerate intraperitoneal chemotherapy received intravenous chemotherapy for the remaining cycles. Despite a National Cancer Institute alert encouraging treatment with the intraperitoneal approach in this setting as a result of this study, a number of factors limited widespread uptake of intraperitoneal chemotherapy (76). These factors include toxicity concerns, increased resource and cost requirements, more cumbersome administration logistics, intraperitoneal port complications, and concerns regarding postoperative healing or performance status (77). In addition, a survey study of Society of Gynecologic Oncology and American Society of Clinical Oncology members showed that most providers giving intraperitoneal chemotherapy did so in an outpatient setting using lower doses than those used in trial-specified protocols (78). Routine use of intraperitoneal chemotherapy has more recently been called into question following reassessment of this approach in a subsequent cooperative group study published in 2019. GOG 252 was an open label, randomized controlled trial that evaluated two intraperitoneal arms (dose dense intravenous paclitaxel with intraperitoneal carboplatin vs. intravenous paclitaxel and intraperitoneal paclitaxel and cisplatin) with one intravenous arm (intravenous carboplatin and paclitaxel) (79). All three arms received bevacizumab. Notably, the dose of intraperitoneal cisplatin used (75 mg/m2) was lower than that used in GOG 172 (100 mg/m2) in order to evaluate the dose more commonly being used in clinical practice. Progression-free survival was not significantly improved after a 7-year follow-up period with either intraperitoneal regimen compared to the intravenous arm when combined with bevacizumab. The reasons behind this are unclear; however, several factors including a lower dose of cisplatin, dose dense paclitaxel regimen, or an effect the bevacizumab may have contributed. As a result of these mixed data, intraperitoneal chemotherapy utilization for advanced ovarian cancer is now more controversial than ever. Enthusiasm for its use has waned considerably since the publication of GOG 252.
HIPEC in upfront advanced ovarian cancer management
HIPEC is a procedure in which cytotoxic chemotherapy is administered as a heated solution into the peritoneal space. Given ovarian cancer’s propensity to proliferate within the peritoneal cavity, the benefit of sustained exposure to high concentrations cytotoxic chemotherapy in the peritoneal cavity without the myelotoxic effects of intravenous treatment presents potential advantages. In addition, HIPEC has the benefit of being administered intra-operatively once, without the need for multiple outpatient administrations. Hyperthermia can also in theory increase the crosslinking effect of platinum based chemotherapy, induce apoptosis, inhibit angiogenesis and penetrate deeper into tumor implants (80-82).
Cytoreductive surgery and HIPEC have become the standard of care among peritoneal malignancies of GI origin such as appendiceal pseudomyxoma peritonei and malignant abdominal mesothelioma (83). In contrast, the use of intraperitoneal chemotherapy including HIPEC in epithelial ovarian cancer continues to be explored and its use is variable among gynecologic oncologists in the treatment of ovarian cancer.
van Driel [2018] reported the first phase III, randomized controlled trial (OVHIPEC trial) that investigated the role of HIPEC for stage III ovarian cancer at the time of interval cytoreductive surgery following neoadjuvant chemotherapy (84). Patients who responded to three cycles of neoadjuvant intravenous carboplatin and paclitaxel were randomized to interval debulking surgery with or without HIPEC with heated 40 °C cisplatin at a dose of 100 mg/m2. Patients undergoing interval cytoreductive surgery with HIPEC experienced a 3.5-month longer recurrence-free period (14.2 vs. 10.7 months). Median overall survival was 12 months longer in the surgery plus HIPEC group compared to the non-HIPEC group (45.7 vs. 33.9 months). The number of patients experiencing grade 3 or 4 adverse events was similar in both arms. The results however are not generalizable to patients with advanced stage peritoneal disease eligible for primary cytoreductive surgery as these patients were not included in this study. Criticisms of the trial include the small study population size (n=245), imbalance between the treatment arms for low-grade cancers, unclear inclusion criteria for selection of interval debulking surgery at participating centers, and randomization after the decision to proceed to surgery was made, not prior to neoadjuvant chemotherapy initiation (85,86). Nevertheless, the OVHIPEC trial provided evidence for the use of HIPEC among patients with abdominal disease who are likely to experience optimal interval cytoreductive surgery. The beneficial effect of HIPEC was similar over a wide variety of subgroups (histologic type, extent of disease, age) (44).
The role of HIPEC at the time of primary cytoreductive surgery was recently explored in a retrospective study (87). Lei et al. [2020] investigated primary cytoreductive surgery with or without HIPEC. The study included five surgery centers in China (87). The methodology of the study involved a lower dose of cisplatin (50 mg/m2) delivered over three administrations (days 1, 3 and 5). Patients undergoing optimal primary cytoreductive surgery with HIPEC experienced a median survival time 11 months longer than the surgery alone cohort. Thus far, all HIPEC randomized controlled trials have largely looked at treatment at the time of interval cytoreduction (Table 2) (84,88-95), however none have yet reported outcomes in the primary surgery setting. The OVHIPEC-2 trial (96) is a randomized open-label trial that began enrolling patients in 2020 with newly diagnosed advanced stage ovarian cancer in several European countries. Following primary cytoreductive surgery with residual disease up to 2.5 mm in maximal dimension, patients are assigned to receive HIPEC or no HIPEC.
Table 2
| Study | Patients | Type | Treatment arms | Efficacy | Safety | Regimen |
|---|---|---|---|---|---|---|
| Lim (88) | 184 | Randomized controlled trial | Arm A: CRS with HIPEC; arm B: CRS without HIPEC | PFS: 21% vs. 16% at 5 years; OS: 51% vs. 49% at 5 years | Not specified | Cisplatin 75 mg/m2 |
| van Driel (84) | 245 | Randomized controlled trial | NACT ×3 cycles; arm A: interval CRS; arm B CRS + HIPEC | PFS: HIPEC 14.2 months vs. control 10.7 months; OS: HIPEC 44.7 months vs. control 33.9 months | Grade 3–4 events: 52% | Cisplatin 100 mg/m2 |
| Coccolini (89) | 54 | Prospective observational study | Primary CRS + HIPEC | DFS: median 12.5 months; OS: median 32.9 months | Grade 3–4 events: 35%; death: 5.6% | Cisplatin 100 mg/m2; paclitaxel 175 mg/m2 |
| Gouy (90) | 30 | Prospective observational study | NACT + CRS, HIPEC | DFS: median 16.7 months; 2 years 71% | Grade 3–4 events: 46% in higher dose regimen; 25% in lower dose regimen | Cisplatin 50–80 mg/m2 |
| Manzanedo (91) | 27 | Prospective observational study | NACT + CRS, HIPEC | DFS: median 12 months; OS: median 40 months | Grade 3–4 events 30%; death 0% | Cisplatin 100 mg/m2 + doxorubicin 15 mg/m2 (n=15); paclitaxel 60 mg/m2 (n=11) |
| Paris (92) | 40 | Prospective observational study | Primary CRS + HIPEC | PFS: 25 months; OS: 92.5% at 25 months | Grade 3–4 events: 20%; death 0% | Cisplatin 75 mg/m2 |
| Lee (93) | 27 | Prospective observational study | NACT + IDS + HIPEC | PFS: 21 months | Grade 3–4 events: 19%; death 0% | Paclitaxel 175 mg/m2 |
| Tentes (94) | 23 | Prospective observational study | CRS + HIPEC | OS: mean 37 months | Grade 3–4 events: 15%; death 5% | Cisplatin 50 mg/m2 + doxorubicin 15 mg/m2 |
| Gonzalez Bayon (95) | 15 | Prospective observational study | NACT + CRS, HIPEC | DFS: median 21 months; OS: median 78 months | Grade 3–4 events: 27%; death 7% | Cisplatin 100 mg/m2 + doxorubicin 30 mg/m2 |
CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; NACT, neoadjuvant chemotherapy; IDS, interval debulking surgery; PFS, progression-free survival; OS, overall survival; DFS, disease-free survival.
Evidence-based guidelines
International evidence-based guidelines vary on the use of HIPEC in treating ovarian cancer. The NCCN recommends the consideration of intraperitoneal cisplatin in patients with stage III ovarian cancer in the interval cytoreductive surgery setting (44). The current NCCN recommended HIPEC regimen is 100 mg/m2 cisplatin as used in the OVHIPEC trial. The European Society of Gynaecological Oncology guidelines, however, do not recommend HIPEC as a standard of care upfront treatment (97). Several questions continue to exist before the more widespread adoption of HIPEC in treating advanced ovarian cancer (98,99). First, there is no single optimal dosing protocol that has been established. Second, the costs vs. benefits of HIPEC have not been explored. HIPEC involves longer operating room time, longer duration of hospitalization compared to intravenous chemotherapy which is the current standard of care. In the United States, use of HIPEC for ovarian cancer increased only modestly following the Dutch trial noted above (100); further data on long-term outcomes and methods to streamline its administration may eventually support more widespread adoption.
Mucinous ovarian cancers
Mucinous ovarian cancer is a rare subtype of epithelial ovarian cancer, accounting for 3% of all ovarian cancer incidence. Mucinous ovarian cancers bear many histological similarities to GI cancer subtypes. Two subcategories of mucinous ovarian cancer have been classified according to histologic growth patterns: (I) the expansile (confluent) subtype and (II) the infiltrative subtype. The expansile subtype exhibits a confluent glandular growth pattern, with little to no stromal invasion; these tumors have a lower metastatic and recurrence potential. The infiltrative subtype on the other hand, exhibits a more obvious destructive stromal invasion pattern and is associated with a higher chance of peritoneal spread and risk of relapse (101,102). When presenting in advanced stages with carcinomatosis, mucinous ovarian cancers demonstrate even worse outcomes than serous ovarian cancers of the same stage. These cancers are typically diagnosed in younger women (median age 53 vs. 61 years for serous ovarian cancer) (101) and comprise the most common histologic subtype of ovarian cancer diagnosed in women under 40 (103). Tobacco use is the only clinical risk factor identified associated with mucinous ovarian cancer risk. Most primary mucinous epithelial ovarian cancers (approximately 65–80%) are diagnosed at an early stage with a good prognosis, especially for the expansile subtype, following surgery (101). Unlike serous epithelial cancers which often produce symptoms following intraperitoneal spread, mucinous ovarian cancers usually present with large primary tumors that produce symptoms while still localized to the ovary of origin. The 5-year survival rate for stage I mucinous ovarian cancer is 90%. In contrast, women with stage III and stage IV mucinous ovarian cancer have a poorer prognosis compared to the more common subtypes such as serous ovarian cancer, with median survival rates between 11 and 16 months (104,105). The mean size at presentation is 16–20 cm. These tumors can become extremely large and fill the entire peritoneal cavity (106). The poor survival for advanced stage mucinous cancers is likely multifactorial, reflecting the aggressiveness of these tumors, resistance to conventional chemotherapy agents, as well as misclassification of many mucinous tumors as ovarian when they may in fact be metastatic from other GI primary sites.
The treatment of mucinous ovarian cancer has been based on regimens used for the more common epithelial subtypes such as serous ovarian cancers. The surgical staging is the same compared to the other epithelial ovarian cancer subtypes. Nodal spread is uncommon in early stage mucinous ovarian cancer (<2%) (107), however is more common in the infiltrative subtype and lymph node evaluation is recommended in these cancers. Discerning histologic subtype at time of surgery with intra-operative frozen section, however, is difficult and adds to the challenge of surgically staging and treating this cancer. Unilateral salpingo-oophorectomy is often considered in younger patients with early stage disease without having any negative impact on prognosis (106).
Before initiating treatment, a comprehensive workup including up-to-date upper GI and colonoscopy screening should be attempted to rule out metastatic disease to the ovaries (Krukenburg tumor). Around 80% of mucinous carcinomas of the ovary are in fact metastatic. The colon and appendix account for the largest portion of metastatic tumors, followed by the pancreas (108). The likelihood of metastasis is even higher if bilateral ovarian tumors are present, carcinoembryogenic antigen (CEA) is elevated, a CA-125/CEA ratio is less than 25, if imaging suggests advanced disease, or if the ovarian tumor is less than 10 cm in size (109,110). In reality however, many ovarian masses are discovered to be mucinous cancers post-operatively due to several reasons. Younger, premenopausal patients will often present to and be treated by a general gynecologist who may perform cystectomy or oophorectomy surgery alone without frozen pathology evaluation. Premenopausal patients are at lower risk for malignancy with the vast majority of adnexal masses in this group being benign. Additionally, CA-125 levels are often not elevated in these cancers.
The chemotherapy regimens used for advanced mucinous ovarian cancer have been the subject of some debate. Among the landmark practice changing clinical trials in ovarian cancer, only a small subset of patients had mucinous ovarian cancer (2–7%) (111-114). Several retrospective studies over the past two decades have shown lower response rates to platinum-based first-line therapy in mucinous ovarian cancers compared to serous ovarian cancers (carboplatin and paclitaxel) and lower overall survival (12–35 vs. 36–94 months) (101,115,116). This is most likely related to a poorer response to these traditional ovarian cancer chemotherapy agents (108,117).
Given that primary mucinous ovarian cancers share many histopathologic similarities to GI cancers (118,119), it has been theorized that many GI cancer regimens may prove more effective for mucinous ovarian cancers than platinum and taxane-based chemotherapy. The GOG launched trial 241 in 2009, a phase III randomized controlled trial which assigned patients to one of four arms for treatment: carboplatin and paclitaxel ± bevacizumab or oxaliplatin and capecitabine ± bevacizumab. The trial ended prematurely in 2013 due to low patient accrual with only 50 patients recruited compared to a target goal of 330 patients. The study’s authors reported an analysis of their limited data (120) which did not show a statistically significant difference in median overall and progression-free survival between the treatment arms; however, no definitive conclusions can be drawn on efficacy or lack thereof between the various arms given the study did not meet its accrual target (121). Importantly, a central review of GOG 241 data showed that 55% of cases were in fact non-ovarian metastases, underscoring the similarities between primary mucinous ovarian cancer and GI mucinous cancers (120).
A recent retrospective study (122) investigated response rates among mucinous ovarian cancer patients receiving adjuvant GI chemotherapy agents oxaliplatin, capecitabine, or 5-fluorouracil compared to platinum-based chemotherapy; bevacizumab use was present among cases in both cohorts. While the GI regimens were significantly more likely to have included bevacizumab, progression-free survival and overall survival in the GI-type chemotherapy cohort were significantly improved (122).
HIPEC in mucinous ovarian cancer
HIPEC is an additional treatment modality that offers potential promise in treating mucinous ovarian cancers. Given the role of HIPEC in metastatic colorectal cancer and pseudomyxoma peritonei, the biological similarities between mucinous ovarian cancer and these GI malignancies suggest that this regimen be a reasonable option to consider, especially in light of the inclusion of HIPEC with interval cytoreductive surgery as a level 2a recommendation in the 2019 NCCN guidelines (44). A 2018 multicenter retrospective analysis investigating the benefit of HIPEC with cytoreductive surgery in rare ovarian cancer subtypes showed 5-year overall survival and 5-year disease-free survival for mucinous ovarian cancer with peritoneal metastasis as 69% and 54%, respectively (123). The study accrued 80 mucinous cancer cases over 15 years at 40 specialized centers, again highlighting the difficulty of studying this tumor type. These survival figures are better than those previously reported for FIGO stage III and IV mucinous ovarian cancers. While the different regimens used across various institutions in the study make it difficult to determine a best single regimen, this variability highlights the need for prospective trials to further elucidate optimal treatments for these cancers. The findings of these studies raise important questions about current treatment regimens for mucinous ovarian cancer. Even with adequate diagnostic measures, these tumors can be difficult to correctly diagnose pre-operatively, making the implementation of upfront HIPEC treatment challenging. The rarity of these tumors and the failure of GOG 241 to accrue adequate patient numbers likely mean that future prospective trials will be difficult to organize. To our knowledge, no prospective trials exist or are planned at present that aim to address the question of optimal primary chemotherapy regimen for advanced mucinous ovarian cancer. One potential strategy given the rarity of this cancer type is inclusion in basket trials enrolling patients with tumors from several anatomic sites. Even with this approach however, the rarity of this tumor type makes accrual to trials an ongoing challenge. Genetic sequencing could potentially identify actionable somatic mutations allowing for use of targeted agents.
Conclusions
Maximal surgical effort combined with intravenous chemotherapy remains the standard of care for upfront treatment in ovarian cancer peritoneal carcinomatosis. Surgical cytoreduction has consistently been shown to be the single most important predictive factor in patient outcomes. Significant work in recent decades has helped optimize the dose, route, and selection of chemotherapy agents used in modern treatment regimens. Despite this, ovarian cancer remains the most fatal gynecologic cancer with most patients experiencing recurrence after upfront treatment. Many challenges and questions remain before HIPEC modalities can experience more widespread adoption among clinicians. Recent advances in our understanding of cancer genetics and novel treatment agents have broadened options for patients beyond just cytotoxic chemotherapy with the advent of antiangiogenic agents and PARP inhibitors. Future work is needed to identify subsets of patients who may benefit from certain treatment modalities. Similarly, rare subtypes of ovarian cancer such as mucinous ovarian cancer have a dearth of high-quality evidence to guide treatment decisions and remain challenging to study prospectively. In the coming years, new approaches and algorithms for patient selection are needed to further identify subsets who may benefit from particular treatment regimens.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrew M. Blakely and Oliver S. Eng) for the series “Peritoneal Carcinomatosis: History and Future” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at: https://dmr.amegroups.com/article/view/10.21037/dmr-22-13/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-13/coif). The series “Peritoneal Carcinomatosis: History and Future” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284-96. [Crossref] [PubMed]
- Momenimovahed Z, Tiznobaik A, Taheri S, et al. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 2019;11:287-99. [Crossref] [PubMed]
- Konstantinopoulos PA, Norquist B, Lacchetti C, et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J Clin Oncol 2020;38:1222-45. [Crossref] [PubMed]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053-64. [Crossref] [PubMed]
- Kuhn E, Kurman RJ, Vang R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. J Pathol 2012;226:421-6. [Crossref] [PubMed]
- Eckert MA, Pan S, Hernandez KM, et al. Genomics of Ovarian Cancer Progression Reveals Diverse Metastatic Trajectories Including Intraepithelial Metastasis to the Fallopian Tube. Cancer Discov 2016;6:1342-51. [Crossref] [PubMed]
- Harter P, Sehouli J, Lorusso D, et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N Engl J Med 2019;380:822-32. [Crossref] [PubMed]
- Barakat RR, Berchuck A, Markman M, et al. Epithelial Ovarian Cancer. In: Principles and Practice of Gynecologic Oncology. 2013. Available online: https://oncology.lwwhealthlibrary.com/book.aspx?bookid=1136
- Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr 1975;42:101-4. [PubMed]
- du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer 2009;115:1234-44. [Crossref] [PubMed]
- Hacker NF, Berek JS, Lagasse LD, et al. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol 1983;61:413-20. [PubMed]
- Hoskins WJ, Bundy BN, Thigpen JT, et al. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol 1992;47:159-66. [Crossref] [PubMed]
- Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol 1998;69:103-8. [Crossref] [PubMed]
- Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol 2007;25:2873-83. [Crossref] [PubMed]
- Vernooij F, Heintz P, Witteveen E, et al. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol 2007;105:801-12. [Crossref] [PubMed]
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006;98:172-80. [Crossref] [PubMed]
- Giede KC, Kieser K, Dodge J, et al. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005;99:447-61. [Crossref] [PubMed]
- Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 1994;170:974-9; discussion 979-80. [Crossref] [PubMed]
- Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248-59. [Crossref] [PubMed]
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:1001-7. [Crossref] [PubMed]
- Angioli R, Palaia I, Zullo MA, et al. Diagnostic open laparoscopy in the management of advanced ovarian cancer. Gynecol Oncol 2006;100:455-61. [Crossref] [PubMed]
- Fagotti A, Fanfani F, Ludovisi M, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol 2005;96:729-35. [Crossref] [PubMed]
- Rutten MJ, van Meurs HS, van de Vrie R, et al. Laparoscopy to Predict the Result of Primary Cytoreductive Surgery in Patients With Advanced Ovarian Cancer: A Randomized Controlled Trial. J Clin Oncol 2017;35:613-21. [Crossref] [PubMed]
- Fagotti A, Ferrandina G, Fanfani F, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol 2006;13:1156-61. [Crossref] [PubMed]
- Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol 2017;145:27-31. [Crossref] [PubMed]
- Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol 2012;29:2005-9. [Crossref] [PubMed]
- Elattar A, Bryant A, Winter-Roach BA, et al. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev 2011;CD007565. [Crossref] [PubMed]
- Nezhat FR, Finger TN, Vetere P, et al. Comparison of perioperative outcomes and complication rates between conventional versus robotic-assisted laparoscopy in the evaluation and management of early, advanced, and recurrent stage ovarian, fallopian tube, and primary peritoneal cancer. Int J Gynecol Cancer 2014;24:600-7. [Crossref] [PubMed]
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 2009;27:5331-6. [Crossref] [PubMed]
- Melamed A, Nitecki R, Boruta DM 2nd, et al. Laparoscopy Compared With Laparotomy for Debulking Ovarian Cancer After Neoadjuvant Chemotherapy. Obstet Gynecol 2017;129:861-9. [Crossref] [PubMed]
- Corrado G, Mancini E, Cutillo G, et al. Laparoscopic Debulking Surgery in the Management of Advanced Ovarian Cancer After Neoadjuvant Chemotherapy. Int J Gynecol Cancer 2015;25:1253-7. [Crossref] [PubMed]
- Gueli Alletti S, Bottoni C, Fanfani F, et al. Minimally invasive interval debulking surgery in ovarian neoplasm (MISSION trial-NCT02324595): a feasibility study. Am J Obstet Gynecol 2016;214:503.e1-6. [Crossref] [PubMed]
- Nitecki R, Rauh-Hain JA, Melamed A, et al. Laparoscopic cytoreduction After Neoadjuvant ChEmotherapy (LANCE). Int J Gynecol Cancer 2020;30:1450-4. [Crossref] [PubMed]
-
FDA Approves New Imaging Drug to Help Identify Ovarian Cancer Lesions 2021 . Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-imaging-drug-help-identify-ovarian-cancer-lesions - Wiltshaw E, Kroner T. Phase II study of cis-dichlorodiammineplatinum(II) (NSC-119875) in advanced adenocarcinoma of the ovary. Cancer Treat Rep 1976;60:55-60. [PubMed]
- Omura G, Blessing JA, Ehrlich CE, et al. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A Gynecologic Oncology Group Study. Cancer 1986;57:1725-30. [Crossref] [PubMed]
- Omura GA, Bundy BN, Berek JS, et al. Randomized trial of cyclophosphamide plus cisplatin with or without doxorubicin in ovarian carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 1989;7:457-65. [Crossref] [PubMed]
- Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst 2000;92:699-708. [Crossref] [PubMed]
- Sandercock J, Parmar MK, Torri V, et al. First-line treatment for advanced ovarian cancer: paclitaxel, platinum and the evidence. Br J Cancer 2002;87:815-24. [Crossref] [PubMed]
- Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003;21:3194-200. [Crossref] [PubMed]
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J Natl Compr Canc Netw 2019;17:896-909. [Crossref] [PubMed]
- Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol 2009;27:1419-25. [Crossref] [PubMed]
- Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009;374:1331-8. [Crossref] [PubMed]
- Clamp AR, James EC, McNeish IA, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019;394:2084-95. [Crossref] [PubMed]
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. [Crossref] [PubMed]
- Hall TR, Dizon DS. Neoadjuvant chemotherapy for advanced epithelial ovarian cancer. Clin Adv Hematol Oncol 2016;14:262-8. [PubMed]
- Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol 2012;124:10-4. [Crossref] [PubMed]
- Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 2016;64:22-31. [Crossref] [PubMed]
- Fagotti A, Ferrandina G, Vizzielli G, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer 2016;59:22-33. [Crossref] [PubMed]
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249-57. [Crossref] [PubMed]
- Fagotti A, Ferrandina MG, Vizzielli G, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer 2020;30:1657-64. [Crossref] [PubMed]
- Narasimhulu DM, Kumar A, Weaver AL, et al. Less guessing, more evidence in identifying patients least fit for cytoreductive surgery in advanced ovarian cancer: A triage algorithm to individualize surgical management. Gynecol Oncol 2020;157:572-7. [Crossref] [PubMed]
- Shim SH, Lee SJ, Kim SO, et al. Nomogram for predicting incomplete cytoreduction in advanced ovarian cancer patients. Gynecol Oncol 2015;136:30-6. [Crossref] [PubMed]
- Vergote I, Coens C, Nankivell M, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol 2018;19:1680-7. [Crossref] [PubMed]
- Reuss A, du Bois A, Harter P, et al. TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int J Gynecol Cancer 2019;29:1327-31. [Crossref] [PubMed]
- Markman M, Liu PY, Wilczynski S, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol 2003;21:2460-5. [Crossref] [PubMed]
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473-83. [Crossref] [PubMed]
- Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 2015;16:928-36. [Crossref] [PubMed]
- Tewari KS, Burger RA, Enserro D, et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J Clin Oncol 2019;37:2317-28. [Crossref] [PubMed]
- Ray-Coquard I, Cibula D, Mirza MR, et al. Final results from GCIG/ENGOT/AGO-OVAR 12, a randomised placebo-controlled phase III trial of nintedanib combined with chemotherapy for newly diagnosed advanced ovarian cancer. Int J Cancer 2020;146:439-48. [Crossref] [PubMed]
- Vergote I, du Bois A, Floquet A, et al. Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol Oncol 2019;155:186-91. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med 2019;381:2416-28. [Crossref] [PubMed]
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019;381:2391-402. [Crossref] [PubMed]
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med 2019;381:2403-15. [Crossref] [PubMed]
- A Phase 3 Comparison of Platinum-based Therapy With TSR-042 and Niraparib Versus Standard of Care (SOC) Platinum-based Therapy as First-line Treatment of Stage III or IV Nonmucinous Epithelial Ovarian Cancer (FIRST). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03602859
-
Durvalumab Treatment in Combination With Chemotherapy and Bevacizumab, Followed by Maintenance Durvalumab, Bevacizumab and Olaparib Treatment in Advanced Ovarian Cancer Patients (DUO-O) 2021 . Available online: https://clinicaltrials.gov/ct2/show/NCT03737643 - A Study in Ovarian Cancer Patients Evaluating Rucaparib and Nivolumab as Maintenance Treatment Following Response to Front-Line Platinum-Based Chemotherapy (ATHENA). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03522246
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol 2021;22:1034-46. [Crossref] [PubMed]
- Zamarin D, Burger RA, Sill MW, et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol 2020;38:1814-23. [Crossref] [PubMed]
- Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 2019;30:1080-7. [Crossref] [PubMed]
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950-5. [Crossref] [PubMed]
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol Oncol 2016;143:3-15. [Crossref] [PubMed]
- Schlappe BA, Mueller JJ, Zivanovic O, et al. Cited rationale for variance in the use of primary intraperitoneal chemotherapy following optimal cytoreduction for stage III ovarian carcinoma at a high intraperitoneal chemotherapy utilization center. Gynecol Oncol 2016;142:13-8. [Crossref] [PubMed]
- Naumann RW, Sukumvanich P, Edwards RP. Practice patterns of intraperitoneal chemotherapy in women with ovarian cancer. Gynecol Oncol 2009;114:37-41. [Crossref] [PubMed]
- Walker JL, Brady MF, Wenzel L, et al. Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol 2019;37:1380-90. [Crossref] [PubMed]
- Ohno S, Siddik ZH, Kido Y, et al. Thermal enhancement of drug uptake and DNA adducts as a possible mechanism for the effect of sequencing hyperthermia on cisplatin-induced cytotoxicity in L1210 cells. Cancer Chemother Pharmacol 1994;34:302-6. [Crossref] [PubMed]
- Meyn RE, Corry PM, Fletcher SE, et al. Thermal enhancement of DNA damage in mammalian cells treated with cis-diamminedichloroplatinum(II). Cancer Res 1980;40:1136-9. [PubMed]
- van de Vaart PJ, van der Vange N, Zoetmulder FA, et al. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer 1998;34:148-54. [Crossref] [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [Crossref] [PubMed]
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med 2018;378:230-40. [Crossref] [PubMed]
- Alter R, Turaga K, Lengyel E. Are We Ready for Hyperthermic Intraperitoneal Chemotherapy in the Upfront Treatment of Ovarian Cancer? JAMA Netw Open 2020;3:e2014184. [Crossref] [PubMed]
- Vergote I, Harter P, Chiva L. Hyperthermic intraperitoneal chemotherapy does not improve survival in advanced ovarian cancer. Cancer 2019;125:4594-7. [Crossref] [PubMed]
- Lei Z, Wang Y, Wang J, et al. Evaluation of Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Stage III Epithelial Ovarian Cancer. JAMA Netw Open 2020;3:e2013940. [Crossref] [PubMed]
- Lim MC, Chang SJ, Yoo HJ, et al. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J Clin Oncol 2017;35:abstr 5520.
- Coccolini F, Campanati L, Catena F, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol 2015;26:54-61. [Crossref] [PubMed]
- Gouy S, Ferron G, Glehen O, et al. Results of a multicenter phase I dose-finding trial of hyperthermic intraperitoneal cisplatin after neoadjuvant chemotherapy and complete cytoreductive surgery and followed by maintenance bevacizumab in initially unresectable ovarian cancer. Gynecol Oncol 2016;142:237-42. [Crossref] [PubMed]
- Manzanedo I, Pereira F, Pérez-Viejo E, et al. Hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) with primary or secondary cytoreductive surgery in the treatment of advanced epithelial ovarian cancer. Minerva Ginecol 2017;69:119-27. [PubMed]
- Paris I, Cianci S, Vizzielli G, et al. Upfront HIPEC and bevacizumab-containing adjuvant chemotherapy in advanced epithelial ovarian cancer. Int J Hyperthermia 2018;35:370-4. [Crossref] [PubMed]
- Lee YJ, Lee JY, Cho MS, et al. Incorporation of paclitaxel-based hyperthermic intraperitoneal chemotherapy in patients with advanced-stage ovarian cancer treated with neoadjuvant chemotherapy followed by interval debulking surgery: a protocol-based pilot study. J Gynecol Oncol 2019;30:e3. [Crossref] [PubMed]
- Tentes AA, Kakolyris S, Kyziridis D, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy in the treatment of advanced epithelial ovarian cancer. J Oncol 2012;2012:358341. [Crossref] [PubMed]
- Gonzalez Bayon L, Steiner MA, Vasquez Jimenez W, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of advanced epithelial ovarian carcinoma: upfront therapy, at first recurrence, or later? Eur J Surg Oncol 2013;39:1109-15. [Crossref] [PubMed]
- Koole S, van Stein R, Sikorska K, et al. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int J Gynecol Cancer 2020;30:888-92. [Crossref] [PubMed]
- Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol 2019;30:672-705. [Crossref] [PubMed]
- Spriggs DR, Zivanovic O. Ovarian Cancer Treatment - Are We Getting Warmer? N Engl J Med 2018;378:293-4. [Crossref] [PubMed]
- Fotopoulou C, Sehouli J, Mahner S, et al. HIPEC: HOPE or HYPE in the fight against advanced ovarian cancer? Ann Oncol 2018;29:1610-3. [Crossref] [PubMed]
- Charo LM, Jou J, Binder P, et al. Current status of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer in the United States. Gynecol Oncol 2020;159:681-6. [Crossref] [PubMed]
- Morice P, Gouy S, Leary A. Mucinous Ovarian Carcinoma. N Engl J Med 2019;380:1256-66. [Crossref] [PubMed]
- Kurman RJ, Carcangiu ML, Herrington CS, et al. editors. WHO Classification of Tumours of Female Reproductive Organs. 2014. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Female-Reproductive-Organs-2014
- Yoshikawa N, Kajiyama H, Mizuno M, et al. Clinicopathologic features of epithelial ovarian carcinoma in younger vs. older patients: analysis in Japanese women. J Gynecol Oncol 2014;25:118-23. [Crossref] [PubMed]
- Mackay HJ, Brady MF, Oza AM, et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int J Gynecol Cancer 2010;20:945-52. [Crossref] [PubMed]
- Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J Natl Cancer Inst 2019;111:60-8. [Crossref] [PubMed]
- Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep 2014;16:389. [Crossref] [PubMed]
- Morice P, Joulie F, Camatte S, et al. Lymph node involvement in epithelial ovarian cancer: analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J Am Coll Surg 2003;197:198-205. [Crossref] [PubMed]
- Shimada M, Kigawa J, Ohishi Y, et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol 2009;113:331-4. [Crossref] [PubMed]
- Sørensen SS, Mosgaard BJ. Combination of cancer antigen 125 and carcinoembryonic antigen can improve ovarian cancer diagnosis. Dan Med Bull 2011;58:A4331. [PubMed]
- Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 2003;27:985-93. [Crossref] [PubMed]
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet 2002;360:505-15. Erratum in: Lancet 2003;361:706. [Crossref] [PubMed]
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1-6. [Crossref] [PubMed]
- Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol 2000;18:3084-92. [Crossref] [PubMed]
- Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol 2000;18:106-15. [Crossref] [PubMed]
- Karabuk E, Kose MF, Hizli D, et al. Comparison of advanced stage mucinous epithelial ovarian cancer and serous epithelial ovarian cancer with regard to chemosensitivity and survival outcome: a matched case-control study. J Gynecol Oncol 2013;24:160-6. [Crossref] [PubMed]
- Hess V, A'Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol 2004;22:1040-4. [Crossref] [PubMed]
- Alexandre J, Ray-Coquard I, Selle F, et al. Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience. Ann Oncol 2010;21:2377-81. [Crossref] [PubMed]
- Kelemen LE, Köbel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol 2011;12:1071-80. [Crossref] [PubMed]
- Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, et al. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer 2006;94:904-13. [Crossref] [PubMed]
- Gore M, Hackshaw A, Brady WE, et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol Oncol 2019;153:541-8. [Crossref] [PubMed]
- Gore ME, Hackshaw A, Brady WE, et al. Multicentre trial of carboplatin/paclitaxel versus oxaliplatin/capecitabine, each with/without bevacizumab, as first line chemotherapy for patients with mucinous epithelial ovarian cancer (mEOC). J Clin Oncol 2015;33:abstr 5528.
- Kurnit KC, Sinno AK, Fellman BM, et al. Effects of Gastrointestinal-Type Chemotherapy in Women With Ovarian Mucinous Carcinoma. Obstet Gynecol 2019;134:1253-9. [Crossref] [PubMed]
- Mercier F, Bakrin N, Bartlett DL, et al. Peritoneal Carcinomatosis of Rare Ovarian Origin Treated by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Multi-Institutional Cohort from PSOGI and BIG-RENAPE. Ann Surg Oncol 2018;25:1668-75. [Crossref] [PubMed]
Cite this article as: Krishnan R, Kurnit KC, Kim JS. Ovarian cancer peritoneal carcinomatosis: a narrative review. Dig Med Res 2022;5:43.