
Tumor regression grade (TRG) for gastric cancer and radiological methods on predicting response to perioperative chemotherapy: a narrative review
Introduction
Gastric cancer (GC) is still ranking third among the most common malignant tumors in terms of mortality rate. In the last decade, with the progression of the multimodal treatment, patients with early-stage disease (stage I) achieve a cure rate of 90%; nonetheless, patients with locally advanced GC have still a poor prognosis (1). The aim of neoadjuvant chemotherapy for locally advanced GC is to downstage the primary tumor improving the rate of radical surgeries. Since the publication of MAGIC and AIO-FLOT-4 trial results, showing that perioperative chemotherapy improves progression free (PFS) and overall survival (OS), it has progressively become the standard of care for locally advanced GC in Western Countries (2-5).
Pathologically, after neoadjuvant treatment and surgery there are two main methods to assess the residual cancer: tumor-node-metastasis (TNM) and tumor regression grade (TRG). TNM is currently used in the routine clinical practice worldwide and one of its limitations is to be founded on the location instead of the quantity of the residual tumor. Conversely TRG, that is a quantitative approach to describe the residual cancer, could be a strong and effective complement to TNM. Between the existing TRG systems there are two different methods to quantify the regressive changes after neoadjuvant chemotherapy: the estimation of the chemo-induced fibrosis in relation to residual tumor (6,7) or the estimation of the percentage of residual tumor related to the original tumor site (8-11).
However, not all patients have effective respond to perioperative chemotherapy. In non-responder, preoperative chemotherapy could be avoided. Thus, there is a need to identify a method to discriminate responder and non-responder patients.
The aim of this study is to review the different TRG classification systems and the current role of imaging for prediction of response to preoperative therapy in GC. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-34/rc).
Methods
As part of this review, a literature search was performed using MEDLINE (PubMed) and Scopus. The terms tumor regression grade, pathologic response, gastric cancer, gastric adenocarcinoma, RECIST 1.1, radiological prediction of response, perioperative, preoperative and neoadjuvant chemotherapy were included. Only papers written in English and published until December 2021 were reviewed. Further details of the literature search are listed in Table 1.
Table 1
| Items | Specifications |
|---|---|
| Date of search | 4th April, 2022 |
| Databases and other sources searched | EMBASE (PubMed), Scopus |
| Search terms used | Tumor regression grade, pathologic response, gastric cancer, gastric adenocarcinoma, RECIST 1.1, radiological prediction of response, perioperative, preoperative and neoadjuvant chemotherapy |
| Time frame | 1950–2021 |
| Inclusion and exclusion criteria | Inclusion: written in English |
| Selection process | Initial selection by Mainardi F and Berardi E then review and amended by Garbarino GM |
RECIST, response evaluation criteria in solid tumors.
Tumor regression modifications after preoperative therapy
During the macroscopic pathological evaluation an estimation of specimens is essential to estimate the extent of the tumor bed (the original site of the tumor) and to assure proper embedding for accurate microscopic investigation. The following step is the assessment of the chemo-induced changes into the tumor area under the microscope, which is the real core of the assessment process. Macroscopically residual tumor with ulcerating or infiltrative features or tumor regression signs like scars can be spotted (Figure 1). By histology, tumor regression after preoperative treatment is mainly represented by subacute and subchronic inflammation. Those cytotoxic effects usually develop between the end of the preoperative treatment and the surgical resection (12) (Figure 2). Microscopically, the morphological chemo-induced changes of the tumor can be described at cellular and stromal level. On the cellular level, several features can be found in the residual neoplastic component: cytoplasmic vacuolization, eosinophilic cytoplasm and marked nuclear atypia, including hyperchromasia, karyorrhexis, pyknosis and enlarged nuclei; oncocytic differentiation and development of neuroendocrine differentiation may be also found. Mitoses are hardly seen in opposition to apoptotic figures (6-8,13-17). Stromal changes embrace modifications like histiocytic reaction with foamy or occasionally hemosiderin-laden macrophages, cholesterol clefts and foreign body reactions, and dystrophic calcifications. Unorganized branch of fibrous tissue with following disarray of the physiological structure is frequently observed. The presence of mucinous substance is widely documented, either as mucin lakes with some scattered residual neoplastic cells or as acellular mucin lakes (6,8,14,17-20). The mucous pool should not be regarded as a viable remaining tumor, but the presence of mucin lakes should be promptly followed by a careful search for viable residual tumor cells (Figure 3) (13). Vascular changes such as obliterative endarteritis and myxohyaline intimal proliferation in vessels have often been reported (10). Treatment-induced changes such as inflammation and oedema have also been reported in adjacent non-neoplastic tissue. The changes in non-tumor epithelia may be similar to those in neoplastic cells, with condensed chromatin, nuclear pleomorphism and eosinophilia (8,13,14). Such changes in non-tumor tissues may hinder the distinction from true neoplastic changes.

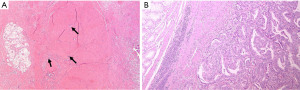
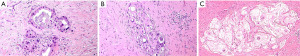
Several histopathologic chemo-induced modifications like calcification, acellular mucinous lakes and the pattern of response (fragmentation vs. bulk reduction) have been proved to be linked with patients’ prognosis (20-22).
TRG systems
TRG systems categorize the quantity of regressive changes after perioperative chemotherapy with the aim to become an objective histopathologic prognostic tool in clinical practice. As previously mentioned, many histopathological detectable modifications are not frequently observed and are not completely specific for tumor regression after chemotherapy. Thus, the available regression classification systems mainly refer to single parameters, which are better reproducible: the proportion of residual tumor, expressed as a percentage, or the estimated ratio of regressed fibrosis to residual tumor, which is typically based on description. The available TRG systems that are those of Dworak (6), Mandard (7), Ryan (23) Becker (8,24) and Japanese Gastric Cancer Association-TRG (15) are summarized in Table 2.
Table 2
| Regression grade | Relation between tumor and fibrosis | Proportion of residual tumor | ||||
|---|---|---|---|---|---|---|
| Mandard | Dworak | Ryan | Becker | JGCA | ||
| Complete | TRG1; no residual cancer cell, total fibrosis | TRG4; no tumor cells, only fibrotic mass | TRG1a; 0% residual tumor | TRG3; 0% residual tumor | ||
| Subtotal | TRG2; rare residual cancer cells, scattered through the fibrosis | TRG3; difficult to find tumor cells microscopically, which scattered in fibrotic tissue | TRG1; no or rare residual cancer cells | TRG1b; <10% residual tumor | TRG2; 1–33% residual tumor | |
| Partial | TRG3; more residual cancer cells, but outgrown by fibrosis | TRG2; easy to find tumor cells microscopically, with dominantly fibrotic changes | TRG2; more residual cancer cells | TRG2; 10–50% Residual tumor | TRG1b; 34–66% residual tumor | |
| No response | TRG4; residual cancer cells outgrowing fibrosis TRG5; absence of regressive changes |
TRG1; dominant tumor mass with obvious fibrosis TRG0; no regression |
TRG3; residual cancer cells outgrowing fibrosis or no regression | TRG3; >50% residual tumor | TRG1a; >67% residual tumor TRG0; 100% residual tumor |
|
TRG, tumor regression grade; JGCA, Japanese Gastric Cancer Association.
Criticisms of TRG
The main issues on histopathologic evaluation of TRG are intra-and inter-observer variability and lack of uniform protocol. The Mandard and Dworak TRG grading systems showed a low interobserver-agreement among expert gastrointestinal pathologists for rectal cancer with kappa values of 0.28 and 0.35, respectively. For gastric and gastroesophageal junction cancers, Becker’s TRG achieved the higher interobserver-agreement (kappa value =0.52). Whereas Mandard and the Japanese grading systems showed kappa values of 0.44 and 0.28, respectively (12). Another crucial limitation of TRG is the over-evaluation of the tumor response even in patients underwent to upfront surgery. Smyth et al. reported a 18% rate of TRG 2–3 (based on Mandard TRG) in patients that did not receive any preoperative chemotherapy (25). A robust and widely accepted TRG system is still needed for gastrointestinal malignancies after neoadjuvant therapy. To overcome the drawbacks and limitations of existing TRG systems in GC, a Delphi survey among international experts proposes a 4-level system for grading regression in the primary tumor, combined with a 3-level system for metastatic lymph nodes. In this system, based mainly on Becker’s TRG, grade 1 corresponds to complete response (no residual tumor), grade 2 to subtotal regression (<10% residual tumor), grade 3 to partial regression (10–50% remaining tumor), and grade 4 to minimal/absent regression (>50% residual tumor). The response in metastatic lymph nodes was classified as: ‘a’ (complete), ‘b’ (partial) or ‘c’ (no response) (26). The main innovation of this classification system is the incorporation of lymph node regression, which provides a complementary prognostic tool.
Prognostic significance of TRG
Pathological complete response (pCR) has been proved to have a better prognosis compared to poor or incomplete response (3,27,28). Koh et al. have proved that patients with pCR had significantly better PFS than patients with non-pCR according to Mandard TRG system (29). Patients with pCR showed a significantly better PFS and OS compared with those with TRG2 to TRG5 (5 years PFS: 68.7% vs. 32.2% (P=0.002); and 5 years OS: 72.7% vs. 31.4% (P=0.002) (29). These results agree with the review of Tomasello et al. that showed that patients achieving a major pathologic response (pCR or near pCR with few residual cells) after preoperative chemotherapy have more than 50% decrease in the risk of death (27). In the same way, both Mandard and Becker TRG systems have been proved to be independent prognostic factors for survival in multivariate analysis (24,30). Controversially Smyth et al. did not demonstrate an independent effect of Mandard TRG on survival in the MAGIC trial (25). Despite the known association between pCR and improved survival, evidence of a linear relationship between the degree of response to chemotherapy and OS is still lacking in the literature. In a single-center cohort of 168 patients with GC treated with preoperative chemotherapy, Mansour et al. found that the degree of histologic response did not independently predict disease-specific survival (31). Other studies that evaluated the prognostic value of Becker’s TRG, Mandard’s TRG, and the Japanese Gastric Cancer Association TRG showed a prognostic effect in univariate analysis, without confirming the prognostic value of any of these TRGs in multivariate analysis (32-40). Xu et al. also showed that histologic type, Lauren classification, vascular or lymphatic invasion, ypN, and post-surgical pathologic stage were significantly associated with survival, but unfortunately, TRG and pathologic T category were not found to be independent factors for OS and disease-free survival (DFS) in patients after perioperative chemotherapy (41). Conversely, Lombardi et al. proved by multivariate analysis that Becker TRG, lymph node ratio and tumor size were independent prognostic factors for DFS and disease specific survival (42). This difference among studies may be due to the difference of anatomical location, the selection of survival index and the tumor type (38). In addition to the status and evaluation of the primary tumor, one of the goals of neoadjuvant chemotherapy is the eradication of metastatic and micro-metastatic disease that may have spread to the lymph nodes (36,39). Nodal stage has also been established as an independent survival predictor in several articles (31-33,41). The clinical application of TRG, as an independent prognostic value in GC, is still ambiguous. Some authors deny that a marked histological response to treatment indicates improved survival, while others believe that pathological TRG has a role as a prognostic marker (31,41,42). Indeed, an internationally and widespread accepted tumor regression grading system for gastrointestinal malignancies following neoadjuvant chemotherapy is still required, as mentioned by the European Society for Medical Oncology Clinical Practice Guidelines on GC (4). The achievement of a critical predictive and prognostic factor could lead to a better selection of patients who may benefit from a neoadjuvant treatment. Conversely, for non-responder patients to preoperative chemotherapy, alternative therapeutic strategies should be identified.
Radiological evaluation and prediction of tumor response
Since 2009, the majority of papers investigating cancer response to neoadjuvant treatments are using the response evaluation criteria in solid tumors (RECIST 1.1): complete response, partial response, stable disease, or progressive disease (43). However, RECIST have several limitations regarding GC. Firstly, the criteria were developed for solid tumors and the stomach is not a parenchymatous organ. Gastric lesion is defined as non-measurable by RECIST because it requires the presence of a measurable lesion, which is often not the case in GC (44). Moreover, RECIST response criteria seem to underestimate histological gastric tumor response compared to the adapted Choi criteria, which appear to better predict survival of these patients (45).
In 2017, the Italian Gastric Cancer Research Group (GIRCG) proposed the maximum tumor diameter reduction rate on computed tomography (CT) scan as a simple, useful and reproducible radiological index to predict TRG after neoadjuvant therapy (46). The key to this technique is to distend the stomach with air or water until the gastric folds appeared mostly flat at the tumor site (Figure 4).
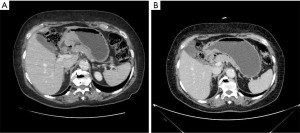
Regarding other morphologic techniques, Ang et al. attempted to evaluate the value of contrast-enhanced ultrasound (CEUS) in patients with locally advanced GC. Despite a sensitivity of 62.9% and a specificity of 56.3%, CEUS was not significantly more accurate than RECIST criteria (P=0.663) (47).
On the other hand, the association of CT scan with endoscopy or endoscopic ultrasonography (EUS) for prediction of GC response to preoperative chemotherapy, resulted in a significant correlation with histopathological response and OS (48,49).
An evident disadvantage of morphological imaging is that it takes time for gross changes in the tumor to manifest themselves. In contrast, metabolic changes precede anatomical changes. Therefore, molecular imaging can play a predominant role in predicting tumor response to preoperative chemotherapy much earlier during treatment.
18F-fluoro-2-deoxyglucose (FDG) positron emission tomography/computed tomography (18F-FDG PET/CT) is widely used to evaluate the response to chemotherapy of several kinds of tumors. Nonetheless, there is still controversy about the pathological response prediction value of 18F-FDG PET/CT in patients treated with neoadjuvant chemotherapy (50-54).
Morgagni et al., in a recent study, recruited 71 patients from 6 Italians centers with noncardia-GC treated with preoperative chemotherapy to better define the usefulness of 18F-FDG PET/CT in predicting pathological tumor response evaluated by Becker TRG and survival (55). Unfortunately, 18F-FDG PET/CT failed to demonstrate its usefulness in predicting cancer regression. In fact, the metabolic response of 54 patients, ranging from 0% to 70%, did not permit to reliably forecast pathologic tumor regression and survival rates were affected by metabolic response (55).
To date, one of the most promising techniques in the evaluation of preoperative therapy for gastric adenocarcinoma is the diffusion weighted imaging (DWI) magnetic resonance imaging (MRI). Indeed, it represents a radiation free value-adding technique and, when compared to 18F-FDG PET/CT, it seems to be more accurate in predicting TRG (56).
Future perspectives
Nowadays, the correct selection of patients for upfront surgery or a perioperative treatment represents one of the most debated points in the field of multimodal approach for gastric malignancies. Both The Cancer Genome Atlas (TCGA) and the Asian Cancer Research Group (ACRG) classified GC in molecular subtypes, showing distinct clinical outcomes and different responses to perioperative chemotherapy for each class (57,58).
Regarding the prediction of treatment response in relation to microsatellite instability (MSI) status, a post hoc analysis of the MAGIC trial validates the poor reaction to chemotherapy in MSI patients (59). In fact, for patients undergoing prior surgery, there was no difference in OS between the microsatellite stability (MSS) and MSI/MRD (mismatch repair-deficient) groups. In addition, MSI patients showed an increased risk of mortality (HR 2.18, 95% CI: 1.08–4.42) when treated with perioperative chemotherapy, questioning its role in this subgroup of patients (59). On the other hand, patients with low microsatellite instability (MSI-L), MSS and/or MMRP (mismatch repair-proficient) may benefit from a radical resection after preoperative chemotherapy (60).
A recent study by Kohlruss et al. aimed to determine the prognostic and predictive significance of Epstein-Barr virus positivity (EBV+) and high microsatellite instability (MSI-H) subgroups in patients undergoing preoperative chemotherapy (61). The authors analyzed 760 adenocarcinomas of the stomach and esophago-gastric junction, demonstrating that MSI-H and EBV+ are not predictive of tumor response to preoperative chemotherapy, but are predictors of a better prognosis. Regarding MSI, MSI-H patients have a favorable prognosis regardless of chemotherapy, while MSI-L patients have a good response to chemotherapy and poor survival when undergoing upfront surgery. These results suggest that MSI-L patients may be ideal candidates for preoperative chemotherapy.
Together with molecular subgroups analysis, another emerging diagnostic tool in the field of precision medicine is radiomics.
Radiomics has already proven effective for predicting the response to treatment of different types of cancer through a variety of imaging modalities (62-64). With regard to GC, CT-based radiomics has already been tested as a potential preoperative prognostic biomarker (65). CT-based radiomics prior to treatment could provide important information on the response rate to preoperative chemotherapy, improving patient selection for multimodal treatment of GC (66-69).
Conclusions
An effective histopathological evaluation method of TRG with an independent prognostic role is urgently needed in clinical practice. A 4-tiered system for grading the regression in the primary tumor, combined with a 3-tiered system for the metastatic lymph nodes achieved a good consensus among experienced pathologists. Diffusion-weighted MRI seems to be one of the most promising imaging techniques for prediction of TRG. As future perspectives, determination of EBV, microsatellites, and/or MMR statuses on the endoscopic biopsies, as well as radiomics features on the CT scan at the time of diagnosis could help to predict the response rate to preoperative therapy and tailor the best approach for each patient with GC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Multidisciplinary Treatment in Gastrointestinal Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-34/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-34/coif). The series “Multidisciplinary Treatment in Gastrointestinal Cancer” was commissioned by the editorial office without any funding or sponsorship. PM served as the unpaid Guest Editor of the series and serves an unpaid editorial board member of Digestive Medicine Research from October 2021 to September 2023. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Al‐Batran S‐E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:4004. [Crossref]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19-23. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg 2005;242:684-92. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg 2005;241:810-7; discussion 817-20. [Crossref] [PubMed]
- Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol 2013;3:262. [Crossref] [PubMed]
- Damjanov I, O’Neil M. Histopathology of colorectal cancer after neoadjuvant chemoradiation therapy. Open Pathol J 2009;3:91-8. [Crossref]
- Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 2004;28:215-23. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Ninomiya Y, Yanagisawa A, Kato Y, et al. Histological indications of a favorable prognosis with far-advanced gastric carcinomas after preoperative chemotherapy. J Cancer Res Clin Oncol 1999;125:699-706. [Crossref] [PubMed]
- Wheeler JM, Warren BF, Mortensen NJ, et al. Quantification of histologic regression of rectal cancer after irradiation: a proposal for a modified staging system. Dis Colon Rectum 2002;45:1051-6. [Crossref] [PubMed]
- Hornick JL, Farraye FA, Odze RD. Prevalence and significance of prominent mucin pools in the esophagus post neoadjuvant chemoradiotherapy for Barrett's-associated adenocarcinoma. Am J Surg Pathol 2006;30:28-35. [Crossref] [PubMed]
- Langer R, Ott K, Feith M, et al. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol 2009;22:1555-63. [Crossref] [PubMed]
- Sannier A, Lefèvre JH, Panis Y, et al. Pathological prognostic factors in locally advanced rectal carcinoma after neoadjuvant radiochemotherapy: analysis of 113 cases. Histopathology 2014;65:623-30. [Crossref] [PubMed]
- Fernández-Aceñero MJ, Estrada Muñoz L, Sastre Varela J, et al. Prognostic influence of histopathological regression patterns in rectal adenocarcinoma receiving neoadjuvant therapy. J Gastrointest Oncol 2017;8:49-54. [Crossref] [PubMed]
- Hav M, Libbrecht L, Geboes K, et al. Prognostic value of tumor shrinkage versus fragmentation following radiochemotherapy and surgery for rectal cancer. Virchows Arch 2015;466:517-23. [Crossref] [PubMed]
- Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141-6. [Crossref] [PubMed]
- Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011;253:934-9. [Crossref] [PubMed]
- Smyth EC, Fassan M, Cunningham D, et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol 2016;34:2721-7. [Crossref] [PubMed]
- Tsekrekos A, Detlefsen S, Riddell R, et al. Histopathologic tumor regression grading in patients with gastric carcinoma submitted to neoadjuvant treatment: results of a Delphi survey. Hum Pathol 2019;84:26-34. [Crossref] [PubMed]
- Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: A meta-analysis of 17 published studies. Eur J Surg Oncol 2017;43:1607-16. [Crossref] [PubMed]
- Zorcolo L, Rosman AS, Restivo A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 2012;19:2822-32. [Crossref] [PubMed]
- Koh YW, Park YS, Ryu MH, et al. Postoperative nodal status and diffuse-type histology are independent prognostic factors in resectable advanced gastric carcinomas after preoperative chemotherapy. Am J Surg Pathol 2013;37:1022-9. [Crossref] [PubMed]
- Derieux S, Svrcek M, Manela S, et al. Evaluation of the prognostic impact of pathologic response to preoperative chemotherapy using Mandard's Tumor Regression Grade (TRG) in gastric adenocarcinoma. Dig Liver Dis 2020;52:107-14. [Crossref] [PubMed]
- Mansour JC, Tang L, Shah M, et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol 2007;14:3412-8. [Crossref] [PubMed]
- Blackham AU, Greenleaf E, Yamamoto M, et al. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J Surg Oncol 2016;114:434-9. [Crossref] [PubMed]
- Fujitani K, Mano M, Hirao M, et al. Posttherapy nodal status, not graded histologic response, predicts survival after neoadjuvant chemotherapy for advanced gastric cancer. Ann Surg Oncol 2012;19:1936-43. [Crossref] [PubMed]
- Becker K, Reim D, Novotny A, et al. Proposal for a multifactorial prognostic score that accurately classifies 3 groups of gastric carcinoma patients with different outcomes after neoadjuvant chemotherapy and surgery. Ann Surg 2012;256:1002-7. [Crossref] [PubMed]
- Messager M, Lefevre JH, Pichot-Delahaye V, et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 2011;254:684-93; discussion 693. [Crossref] [PubMed]
- Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653-60. [Crossref] [PubMed]
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32:2983-90. [Crossref] [PubMed]
- Tong Y, Liu D, Zhang J. Connection and distinction of tumor regression grading systems of gastrointestinal cancer. Pathol Res Pract 2020;216:153073. [Crossref] [PubMed]
- Pereira MA, Ramos MFKP, Dias AR, et al. Lymph node regression after neoadjuvant chemotherapy: A predictor of survival in gastric cancer. J Surg Oncol 2020;121:795-803. [PubMed]
- Gaca JG, Petersen RP, Peterson BL, et al. Pathologic nodal status predicts disease-free survival after neoadjuvant chemoradiation for gastroesophageal junction carcinoma. Ann Surg Oncol 2006;13:340-6. [Crossref] [PubMed]
- Xu X, Zheng G, Zhang T, et al. Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response. Cancer Chemother Pharmacol 2019;84:635-46. [Crossref] [PubMed]
- Lombardi PM, Mazzola M, Achilli P, et al. Prognostic value of pathological tumor regression grade in locally advanced gastric cancer: New perspectives from a single-center experience. J Surg Oncol 2021;123:923-31. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Kurokawa Y, Shibata T, Sasako M, et al. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer 2014;17:514-21. [Crossref] [PubMed]
- Liu K, Li G, Fan C, et al. Adapted Choi response criteria for prediction of clinical outcome in locally advanced gastric cancer patients following preoperative chemotherapy. Acta Radiol 2012;53:127-34. [Crossref] [PubMed]
- Mazzei MA, Bagnacci G, Gentili F, et al. Gastric Cancer Maximum Tumour Diameter Reduction Rate at CT Examination as a Radiological Index for Predicting Histopathological Regression after Neoadjuvant Treatment: A Multicentre GIRCG Study. Gastroenterol Res Pract 2018;2018:1794524. [Crossref] [PubMed]
- Ang J, Hu L, Huang PT, et al. Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy. World J Gastroenterol 2012;18:7026-32. [Crossref] [PubMed]
- Lorenzen S, Blank S, Lordick F, et al. Prediction of response and prognosis by a score including only pretherapeutic parameters in 410 neoadjuvant treated gastric cancer patients. Ann Surg Oncol 2012;19:2119-27. [Crossref] [PubMed]
- Heger U, Bader F, Lordick F, et al. Interim endoscopy results during neoadjuvant therapy for gastric cancer correlate with histopathological response and prognosis. Gastric Cancer 2014;17:478-88. [Crossref] [PubMed]
- Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 2003;21:4604-10. [Crossref] [PubMed]
- Ott K, Herrmann K, Lordick F, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res 2008;14:2012-8. [Crossref] [PubMed]
- Won E, Shah MA, Schöder H, et al. Use of positron emission tomography scan response to guide treatment change for locally advanced gastric cancer: the Memorial Sloan Kettering Cancer Center experience. J Gastrointest Oncol 2016;7:506-14. [Crossref] [PubMed]
- Ott K, Herrmann K, Schuster T, et al. Molecular imaging of proliferation and glucose utilization: utility for monitoring response and prognosis after neoadjuvant therapy in locally advanced gastric cancer. Ann Surg Oncol 2011;18:3316-23. [Crossref] [PubMed]
- Vallböhmer D, Hölscher AH, Schneider PM, et al. [18F]-fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J Surg Oncol 2010;102:135-40. [Crossref] [PubMed]
- Morgagni P, Bencivenga M, Colciago E, et al. Limited Usefulness of 18F-FDG PET/CT in Predicting Tumor Regression After Preoperative Chemotherapy for Noncardia Gastric Cancer: The Italian Research Group for Gastric Cancer (GIRCG) Experience. Clin Nucl Med 2020;45:177-81. [Crossref] [PubMed]
- Giganti F, De Cobelli F, Canevari C, et al. Response to chemotherapy in gastric adenocarcinoma with diffusion-weighted MRI and (18) F-FDG-PET/CT: correlation of apparent diffusion coefficient and partial volume corrected standardized uptake value with histological tumor regression grade. J Magn Reson Imaging 2014;40:1147-57. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197-203. [Crossref] [PubMed]
- Petrillo A, Pompella L, Tirino G, et al. Perioperative Treatment in Resectable Gastric Cancer: Current Perspectives and Future Directions. Cancers (Basel) 2019;11:399. [Crossref] [PubMed]
- Kohlruss M, Grosser B, Krenauer M, et al. Prognostic implication of molecular subtypes and response to neoadjuvant chemotherapy in 760 gastric carcinomas: role of Epstein-Barr virus infection and high- and low-microsatellite instability. J Pathol Clin Res 2019;5:227-39. [Crossref] [PubMed]
- De Cecco CN, Ganeshan B, Ciolina M, et al. Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 2015;50:239-45. [Crossref] [PubMed]
- Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol 2016;61:R150-66. [Crossref] [PubMed]
- Ferrari R, Mancini-Terracciano C, Voena C, et al. MR-based artificial intelligence model to assess response to therapy in locally advanced rectal cancer. Eur J Radiol 2019;118:1-9. [Crossref] [PubMed]
- Giganti F, Antunes S, Salerno A, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol 2017;27:1831-9. [Crossref] [PubMed]
- Giganti F, Marra P, Ambrosi A, et al. Pre-treatment MDCT-based texture analysis for therapy response prediction in gastric cancer: Comparison with tumour regression grade at final histology. Eur J Radiol 2017;90:129-37. [Crossref] [PubMed]
- Li Z, Zhang D, Dai Y, et al. Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: A pilot study. Chin J Cancer Res 2018;30:406-14. [Crossref] [PubMed]
- Mazzei MA, Di Giacomo L, Bagnacci G, et al. Delta-radiomics and response to neoadjuvant treatment in locally advanced gastric cancer-a multicenter study of GIRCG (Italian Research Group for Gastric Cancer). Quant Imaging Med Surg 2021;11:2376-87. [Crossref] [PubMed]
- Garbarino GM, Zerunian M, Berardi E, et al. Perioperative Chemotherapy with FLOT Scheme in Resectable Gastric Adenocarcinoma: A Preliminary Correlation between TRG and Radiomics. Applied Sciences 2021;11:9211. [Crossref]
Cite this article as: Garbarino GM, Mainardi F, Berardi E, Zerunian M, Polici M, Campanelli M, Lisi G, Laracca GG, Pecoraro A, Costa G, Caruso D, Laghi A, Mazzuca F, Pilozzi E, Mercantini P. Tumor regression grade (TRG) for gastric cancer and radiological methods on predicting response to perioperative chemotherapy: a narrative review. Dig Med Res 2023;6:2.


