
Roles of adipose-derived stem cells in cell-based therapy: current status and future scope—a narrative review
Introduction
Stem cell-based regenerative medicine is a new therapeutic approach for repair or replacement of tissues and organs that have been depleted or damaged by genetic, traumatic, or age-related degenerative disorders by creating functional tissues or improving patients’ quality of life (QOL) and appearance (1,2). The therapeutic rationale is based on the idea that stem cells have the potential to replace damaged or dead cells with newly differentiated progeny, making themselves components of tissue reconstruction (cell replacement therapy) (3,4), and on their ability to contribute to tissue repair and regeneration by autocrine and paracrine actions through secretion of growth factors, cytokines, extrecellular matrix (ECM) molecules, and exosomes (cell supportive therapy) (5,6). Biological functions may be improved by stem cells by direct transplantation in vivo into host tissue or if combined with a therapeutic gene or biomaterial to generate tissue ex vivo (7-10). For clinical application, stem cells should meet several criteria and are generally expected to be abundant, minimally invasive to the patient, and have strong potential to proliferate and differentiate into multiple cell types (11,12). From this perspective, adipose-derived stem cells (ADSCs), a type of mesenchymal stem cell (MSC) isolated from adipose tissue, are an ideal source of cells for treatment of many diseases (11-14). In this review, the current challenges for use of ADSCs in regenerative medicine are discussed, with the goal of understanding directions for clinical applications in transplantation, gene therapy, and tissue engineering. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-32/rc).
Methods
To explicate recent developments and progress in ADSCs in cell-based therapy, we performed a thorough review of the literature published from March 1968 to May 2022 by independent searches using the MEDLINE database in NIH National Library of Medicine PubMed (Table 1). This publicly available and institutionally accessed database was used to search indexed and published articles. Editorials, technical reports and expert opinions were excluded from the literature search. Studies presented at international meetings and conferences, but not published in standard journals, were also excluded.
Table 1
| Items | Specification |
|---|---|
| Date of search | 02 June, 2022 |
| Databases and other sources searched | NIH National Library of Medicine PubMed, MEDLINE database |
| Search terms used | Adipose; Fat; Stem cells; Mesenchymal stem cells; Clinical application |
| Timeframe | March 1968 to May 2022 |
| Inclusion and exclusion criteria | English only |
Properties of multipotency and self-renewal of ADSCs
ADSCs are mesoderm-derived adult stem cells that commonly express markers of a mesenchymal phenotype, such as CD73, CD90, and CD105), but are negative for markers such as CD31, CD45, and HLA-DR; however, a single cell surface marker that uniquely identifies an ADSC has not been found (15,16). ADSCs have strong self-renewal ability based on results from colony forming unit formation (CFU-F) assays (17), and are multipotent, as indicated by their differentiation into various mesenchymal cell lineages, including fat, bone, cartilage, and muscle (11-13,15) (Figure 1). It is also of note that ASCs are responsive to inducers of differentiation of non-mesenchymal cell lineages, and can differentiate into desired cells beyond the germline (Figure 2) (11-17).
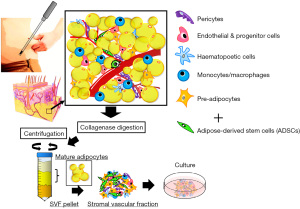
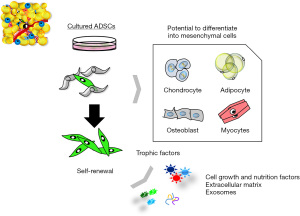
Mechanisms of cell therapy using ADSCs
Behavior and fate of transplanted ADSCs at the site of regeneration
There are several possible mechanisms underlying promotion of tissue regeneration by ADSCs. Some studies have suggested that ADSCs act through differentiation into specific cell types, thereby replacing defective cell populations in vivo (cell replacement therapy). However, most studies in animal models, in which ADSCs can be tracked in vivo, have failed to show this behavior using high-quality cell tracking methods, except for the classic mesenchymal phenotype of differentiation into adipocytes, osteoblasts, and chondrocytes (18). Most studies have shown that differentiation of MSCs may lead to “intermediate two phenotype cells” that co-express certain cellular markers, but without evidence of acquisition of actual functionality (19). Another possibility is that cell fusion is the primary mechanism for forming new functional cells (20), but Aurich et al. found no fusion of host hepatocytes with transplanted ADSCs that differentiated into hepatocytes (21). We have used the Cre-loxP-mediated fate tracking system to visualize in vivo survival of transplanted ADSCs and to investigate differentiation into Schwann lineage cells at a peripheral nerve injury site. We found that transplanted ADSCs did not differentiate into Schwann cells (SCs), but did promote peripheral nerve regeneration at the injured site (Figure 3) (22).
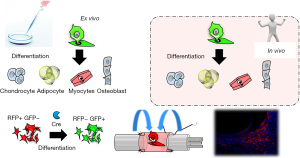
ADSCs may also contribute to tissue regeneration through regulation of effects in the host stem cell niche, such as promotion of activation of endogenous stem cells at the site of injury, as a source of free radical scavengers that isolate and remove toxic substances and allow surviving cells to recover their functions (23). Thus, at present, it is widely believed that ADSCs promote cell regeneration in tissues and organs mainly through release of cytokines, growth factors and free radical scavengers (18).
Paracrine and immunomodulatory properties of ASCs
ADSCs promote tissue regeneration by secreting cytokines and growth and nutrient factors that restore normal tissue function or reduce tissue damage (24,25). Among the cytokines produced by ADSCs, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), granulocyte/macrophage colony-stimulating factor, hypoxia-inducible factor 1α (HIF1α), hepatocyte growth factor (HGF), insulin-like growth factor (IGF-1), platelet-derived growth factor-BB (PDGF-BB), and fibroblast growth factor (FGF2) have crucial roles in formation of tissue structures due to their angiogenic properties and ability to induce tissue neovascularization (26,27). Anti-apoptotic factors such as IGF-1 also protect cardiomyocytes from apoptosis (28). ADSCs also have neuroprotective effects by promoting regeneration of peripheral and central nervous system cells through secretion of brain-derived neurotrophic factor, glial-derived neurotrophic factor, nerve growth factor, and IGF (24). We have shown that undifferentiated ADSCs release neurotrophic factors to a similar extent to that of SCs and other glial cells, and have an effect of elongation of nerve axons, which suggests that ADSCs promote peripheral nerve regeneration partly through paracrine secretion of trophic factors and regardless of donor age or anatomic site of origin (29).
Immunomodulatory properties of ASCs
Evidence for the immunomodulatory properties of ADSCs has accumulated from in vitro and in vivo studies (30,31). ADSCs inhibit the inflammatory process in wound healing by inducing and activating quiescent native MSCs or other support cells at the site of local injury to secrete anti-inflammatory proteins (32). For instance, ADSCs inhibit production of pro-inflammatory cytokines, promote production of anti-inflammatory cytokines, and induce formation of antigen-specific regulatory T cells (33). In addition, control of the polarity of M1/M2 macrophages by ADSCs can promote wound healing (34,35). These cells also protect against organ rejection and prevent graft-versus-host disease after allogeneic stem cell transplantation (36). However, the antiapoptotic and immunomodulatory properties of ADSCs in their use in immune tolerance are still an unexplored area and further studies are needed.
Potential use of ADSCs in gene therapy
Gene therapy is an innovative method that has the potential for introducing genes into genetically defective host cells, which may produce unprecedented clinical benefits in incurable diseases. Using replication-defective retroviruses, adeno-associated vectors and lentiviruses, genes can be introduced into the DNA of recipient cells to change their traits and produce new functions (37,38). Gene transfer can produce cells that can generate therapeutic proteins in vivo and this can be regulated to an extent. Combination of stem cells, which are highly proliferative and viable, with a gene transfer system has increased the therapeutic potential of gene therapy (39-42), and ADSCs are also gaining prominence as a stem cell source for this purpose (43).
ADSC exosomes
Exosomes are extracellular vesicles, microvesicles, and apoptotic bodies of diameter 40–150 nm that are produced by eukaryotic cells and can be isolated from a cell culture medium by ultracentrifugation or precipitation with polyethylene glycol. Exosomes contain proteins, lipids, cytokines, and nucleic acids, including DNA, microRNAs, lncRNA, circRNA, and other non-coding RNAs, and are associated with immunomodulation, mediation of cell survival, proliferation, migration, division, apoptosis, and physiological homeostasis (44,45).
Exosome research has expanded rapidly, with an increased number of publications on the function and application of MSC-derived exosomes (MSC-EXOs) as potential cell-free therapeutics. ADSC exosomes (ADSC-EXOs) contain important paracrine components that are released from ADSCs and have various biological activities. A “vital network”, in which growth factors, proteases, progenitor cells, and immune cells producing proinflammatory cytokines work together, is thought to be required for tissue regeneration (46). ADSC-EXOs play important roles in these networks as intercellular messengers. Furthermore, by encapsulation of bioactive substances, ADSC-EXOs can be used for multiple tissue regeneration processes, including mechanical repair for cell survival, migration, proliferation, and promotion of neovascularization. ADSC-EXOs have a good biosafety profile with low immunogenicity, and differ from other MSC-EXOs in promoting proliferation, differentiation and immunosuppressive pathways in target cells (47,48).
ADSC-EXOs have similar functions to those of ADSCs, including promotion of repair of heart muscle, kidney, urethral, liver, skeletal muscle, and other tissues and organs (49-52). Practically, it has been shown that ADSC-EXOs induce proliferation and migration of vascular endothelial cells and angiogenesis (53,54). ADSC-EXOs can also promote various types of wound healing and are being developed as agents for treating diabetic skin ulcers and improving the fat grafting rate for soft tissue defects. These cells also have influential roles as a carrier and combined scaffold for treatment, leading to scarless cutaneous repair (55). Several studies have examined the detailed mechanism of promotion of wound healing. Thus, Choi et al. showed that ADSCs-EXOs seem to induce enrichment of fibroblast microRNAs within the fibroblasts that contribute to healing (56). In a murine wound model, Wang et al. suggested that intravenous injection of ADSCs-EXOs resulted in reduced scar size and changes in metalloproteinases that may improve healing (57). Ren et al. found that ADSC-derived microvesicles stimulate proliferation and migration of fibroblasts, keratinocytes, and endothelial cells in vitro and in vivo, mainly via the AKT and ERK signaling pathways (58), and ADSC-EXOs have also been shown to promote proliferation and migration and inhibit neuronal cell apoptosis by activating the PI3K/AKT pathway (59).
The roles of ADSC-EXOs have been examined in tissue regeneration in plastic and cosmetic surgery, such as improvement of dermatitis, scar removal, bone tissue repair and regeneration, obesity, fat grafting, breast reconstruction, and anti-aging treatment of skin (46). Elucidation of the biological properties of ADSC-EXOs is likely to lead to new strategies for tissue regeneration in these fields. Compared with cell-based therapy, ADSC-EXOs have high stability and are easily stored. In addition, they are controllable and less prone to rejection and unexpected homing to organs, which may permit development of clinical applications of non-autologous ADSC-EXOs. Thus, future use of various forms of ADSC-EXOs is likely to become more common for wounds, reconstructive surgery, and aesthetic treatment.
Three current strategies for regenerative medicine
The characteristics and utilization of ADSCs described above suggest three therapeutic strategies for regenerative medicine: (I) transplantation, in which autologous ADSCs are grown in vitro and transplanted directly into the host tissue, or first combined with biomaterials and then transplanted into the host; (II) cell supportive therapy, using culture supernatants or molecular factories such as exosomes; and (III) gene/cell therapy, in which autologous ADSCs are grown in vitro, transduced with viral vectors carrying therapeutic genes, and then implanted into the host tissue (Figure 4).
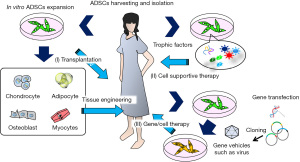
Application of regenerative therapy for wound healing using ADSCs
Skin ulcer
In wounds of the skin and soft tissues, there is damage to the structure and homeostasis of the tissues due to trauma or physical or chemical effects such as heat or radiation (60,61). Wound healing requires a series of molecular events that manifest as inflammation, neovascularization, formation of scar tissue, and tissue remodeling, and these events are strongly regulated by growth factors such as transforming growth factor-beta, FGF, VEGF, and PDGF (62,63). In many cases, wound repair is preceded by scar formation, which promotes collagenous ECM deposition and sometimes recruitment of collagen-secreting fibroblasts, with angiogenesis required for these activities (64). ADSCs have well-known angiogenic effects, including in promotion of wound healing in intractable ulcers caused by ischemia in diseases such as diabetes (65-67), and can also control inflammation at the wound site by altering macrophage polarization (68,69).
Several preclinical studies of wound healing have reported beneficial effects of cell-based therapy using ADSCs (70-73). Hamada et al. demonstrated that transplantation of human ADSC (hADSC) sheets combined with artificial skin in rat increased blood vessel density and dermal thickness, and found that this accelerated wound healing compared with that in controls due to secretion of angiogenic growth factors by the hADSC sheets (70). In immunohistochemical analysis, there was more frequent neovascularization in xenografted rats in the transplantation group, and the transplanted hADSCs were localized to the periphery of new blood vessels. This xenograft model may be useful for identification of factors produced by human cell tissue-based products in use of these products to accelerate wound healing. The same research group also demonstrated that an artificial dermis can maintain autogenetic ADSCs, which can promote vascularization capacity and enhance wound healing in a wound with exposed bone (71). In an excisional wound healing model in rat, Nie et al. showed that ADSCs secrete pro-angiogenic mediators (e.g., VEGF-A, HGF, and FGF) that promote neovascularization and re-epithelial regeneration of wounds, which accelerates wound repair (72). A recent study showed that combination therapy of photobiomodulation and ADSCs is an effective and promising treatment by stimulating skin injury repair and modulating the inflammatory response in an MRSA-infected wound model in rats with diabetes mellitus type 1 (73). Taken together, these findings suggest that ADSCs have considerable potential as therapy to promote wound healing.
Cell therapy for radiation-induced skin damage
Radiation therapy has been replaced by tumor resection as the current mainstay for prevention of cancer growth, recurrence, and metastasis. Recurrence is reduced in a radiation dose-dependent manner, but there is a serious problem of deterministic effects in surrounding healthy tissue. In particular, the skin, subcutaneous tissues, and muscles are affected by radiation exposure under certain circumstances (74,75). Specific adverse events include fibrosis, atrophy, minor damage to small blood vessels (ischemia), and dermal thickening (75-77). These radiation-induced injuries cause many clinical manifestations in the skin and subcutis, including radiation dermatitis, scar contracture, lymphedema, and refractory wound healing. In addition, the elasticity and extensibility of the skin are impaired, skin appendages and hair follicles are lost, and joint movement is restricted (77). Impaired tissue regeneration/reconstruction and wound healing after radiation therapy can cause tissue dysfunction and decompensation, and may lead to more serious conditions such as intractable skin ulcers and osteomyelitis many years after radiation exposure (77,78).
Progression of skin disorders has been related to adipose tissue under the skin or ADSCs in this tissue. In vitro studies have shown that ADSCs exposed to radiation are damaged, senescent, and lose their proliferative and differentiation potential (79). Adipose tissue beneath the skin has a function of reducing skin damage from radiation, but this tissue itself remodels and atrophies due to exposure (80). Thus, adipose tissue and its component ADSCs are damaged by exposure and cannot adequately support skin regeneration, further worsening the condition. This suggests that promotion of tissue normalization may be achieved by replenishing the deficient ADSCs.
Cell therapy has been proposed by the International Atomic Energy Agency (IAEA 2009) as treatment for radiation-induced skin damage (81). The efficacy of products derived from adipose tissue containing ADSCs for radiation skin disorder has been shown in preclinical studies (82-85). In a rat model of chronic radiation wounds, Huang et al. showed that ADSCs produced neovascularization at the ulcer surface after transplantation (83). In regenerative medicine, selective use of cell populations from ADSCs is particularly effective, with Borrelli et al. reporting that CD74+ cells, which are known to have antifibrotic effects, ameliorate radiation skin impairment in vitro and in vivo (84). Among adipose-derived stromal cells, it has also been proven that the CD34+CD146+ population promotes angiogenesis and wound healing (85).
Radiation-induced qualitative and pathological damage of tissues can be partially recovered by fat grafting, with this effect thought to be derived from MSCs and vascular endothelial cells contained in the grafted tissue. In an acute radiation dermatitis model in the back skin of mice irradiated at 45 Gy, Sultan et al. showed that fat grafting suppressed inflammation and slowed progression of fibrosis (86). Radiation causes significant skin thinning, depleted blood vessels, and increased scar formation, and fat grafting increased the skin thickness, collagen content, and vascular density in irradiated skin (87,88). Qualitative changes in tissues, including blood circulation, and partial improvement of extensibility and healing ability also occur. Similar effects are obtained with micronized fat adipose transfer, using fractured stromal tissue with mature adipocytes removed (89). These findings suggest that adipose tissue utilizes its various cells and components for different purposes.
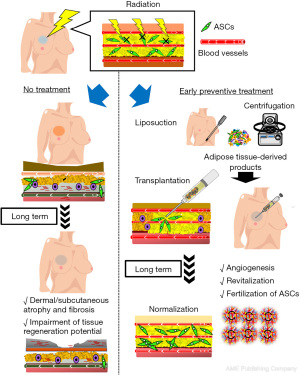
Fractionated radiotherapy was developed to maximize therapeutic efficacy and reduce deterministic effects on healthy tissues that result in long-term tissue damage (78). Yoshimura and colleagues applied the commonly practiced fractionated irradiation protocol to nude mice and found that radiation damage was dose-dependent, with mice exposed to a total dose of ≥15 Gy developing spontaneous skin ulcers. Injection of suctioned fat tissue or micronized tissue made the ulcer heal faster than in untreated controls, and these therapeutic effects were comparable to those of cultured ADSCs. More recently, our group showed that prophylactic administration of products containing ADSCs immediately after radiotherapy prevents development of long-term functional disorders in irradiated tissues. These findings could have a substantial impact on anticancer radiotherapy; thus, next-generation radiotherapy may need to be combined with stem cell therapy. Such prophylactic treatment has the potential to improve wound healing of irradiated tissue and the clinical outcomes of reconstructive surgery after radiotherapy (Figure 5) (90).
Peripheral nerve regeneration
Successful nerve regeneration requires a high level of coordinated interactions between the cells involved in regeneration, extracellular matrix components, and nerve growth factors. The essential point of peripheral nerve regeneration therapy is to restore peripheral sensory and motor functions as soon as possible after injury (91-93). With the development of stem cell biology, the dream of cell therapy through relocation of effective cells to the site of nerve damage at the right time and place and in the required amounts is gradually turning into reality. Many cell types are involved in maintenance and regeneration of the peripheral nervous system, but Schwann cells (SCs) play the most pivotal roles (93-95), and there is also no alternative to SCs for the unique function of myelinization. Thus, treatment of peripheral nerve injuries has focused on using SCs as cell-based therapy. However, isolating SCs has significant drawbacks in clinical application, including the main disadvantage of sacrificing a functional nerve. Therefore, there is a need for technological advances in methods to acquire SCs, including lineage-specific differentiation and reprogramming to generate SCs from various cell types (96,97). Among many options, ADSCs are promising as alternatives due to easily accessible harvesting in adequate amounts through a minimally invasive procedure. For instance, rat ADSCs can be induced into SC-like cells (SCLCs) (22,98,99), and this method can be applied to human ADSCs. However, strong evidence that SCLCs have the same functional effects as genuine SCs, such as myelin protein formation, has yet to be obtained (100).
More recently, the idea that ADSCs do not necessarily need to be differentiated into SCLCs has been proposed for clinical application (22,101,102). In fact, the neuroregenerative effects of undifferentiated ADSCs (uADSCs) have been confirmed in many studies, including by our group (13,101-103). It is gradually becoming understood that uADSCs secrete trophic factors and cytokines, and application of these functions to nerve disorder therapy is regarded as cell supportive therapy (13,103,104). We found that uADSCs produce and release abundant factors that promote peripheral nerve regeneration (BDNF, NGF, VEGF, GGF, IGF, HGF), and that these ADSC-derived factors promote SC survival and proliferation, as well as dorsal root ganglion cell survival and protrusion. These effects were comparable to those of astrocytes and SCs (103). Moreover, the ability of uADSCs to produce and release factors related to peripheral nerve regeneration was maintained regardless of the site of collection or age of the ADSCs. These results suggest that uADSCs release peripheral nerve regeneration-related factors in vitro to a similar extent to that of dADSCs. Furthermore, in 2015, we used Cre/Floxed-reporter mice to track and mark neural crest-derived ADSCs, and found that a portion of ADSCs comprised neural crest cell-derived cells, after which we examined the biological properties of each cell population (29). These results suggest that uADSCs may already contain a population of cells with specific neuroregenerative properties.
In 2017, our research group transplanted ADSCs and SCs with gelatin hydrogel tubes at an artificially blunted sciatic nerve lesion in mice. We used Cre-loxP-mediated fate tracking to visualize survival in vivo of transplanted ADSCs and to investigate whether they differentiated into a SC lineage at the peripheral nerve injury site. The ADSCs were found to promote regeneration of axons, formation of myelin, and restoration of denervation muscle atrophy to levels comparable to those achieved by SC transplantation. The ADSCs survived for at least four weeks after transplantation, without differentiating into SCs (105). In this way, it was proven that cell supportive therapy contributes to peripheral nerve regeneration by functioning as a molecular factory that produces and releases nerve regeneration-related factors. The scope of application of ADSCs for nerve regeneration medicine may extend beyond peripheral nerve damage, diabetes-induced neuropathy, and neurodegenerative diseases to spinal cord and brain damage (105-109). There are still many problems to be solved, such as oncological risk and the time and cost required for cell preparation, but future development of cell-based regenerative therapy using ADSCs is very likely to lead to novel clinical applications in peripheral nerve repair therapies.
Ischemic diseases
ADSCs are a type of MSC that may differentiate into fat, cartilage, bone, and smooth muscle after transplantation, but the evidence for this process is less than satisfactory. Differentiation into other cell types is a matter of further debate. Accordingly, the primary mechanism of the function of ADSCs in therapy for internal organs, blood vessels, skin, and nerves seem to be a paracrine effect (110,111). However, the therapeutic value of the ADSC secretome is limited without long-term cell retention and engraftment after transplantation. Therefore, various strategies have been proposed, including genetic, pharmacological, physiological, physical, and cytokine preconditioning, and tissue engineering, to modulate and stimulate ADSCs to release specific trophic factors (112-116). There are several methods that promote therapeutic paracrine and immunomodulatory properties, but optimal preconditioning and the exact triggering and regulatory mechanisms remain elusive, and the functional proteins and RNAs in the secretome are yet to be completely identified (111,113,117). Also, it is still an open question whether paracrine and immunomodulatory therapy alone is sufficient for cardiac repair. Therefore, further studies are needed to determine these pivotal mechanisms and to establish better therapeutic approaches.
Periodontal tissue regeneration
Bacterial biofilms formed on dental root surfaces occasionally cause periodontitis, which irreversibly destroys periodontal tissues such as alveolar bone, root cementum, periodontal ligament, and gingiva. As a result, oral functions such as mastication and occlusion are impaired, ultimately forcing the tooth to be removed (118). Normal periodontitis treatment primarily consists of coping therapies such as biofilm removal and debridement of necrotic root tissue. However, these treatments are often insufficient for patients with severe periodontitis. Curative treatment of periodontal disease requires regeneration of destroyed periodontal tissues such as alveolar bone, cementum, and periodontal ligament. Therefore, as a clinical approach for patients with severe periodontitis, development of therapies to induce periodontal tissue regeneration using MSCs is currently under investigation (119). MSC transplants isolated from the pulp or periodontal ligament have been investigated in preclinical and clinical trials, and their therapeutic efficacy has been confirmed (120,121). However, these treatments require wisdom teeth that can be extracted, and these teeth must be unaffected by decay or periodontitis, which greatly limits the number of patients. As a replacement, ADSCs have been shown to have therapeutic efficacy comparable to that of pulp stem cells (122-125). This new treatment method is expected to promote periodontal tissue regeneration with various growth factors secreted from ADSCs, and also to regenerate new blood vessels in damaged tissues.
Sawada and colleagues showed that autologous transplantation of ADSCs is effective for periodontal tissue regeneration in an experimental periodontitis model in beagles, and found that factors released from ADSCs promote periodontal ligament cell differentiation (123). Requicha et al. demonstrated that the proposed double-layer scaffold supports proliferation and selectively promotes osteogenic differentiation of ADSCs seeded onto functionalized mesh, which means the 3D structure and asymmetric composition of the scaffold in combination with ADSCs may provide an alternative therapy to treat periodontal defects more efficiently (124). Takedachi et al. conducted a single-arm, exploratory Phase I clinical trial of the therapeutic effect of ADSCs in 12 patients with periodontal disease and found that the depth of periodontal pockets was reduced and alveolar bone regeneration was induced. The next challenge is that, unlike other diseases, in which a single administration of MSCs is sufficient, several surgeries are needed for periodontal tissue regeneration, and countermeasures against adverse effects are required. Therefore, it will be desirable to study the use of allogeneic ADSCs in the future (125).
Treatment of internal organ failure and infection
ADSCs have been proven in many studies to have immunomodulatory, proangiogenic, neurotrophic, and epithelization activities, and can potentially be used for neurodegenerative, cardiovascular, respiratory, inflammatory and autoimmune diseases, as well as wound healing. More recently, the indication has been further expanded into new areas, such as treatment of internal organ failure, including the liver and lungs, and related pneumonia. Hepatocyte dysfunction often leads to intractable liver diseases, and orthotopic liver transplantation is currently the only treatment available for patients with end-stage liver disease. However, this method is limited due to the invasiveness associated with the need for donor organs. Hepatocyte transplantation may be an alternative to full liver transplantation for liver failure, and recent studies have suggested that stem cell-derived hepatocytes could be used as therapeutic liver cells (126-128). Some studies have examined the possibility of therapeutic application of liver fibrosis using the antifibrotic effect of undifferentiated ADSCs before they are induced to differentiate (129-131).
COVID-19 is a viral pneumonia that is currently having a huge impact worldwide. This disease has the potential to cause multiple organ damage and there is currently no specific treatment or drug available. However, several positive findings for the application of stem cells as treatment for COVID-19 have now been reported (132-134) and clinical trials have been started to investigate the safety and efficacy of stem cell or stem cell-derived exosome transplantation therapy for patients with COVID-19. Indications under investigation are COVID-19-related diseases such as severe pneumonia, respiratory failure, ARDS, and pulmonary fibrosis (135). Adipose tissue has yielded the most promising results among use of stromal vascular fraction (SVF), placental cells, natural killer cells and platelet lysates (134,136,137), and aerosol inhalation and intravenous routes are being investigated as methods of administration (138). Thus, ADSCs, SVF, and other adipose tissue-derived products, including micronized fat tissue, are likely to become more recognized as a relatively safe way to suppress immune responses in severe COVID-19-related diseases associated with cytokine storm and other conditions (135-137). First, however, it is important to have a standardized and universal protocol for administration and a detailed mechanistic understanding of the effects of these therapies. In this regard, there is a particular need for studies with a high level of evidence, especially prospective randomized controlled trials that include ethical considerations.
Future challenges in clinical application of ADSCs
The emergence of ADSC therapy provides a novel means for tissue regeneration. Numerous clinical and preclinical studies have shown the vital role of ADSCs in reconstructing and repairing target organs, such as bone, cartilage, myocardium, liver, nervous system, and skin. ADSCs are MSCs that may differentiate into fat, cartilage, bone, and smooth muscle after transplantation, but there is limited evidence for this behavior. Differentiation into other cell types is also in question. A paracrine mechanism seems to underlie the function of ADSCs in therapy for internal organs, blood vessels, skin, and nerves. However, the therapeutic value of trophic factors derived from ADSCs is limited without long-term cell retention and engraftment after transplantation. Strategies including genetic, pharmacological, cellular and tissue engineering, use of biomaterials, and cytokine preconditioning have been proposed to modulate and stimulate ADSCs to release the secretome for a certain period. These methods enhance the therapeutic value of the secretome, but optimal preconditioning and the triggering and regulatory mechanisms are uncertain, and the functional proteins and RNAs in the secretome are not fully understood. It is also unclear if secretome therapy using ADSCs alone is sufficient. Therefore, further studies are needed to evaluate these mechanisms and develop better therapeutic approaches. Additionally, many safety issues need to be addressed, from the preparation of ADSCs to their application, and additional studies are required to identify appropriate scaffolds and potent bioactive factors to induce an optimal microenvironment for ADSC proliferation and differentiation. Long-term studies are needed to confirm implant-tissue interactions, resorption and hierarchical structure, and finally to produce a clinically viable method. Due to differences between preclinical studies and clinical trials, the oncogenicity of ADSC differentiation also warrants further research. Despite the current challenges, the remarkable pace of progress in this field suggests that ADSC-based approaches will play increasingly important roles in regenerative medicine.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tevfik Tolga Sahin; Egemen Cicek and Basri Satilmis) for the series “Therapeutic Efficacy of Adipose Tissue Derived Mesenchymal Stem Cells in Diseases and Surgery of Gastrointestinal Tract” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-32/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-32/coif). The series “Therapeutic Efficacy of Adipose Tissue Derived Mesenchymal Stem Cells in Diseases and Surgery of Gastrointestinal Tract” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bacakova L, Zarubova J, Travnickova M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv 2018;36:1111-26. [Crossref] [PubMed]
- Martino S, D'Angelo F, Armentano I, et al. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol Adv 2012;30:338-51. [Crossref] [PubMed]
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol 2011;12:79-89. [Crossref] [PubMed]
- Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res 2014;163:399-408. [Crossref] [PubMed]
- Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med 2010;5:121-43. [Crossref] [PubMed]
- De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 2012;12:574-91. [Crossref] [PubMed]
- Nguyen PK, Rhee JW, Wu JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol 2016;1:831-41. [Crossref] [PubMed]
- Mathiasen AB, Kastrup J. Non-invasive in-vivo imaging of stem cells after transplantation in cardiovascular tissue. Theranostics 2013;3:561-72. [Crossref] [PubMed]
- Morena F, Argentati C, Calzoni E, et al. Ex-Vivo Tissues Engineering Modeling for Reconstructive Surgery Using Human Adult Adipose Stem Cells and Polymeric Nanostructured Matrix. Nanomaterials (Basel) 2016;6:57. [Crossref] [PubMed]
- Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013;341:1233158. [Crossref] [PubMed]
- Barba M, Di Taranto G, Lattanzi W. Adipose-derived stem cell therapies for bone regeneration. Expert Opin Biol Ther 2017;17:677-89. [Crossref] [PubMed]
- Nordberg RC, Loboa EG. Our Fat Future: Translating Adipose Stem Cell Therapy. Stem Cells Transl Med 2015;4:974-9. [Crossref] [PubMed]
- Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012;30:804-10. [Crossref] [PubMed]
- Kolaparthy LK, Sanivarapu S, Moogla S, et al. Adipose Tissue - Adequate, Accessible Regenerative Material. Int J Stem Cells 2015;8:121-7. [Crossref] [PubMed]
- Bateman ME, Strong AL, Gimble JM, et al. Concise Review: Using Fat to Fight Disease: A Systematic Review of Nonhomologous Adipose-Derived Stromal/Stem Cell Therapies. Stem Cells 2018;36:1311-28. [Crossref] [PubMed]
- Feisst V, Meidinger S, Locke MB. From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning 2015;8:149-62. [PubMed]
- Yang HJ, Kim KJ, Kim MK, et al. The stem cell potential and multipotency of human adipose tissue-derived stem cells vary by cell donor and are different from those of other types of stem cells. Cells Tissues Organs 2014;199:373-83. [Crossref] [PubMed]
- Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: an update on the transition to clinical translation. Regen Med 2012;7:225-35. [Crossref] [PubMed]
- Rose RA, Jiang H, Wang X, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells 2008;26:2884-92. [Crossref] [PubMed]
- Utsunomiya T, Shimada M, Imura S, et al. Human adipose-derived stem cells: potential clinical applications in surgery. Surg Today 2011;41:18-23. [Crossref] [PubMed]
- Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 2009;58:570-81. [Crossref] [PubMed]
- Sowa Y, Kishida T, Imura T, et al. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast Reconstr Surg 2016;137:318e-30e. [Crossref] [PubMed]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;6:230-47. [Crossref] [PubMed]
- Salgado AJ, Reis RL, Sousa NJ, et al. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 2010;5:103-10. [Crossref] [PubMed]
- Dubey NK, Mishra VK, Dubey R, et al. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int J Mol Sci 2018;19:2200. [Crossref] [PubMed]
- Gir P, Oni G, Brown SA, et al. Human adipose stem cells: current clinical applications. Plast Reconstr Surg 2012;129:1277-90. [Crossref] [PubMed]
- Sterodimas A, de Faria J, Nicaretta B, et al. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg 2010;63:1886-92. [Crossref] [PubMed]
- Sadat S, Gehmert S, Song YH, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun 2007;363:674-9. [Crossref] [PubMed]
- Sowa Y, Imura T, Numajiri T, et al. Adipose stromal cells contain phenotypically distinct adipogenic progenitors derived from neural crest. PLoS One 2013;8:e84206. [Crossref] [PubMed]
- Baer PC. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells 2014;6:256-65. [Crossref] [PubMed]
- Nagaya R, Mizuno-Kamiya M, Takayama E, et al. Mechanisms of the immunosuppressive effects of mouse adipose tissue-derived mesenchymal stromal cells on mouse alloreactively stimulated spleen cells. Exp Ther Med 2014;7:17-22. [Crossref] [PubMed]
- Caplan AI. MSCs: The Sentinel and Safe-Guards of Injury. J Cell Physiol 2016;231:1413-6. [Crossref] [PubMed]
- Gonzalez-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 2010;69:241-8. [Crossref] [PubMed]
- Liu J, Qiu P, Qin J, et al. Allogeneic adipose-derived stem cells promote ischemic muscle repair by inducing M2 macrophage polarization via the HIF-1α/IL-10 pathway. Stem Cells 2020;38:1307-20. [Crossref] [PubMed]
- Yu S, Cheng Y, Zhang L, et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther 2019;10:333. [Crossref] [PubMed]
- Yañez R, Lamana ML, García-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 2006;24:2582-91. [Crossref] [PubMed]
- Naldini L. Ex vivo gene transfer and correction for cell-based therapies. Nat Rev Genet 2011;12:301-15. [Crossref] [PubMed]
- Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med 2001;7:33-40. [Crossref] [PubMed]
- Alenzi FQ, Lotfy M, Tamimi WG, et al. Review: Stem cells and gene therapy. Lab Hematol 2010;16:53-73. [Crossref] [PubMed]
- Altanerova U, Jakubechova J, Benejova K, et al. Prodrug suicide gene therapy for cancer targeted intracellular by mesenchymal stem cell exosomes. Int J Cancer 2019;144:897-908. [Crossref] [PubMed]
- Rostami M, Haidari K, Shahbazi M. Genetically Engineered Adipose Mesenchymal Stem Cells Using HIV-Based Lentiviral Vectors as Gene Therapy for Autoimmune Diseases. Cell Reprogram 2018;20:337-46. [Crossref] [PubMed]
- Choi SA, Lee JY, Wang KC, et al. Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. Eur J Cancer 2012;48:129-37. [Crossref] [PubMed]
- Barranco C. Stem cells: Mesenchymal stem cells from adipose tissue could be used to deliver gene therapy to the liver. Nat Rev Gastroenterol Hepatol 2011;8:64. [Crossref] [PubMed]
- Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009;21:575-81. [Crossref] [PubMed]
- Hong P, Yang H, Wu Y, et al. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther 2019;10:242. [Crossref] [PubMed]
- Xiong M, Zhang Q, Hu W, et al. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front Cell Dev Biol 2020;8:574223. [Crossref] [PubMed]
- Wu J, Yang Q, Wu S, et al. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng Regen Med 2021;18:685-91. [Crossref] [PubMed]
- Liu Y, Wang C, Wei M, et al. Multifaceted Roles of Adipose Tissue-Derived Exosomes in Physiological and Pathological Conditions. Front Physiol 2021;12:669429. [Crossref] [PubMed]
- Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant 2019;54:789-92. [Crossref] [PubMed]
- Wang L, Cheng W, Zhu J, et al. Electrospun nanoyarn and exosomes of adipose-derived stem cells for urethral regeneration: Evaluations in vitro and in vivo. Colloids Surf B Biointerfaces 2022;209:112218. [Crossref] [PubMed]
- Byun SE, Sim C, Chung Y, et al. Skeletal Muscle Regeneration by the Exosomes of Adipose Tissue-Derived Mesenchymal Stem Cells. Curr Issues Mol Biol 2021;43:1473-88. [Crossref] [PubMed]
- Mou S, Zhou M, Li Y, et al. Extracellular Vesicles from Human Adipose-Derived Stem Cells for the Improvement of Angiogenesis and Fat-Grafting Application. Plast Reconstr Surg 2019;144:869-80. [Crossref] [PubMed]
- Wu SC, Kuo PJ, Rau CS, et al. Increased Angiogenesis by Exosomes Secreted by Adipose-Derived Stem Cells upon Lipopolysaccharide Stimulation. Int J Mol Sci 2021;22:8877. [Crossref] [PubMed]
- Han Y, Ren J, Bai Y, et al. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol 2019;109:59-68. [Crossref] [PubMed]
- Ogawa R, Tanaka C, Sato M, et al. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun 2010;398:723-9. [Crossref] [PubMed]
- Choi HS, Kim HJ, Oh JH, et al. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson's disease. Neurobiol Aging 2015;36:2885-92. [Crossref] [PubMed]
- Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep 2017;7:13321. [Crossref] [PubMed]
- Ren S, Chen J, Duscher D, et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther 2019;10:47. [Crossref] [PubMed]
- Xie Y, Chen Y, Zhu Y, et al. Adipose Mesenchymal Stem Cell-Derived Exosomes Enhance PC12 Cell Function through the Activation of the PI3K/AKT Pathway. Stem Cells Int 2021;2021:2229477. [Crossref] [PubMed]
- Devalia HL, Mansfield L. Radiotherapy and wound healing. Int Wound J 2008;5:40-4. [Crossref] [PubMed]
- Fry DE. Pressure Irrigation of Surgical Incisions and Traumatic Wounds. Surg Infect (Larchmt) 2017;18:424-30. [Crossref] [PubMed]
- Grazul-Bilska AT, Johnson ML, Bilski JJ, et al. Wound healing: the role of growth factors. Drugs Today (Barc) 2003;39:787-800. [Crossref] [PubMed]
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008;453:314-21. [Crossref] [PubMed]
- Jones VM, Suarez-Martinez AD, Hodges NA, et al. A clinical perspective on adipose-derived cell therapy for enhancing microvascular health and function: Implications and applications for reconstructive surgery. Microcirculation 2021;28:e12672. [Crossref] [PubMed]
- Inoue O, Usui S, Takashima SI, et al. Diabetes impairs the angiogenic capacity of human adipose-derived stem cells by reducing the CD271+ subpopulation in adipose tissue. Biochem Biophys Res Commun 2019;517:369-75. [Crossref] [PubMed]
- Fromer MW, Chang S, Hagaman ALR, et al. The endothelial cell secretome as a novel treatment to prime adipose-derived stem cells for improved wound healing in diabetes. J Vasc Surg 2018;68:234-44. [Crossref] [PubMed]
- Kim SM, Kim YH, Jun YJ, et al. The effect of diabetes on the wound healing potential of adipose-tissue derived stem cells. Int Wound J 2016;13:33-41. [Crossref] [PubMed]
- Xu M, Fang S, Xie A. Posttranscriptional control of PLOD1 in adipose-derived stem cells regulates scar formation through altering macrophage polarization. Ann Transl Med 2021;9:1573. [Crossref] [PubMed]
- Zomer HD, Jeremias TDS, Ratner B, et al. Mesenchymal stromal cells from dermal and adipose tissues induce macrophage polarization to a pro-repair phenotype and improve skin wound healing. Cytotherapy 2020;22:247-60. [Crossref] [PubMed]
- Hamada M, Iwata T, Kato Y, et al. Xenogeneic transplantation of human adipose-derived stem cell sheets accelerate angiogenesis and the healing of skin wounds in a Zucker Diabetic Fatty rat model of obese diabetes. Regen Ther 2017;6:65-73. [Crossref] [PubMed]
- Hamada T, Matsubara H, Yoshida Y, et al. Autologous adipose-derived stem cell transplantation enhances healing of wound with exposed bone in a rat model. PLoS One 2019;14:e0214106. [Crossref] [PubMed]
- Nie C, Yang D, Xu J, et al. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 2011;20:205-16. [Crossref] [PubMed]
- Ebrahimpour-Malekshah R, Amini A, Zare F, et al. Combined therapy of photobiomodulation and adipose-derived stem cells synergistically improve healing in an ischemic, infected and delayed healing wound model in rats with type 1 diabetes mellitus. BMJ Open Diabetes Res Care 2020;8:e001033. [Crossref] [PubMed]
- Spałek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol 2016;9:473-82. [Crossref] [PubMed]
- Bray FN, Simmons BJ, Wolfson AH, et al. Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatol Ther (Heidelb) 2016;6:185-206. [Crossref] [PubMed]
- Kim JH, Kolozsvary AJ, Jenrow KA, et al. Mechanisms of radiation-induced skin injury and implications for future clinical trials. Int J Radiat Biol 2013;89:311-8. [Crossref] [PubMed]
- Zawaski JA, Yates CR, Miller DD, et al. Radiation combined injury models to study the effects of interventions and wound biomechanics. Radiat Res 2014;182:640-52. [Crossref] [PubMed]
- Jagetia GC, Rajanikant GK. Acceleration of wound repair by curcumin in the excision wound of mice exposed to different doses of fractionated γ radiation. Int Wound J 2012;9:76-92. [Crossref] [PubMed]
- Jeong W, Yang X, Lee J, et al. Serial changes in the proliferation and differentiation of adipose-derived stem cells after ionizing radiation. Stem Cell Res Ther 2016;7:117. [Crossref] [PubMed]
- Xiao Y, Mo W, Jia H, et al. Ionizing radiation induces cutaneous lipid remolding and skin adipocytes confer protection against radiation-induced skin injury. J Dermatol Sci 2020;97:152-60. [Crossref] [PubMed]
- Galvez-Martín P, Martin JM, Ruiz AM, et al. Encapsulation in Cell Therapy: Methodologies, Materials, and Clinical Applications. Curr Pharm Biotechnol 2017;18:365-77. [Crossref] [PubMed]
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006;99:1285-97. [Crossref] [PubMed]
- Huang SP, Huang CH, Shyu JF, et al. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J Biomed Sci 2013;20:51. [Crossref] [PubMed]
- Borrelli MR, Patel RA, Adem S, et al. The antifibrotic adipose-derived stromal cell: Grafted fat enriched with CD74+ adipose-derived stromal cells reduces chronic radiation-induced skin fibrosis. Stem Cells Transl Med 2020;9:1401-13. [Crossref] [PubMed]
- Deleon NMD, Adem S, Lavin CV, et al. Angiogenic CD34+CD146+ adipose-derived stromal cells augment recovery of soft tissue after radiotherapy. J Tissue Eng Regen Med 2021;15:1105-17. [Crossref] [PubMed]
- Sultan SM, Stern CS, Allen RJ Jr, et al. Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg 2011;128:363-72. [Crossref] [PubMed]
- Dassoulas KR, Wang JS, Stovall MM, et al. A Novel Small-Animal Model of Irradiated, Implant-Based Breast Reconstruction. Plast Reconstr Surg 2018;141:1416-25. [Crossref] [PubMed]
- Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plast Reconstr Surg 2014;134:249-57. [Crossref] [PubMed]
- Feng J, Doi K, Kuno S, et al. Micronized cellular adipose matrix as a therapeutic injectable for diabetic ulcer. Regen Med 2015;10:699-708. [Crossref] [PubMed]
- Sowa Y, Inafuku N, Kishida T, et al. Prophylactic application of human adipose tissue-derived products to prevent radiation disorders. Plast Reconstr Surg 2022; In Press.
- Kouyoumdjian JA. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve 2006;34:785-8. [Crossref] [PubMed]
- Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials Microsurgery 2010;30:574-88. review. [Crossref] [PubMed]
- Xu Y, Zhang Z, Chen X, et al. A Silk Fibroin/Collagen Nerve Scaffold Seeded with a Co-Culture of Schwann Cells and Adipose-Derived Stem Cells for Sciatic Nerve Regeneration. PLoS One 2016;11:e0147184. [Crossref] [PubMed]
- Torigoe K, Tanaka HF, Takahashi A, et al. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp Neurol 1996;137:301-8. [Crossref] [PubMed]
- Mosahebi A, Woodward B, Wiberg M, et al. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia 2001;34:8-17. [Crossref] [PubMed]
- Sowa Y, Kishida T, Tomita K, et al. Direct Conversion of Human Fibroblasts into Schwann Cells that Facilitate Regeneration of Injured Peripheral Nerve In Vivo. Stem Cells Transl Med 2017;6:1207-16. [Crossref] [PubMed]
- Kitada M, Murakami T, Wakao S, et al. Direct conversion of adult human skin fibroblasts into functional Schwann cells that achieve robust recovery of the severed peripheral nerve in rats. Glia 2019;67:950-66. [Crossref] [PubMed]
- Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 2007;207:267-74. [Crossref] [PubMed]
- di Summa PG, Kingham PJ, Raffoul W, et al. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 2010;63:1544-52. [Crossref] [PubMed]
- Faroni A, Smith RJ, Lu L, et al. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur J Neurosci 2016;43:417-30. [Crossref] [PubMed]
- Zhang R, Rosen JM. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen Res 2018;13:757-63. [Crossref] [PubMed]
- Erba P, Mantovani C, Kalbermatten DF, et al. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg 2010;63:e811-7. [Crossref] [PubMed]
- Sowa Y, Imura T, Numajiri T, et al. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev 2012;21:1852-62. [Crossref] [PubMed]
- Tomita K, Madura T, Sakai Y, et al. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience 2013;236:55-65. [Crossref] [PubMed]
- Rafiei Alavi SN, Madani Neishaboori A, Hossein H, et al. Efficacy of adipose tissue-derived stem cells in locomotion recovery after spinal cord injury: a systematic review and meta-analysis on animal studies. Syst Rev 2021;10:213. [Crossref] [PubMed]
- Ryu S, Lee JM, Bae CA, et al. Therapeutic efficacy of neuregulin 1-expressing human adipose-derived mesenchymal stem cells for ischemic stroke. PLoS One 2019;14:e0222587. [Crossref] [PubMed]
- Amini N, Vousooghi N, Alizade A, et al. Transplantation of Adipose Tissue-Derived Stem Cells into Brain Through Cerebrospinal Fluid in Rat Models: Protocol Development and Initial Outcome Data. Curr Stem Cell Res Ther 2019;14:191-5. [Crossref] [PubMed]
- Zhao K, Li R, Bi S, et al. Combination of mild therapeutic hypothermia and adipose-derived stem cells for ischemic brain injury. Neural Regen Res 2018;13:1759-70. [Crossref] [PubMed]
- Kappy NS, Chang S, Harris WM, et al. Human adipose-derived stem cell treatment modulates cellular protection in both in vitro and in vivo traumatic brain injury models. J Trauma Acute Care Surg 2018;84:745-51. [Crossref] [PubMed]
- Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther 2017;8:125. [Crossref] [PubMed]
- Li X, Ma T, Sun J, et al. Harnessing the secretome of adipose-derived stem cells in the treatment of ischemic heart diseases. Stem Cell Res Ther 2019;10:196. [Crossref] [PubMed]
- Song SH, Lee MO, Lee JS, et al. Genetic modification of human adipose-derived stem cells for promoting wound healing. J Dermatol Sci 2012;66:98-107. [Crossref] [PubMed]
- Shevchenko EK, Makarevich PI, Tsokolaeva ZI, et al. Transplantation of modified human adipose derived stromal cells expressing VEGF165 results in more efficient angiogenic response in ischemic skeletal muscle. J Transl Med 2013;11:138. [Crossref] [PubMed]
- Deveza L, Choi J, Imanbayev G, et al. Paracrine release from nonviral engineered adipose-derived stem cells promotes endothelial cell survival and migration in vitro. Stem Cells Dev 2013;22:483-91. [Crossref] [PubMed]
- Lee TJ, Shim MS, Yu T, et al. Bioreducible Polymer Micelles Based on Acid-Degradable Poly(ethylene glycol)-poly(amino ketal) Enhance the Stromal Cell-Derived Factor-1α Gene Transfection Efficacy and Therapeutic Angiogenesis of Human Adipose-Derived Stem Cells. Int J Mol Sci 2018;19:529. [Crossref] [PubMed]
- Xu Y, Shi T, Xu A, et al. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J Cell Mol Med 2016;20:1203-13. [Crossref] [PubMed]
- Luo Q, Guo D, Liu G, et al. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol Biochem 2017;44:2105-16. [Crossref] [PubMed]
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [Crossref] [PubMed]
- Novello S, Debouche A, Philippe M, et al. Clinical application of mesenchymal stem cells in periodontal regeneration: A systematic review and meta-analysis. J Periodontal Res 2020;55:1-12. [Crossref] [PubMed]
- Tassi SA, Sergio NZ, Misawa MYO, et al. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J Periodontal Res 2017;52:793-812. [Crossref] [PubMed]
- Ferrarotti F, Romano F, Gamba MN, et al. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J Clin Periodontol 2018;45:841-50. [Crossref] [PubMed]
- Dziedzic DSM, Mogharbel BF, Ferreira PE, et al. Transplantation of Adipose-derived Cells for Periodontal Regeneration: A Systematic Review. Curr Stem Cell Res Ther 2019;14:504-18. [Crossref] [PubMed]
- Sawada K, Takedachi M, Yamamoto S, et al. Trophic factors from adipose tissue-derived multi-lineage progenitor cells promote cytodifferentiation of periodontal ligament cells. Biochem Biophys Res Commun 2015;464:299-305. [Crossref] [PubMed]
- Requicha JF, Viegas CA, Muñoz F, et al. A tissue engineering approach for periodontal regeneration based on a biodegradable double-layer scaffold and adipose-derived stem cells. Tissue Eng Part A 2014;20:2483-92. [Crossref] [PubMed]
- Takedachi M, Sawada K, Sakura K, et al. Periodontal tissue regeneration by transplantation of autologous adipose tissue-derived multi-lineage progenitor cells. Sci Rep 2022;12:8126. [Crossref] [PubMed]
- Taléns-Visconti R, Bonora A, Jover R, et al. Human mesenchymal stem cells from adipose tissue: Differentiation into hepatic lineage. Toxicol In Vitro 2007;21:324-9. [Crossref] [PubMed]
- Lee JH, Lee KH, Kim MH, et al. Possibility of undifferentiated human thigh adipose stem cells differentiating into functional hepatocytes. Arch Plast Surg 2012;39:593-9. [Crossref] [PubMed]
- Saito Y, Ikemoto T, Tokuda K, et al. Effective three-dimensional culture of hepatocyte-like cells generated from human adipose-derived mesenchymal stem cells. J Hepatobiliary Pancreat Sci 2021;28:705-15. [Crossref] [PubMed]
- Yang N, Ma W, Ke Y, et al. Transplantation of adipose-derived stem cells ameliorates Echinococcus multilocularis-induced liver fibrosis in mice. PLoS Negl Trop Dis 2022;16:e0010175. [Crossref] [PubMed]
- De Luna-Saldivar MM, Marino-Martinez IA, Franco-Molina MA, et al. Advantages of adipose tissue stem cells over CD34+ mobilization to decrease hepatic fibrosis in Wistar rats. Ann Hepatol 2019;18:620-6. [Crossref] [PubMed]
- Han HS, Lee H, You D, et al. Human adipose stem cell-derived extracellular nanovesicles for treatment of chronic liver fibrosis. J Control Release 2020;320:328-36. [Crossref] [PubMed]
- Choudhery MS, Harris DT. Stem cell therapy for COVID-19: Possibilities and challenges. Cell Biol Int 2020;44:2182-91. [Crossref] [PubMed]
- Gupta A, Kashte S, Gupta M, et al. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Hum Cell 2020;33:907-18. [Crossref] [PubMed]
- Jeyaraman M, Ranjan R, Kumar R, et al. Cellular Therapy: Shafts of Light Emerging for COVID-19. Stem Cell Investig 2020;7:11. [Crossref] [PubMed]
- Li Z, Niu S, Guo B, et al. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif 2020;53:e12939. [Crossref] [PubMed]
- Mazini L, Ezzoubi M, Malka G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: flow chart and regulation updates before and after COVID-19. Stem Cell Res Ther 2021;12:1. [Crossref] [PubMed]
- Gentile P, Sterodimas A. Adipose Stem Cells (ASCs) and Stromal Vascular Fraction (SVF) as a Potential Therapy in Combating (COVID-19)-Disease. Aging Dis 2020;11:465-9. [Crossref] [PubMed]
- Copcu HE. Three states of stromal cells-solid, liquid, and aerosol-and innovative delivery methods not previously reported. Arch Plast Surg 2021;48:549-52. [Crossref] [PubMed]
Cite this article as: Sowa Y, Mazda O, Tsuge I, Inafuku N, Kishida T, Morimoto N. Roles of adipose-derived stem cells in cell-based therapy: current status and future scope—a narrative review. Dig Med Res 2022;5:57.


