
Physiology of pain—a narrative review on the pain pathway and its application in the pain management
Introduction
Pain is a vital physiological function that has evolved to alert the individual to the presence of noxious stimuli, minimising the risk of tissue damage and protecting against ongoing tissue injury. However, uncontrolled or inappropriate pain may have negative and far-reaching consequences. For instance, in the case of persistent intractable pain, individuals may adopt avoidance behaviour, leading to detrimental changes in mobility, mood and social function. Pain has long been recognised to cause significant socioeconomical burden. In the recent Global Burden of Disease [2019] study, pain related conditions such as chronic back pain, headache disorder, depressive order and road traffic accidents were all identified within the top 10 causes for disabilities in both 10–24 years and 25–49 years groups globally (1).
In major surgeries, poorly controlled postoperative pain is associated with an array of adverse outcomes. It increases the risk of wound infections, delays wound healing and increases the risk of cardiovascular and pulmonary complications (2). Furthermore, acute post-surgical pain can go on to become chronic pain (3,4). A multicentre observational study from 2015 examined pain following 18 different surgical procedures and found that severe pain on postoperative day 1 correlated positively with the development of chronic post-surgical pain (CPSP) at 12 months post-surgery. A 10% increase in the time spent in severe pain on postoperative day 1 was associated with a 30% increase in the incidence of CPSP at 12 months (3).
CPSP is associated with impaired physical function and reduced quality of life scores. This in turn leads to an increase in morbidity and mortality, is detrimental to patient experience and increases the burden on healthcare and social systems (2,4-6). For these reasons, optimal control of perioperative pain is a fundamental part of any successful enhanced recovery programme (7).
An appreciation of the basic physiological mechanisms via which pain is sensed, transmitted and processed is essential for understanding how to correctly assess, diagnose and manage it. The physiology of pain remain to be focus of current research and clinical studies which in turn provide us with better understandings of the pain physiology. This review article will provide an updated overview of the physiology of pain which is fundamental to the management of acute and chronic pain. Understanding the physiology has allowed the development of a variety of pharmacological and non-pharmacological analgesic strategies and ‘multimodal analgesia’—that targets pain via multiple different parts of the pain pathway—has become the standard of practice in perioperative care. As research in this area improves our knowledge of the physiology, we can provide patients with better pain management and prevention of CPSP with novel agents and interventions. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-100/rc).
Methods
We performed a literature search on PubMed and Google Scholar using the terms ‘pain physiology’, ‘acute pain’, ‘chronic pain’, ‘chronic post-surgical pain’, ‘nociception’, ‘peripheral and central sensitisation’, ‘descending modulation pathway’, ‘pain management’ and ‘multimodal analgesia’. We included any studies, review articles and editorials published in English from 1st January 2000 to 1st December 2021. Due to the large number of articles being published on the subject, review articles that were published in well-established journals in the field were prioritised. All papers reviewed and their key references are crossed checked with additional reference to ensure a balanced and high quality literature review on the subjects. We also referenced the ‘physiology of pain’ chapter from the textbook—Fundamentals of anaesthesia (3rd ed.) and the International Association for the Study of Pain (IASP) website, both are recognised as the ‘gold standard’ in educating clinicians in the study of ‘Pain’. They provide reliable basic scientific foundations and the etymology of pain (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 01/09/2021 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used (including MeSH and free text search terms and filters) | ‘pain physiology’, ‘acute pain’, ‘chronic pain’, ‘chronic post-surgical pain’, ‘nociception’, ‘peripheral and central sensitisation’, ‘descending modulation pathway’, ‘pain management’ and ‘multimodal analgesia’ |
| Timeframe | 01/01/2000–01/12/2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Studies, review articles and editorials |
| English only | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Author 1 (S Liu) conducted the selection process independently. Articles from peer reviewed journal, most recently published, most related to the key words searched and higher number of citations are prioritized selected |
| Any additional considerations, if applicable | Key references also taken from text book—Fundamentals of anaesthesia (3rd ed.) chapter—‘Physiology of pain’ and the IASP. They are selected for being widely accepted as reliable information source by clinicians |
IASP, International Association for the Study of Pain.
Discussion
Classification of pain
There are many ways of classifying pain. Examples include by duration, aetiology or anatomical location (8).
Duration
Pain is commonly described as acute or chronic pain. Acute pain is pain provoked by tissue damage and serves as a warning sign of an ongoing noxious process such as a medical condition or injury (e.g., surgical trauma). Acute pain resolves following treatment of the underlying cause and healing of the damaged tissue. Chronic pain is defined as pain that persists or recurs for longer than three months, and it persists beyond the period of time normally expected for healing and recovery from tissue injury (9). Chronic pain is debilitating and commonly associated with depression, social and behaviour dysfunction, it often continues to deteriorate over years if left untreated (4). Among surgical patients, it is paramount to reduce the risks of developing chronic pain in susceptible patients with a strategic surgical, anaesthetic and recovery plan.
Aetiology
Pain may also be classified as physiological or pathological. Physiological pain occurs in the absence of actual tissue or nerve damage, serving as a warning sign for impending injury. Examples include muscle cramp and abdominal colic.
In pathological pain, tissue or nerve damage occurs. The activation of the pain system is commonly accompanied by its sensitisation. This can be regarded as a form of positive feedback control, in contrast to the more usual negative feedback control that maintains many of our physiological systems in homoeostasis. Sensitisation is recognised as occurring both peripherally at nociceptor level and centrally in the dorsal horn of the spinal cord. Peripheral sensitisation involves an increase in the responsiveness and reduction in activation threshold of peripheral nociceptive neurons (10). Central sensitization is the increased responsiveness of the nociceptive neurons in the central nervous system. The pathophysiology of both peripheral and central sensitisations will be detailed later in this article. Sensitisation results in the clinical phenomena of hyperalgesia and allodynia. Hyperalgesia describes increased pain on supra-threshold stimulation (10). Allodynia is the misperception of pain to a stimulus that does not normally provoke pain (10). This is caused either by a faulty connection between the non-nociceptive and nociceptive pathways or by an exaggerated response from particular neurons in the spinal cord that in normal conditions are dormant [wide dynamic range (WDR) neurons in the dorsal horn lamina V]. Aβ fibres that transduce non-nociceptive stimuli, such as light touch, usually synapse in the dorsal horn at laminae III and IV; in individuals with allodynia, they have also been shown to synapse with nociceptive neurons in lamina II. The spinal cord was therefore believed to be a key area in the aetiology of allodynia, however, further studies have revealed that faulty wiring can occur anywhere from the peripheral to the central nervous system. Indeed the phenomenon of allodynia occurring in patients following a thalamic stroke provides evidence that the aetiology can locate as high as cerebellum (11).
Location
Pain may also be classified as either somatic or visceral (or a combination of both) depending on the anatomical nature of the tissue involved. Somatic pain from joints, muscle or skin is usually well localised. Visceral pain from internal organs is often poorly localised and diffuse due to inputs from multiple spinal segments and the convergence of afferents from neighbouring organs (viscerovisceral convergence). In addition, visceral pain is often associated with referred pain (e.g., shoulder-tip pain following irritation of the diaphragm) as result of the convergence of visceral and somatic afferents. It is also associated with autonomic symptoms as a result of visceral afferents traversing autonomic plexuses at the spinal level (8).
Pain pathways
Nociception and peripheral sensitisation
The primary afferent neurons involved in the detection and transmission of pain are referred to as nociceptors. They have two main functions:
- The detection and encoding of noxious stimuli and the subsequent signal transduction from the periphery to the central nervous system.
- Synthetic function—nociceptors synthesise and release neuropeptides [e.g., substance P, calcitonin gene-related peptide (CGRP)], neurotrophins (molecules that modulate nociceptor gene expression), neurotransmitters (e.g., glutamate) and inflammatory mediators. All of these modulate and facilitate the transduction of noxious stimuli.
Nociceptors’ cell bodies are located in the dorsal root ganglia and they synapse with second-order neurons within the dorsal horn of the spinal cord. The exception to this is the trigeminal nerve (which carries nociception from face and head) whose cell bodies are located outside of the spinal cord, in the trigeminal ganglion, and which synapses with the central nervous system at the nucleus caudalis in the medulla.
Nociceptors are unspecialised free nerve endings that transduce local ‘noxious’ information into afferent action potentials via cell membranous ion channels. Two types of ion channels are important in the generation and magnitude of an action potential: transient receptor potential (TRP) and voltage-gated ion channels (8). Following injury, local tissue homeostasis is interrupted. Damaged tissue, activated mast cells, neutrophils and macrophages all release inflammatory mediators such as hydrogen ions (protons), sodium ions, serotonin, cytokines, bradykinin, histamine, prostaglandins and leukotrienes. These activate and sensitize local TRP channels on nociceptors. The TRP channels may also be directly activated by pressure and heat. Once activated they transduce the physical or chemical stimuli into transient action potentials via modulation of ion entry (Ca2+, Mg2+) into the neuron. These transient action potentials in turn activate the voltage-gated ion channels and thus initiate a cascade which leads to the formation of a supra-threshold action potential. Different voltage-gated channels open at various different thresholds of membrane potential allowing a noxious stimulus to be encoded into action potentials with a rate that varies according to the intensity of the stimulus.
TRP channels play an important role in the development of hyperalgesia as they can be sensitized by inflammatory mediators (12). Specific TRP channels such as TRP ankyrin 1 (TRPA1) have been identified as potential therapeutic targets in recent analgesia research owing to their role in the mediation of prolonged hypersensitivity and their expression in glial cells (non-neuronal support cells found throughout the peripheral and central nervous system) which play a critical role in sustaining chronic pain (13).
Furthermore, the local inflammatory response may be amplified by ‘neurogenic inflammation’ which is orchestrated by the synthesis and release of a variety of pro-inflammatory mediators such as substance P, CGRP, neurokinin A and nitric oxide from the nociceptors (12). These act on other ‘silent’ C fibres to lower their activation threshold and increase the excitability of their neurones. This results in peripheral sensitization. The inflammatory response can also expand systemically, which in turn activates the sympathetic system leading to the release of catecholamines such as noradrenaline. Noradrenaline itself can activate nociceptors setting in place a positive feedback loop. In summary, the pain pathway is initiated with nociceptor activation; the subsequent triggering of inflammatory, neural and endocrine systems can potentiate and modulate the response with via a plethora of complex biochemical changes (14). The early control of the local inflammatory response is paramount in preventing peripheral sensitization (12). In patients with more challenging analgesic needs it may be necessary to target the inflammatory, nervous and endocrine systems simultaneously.
There are two types of primary nociceptive afferent: Aδ and C fibres. Their cell bodies are located in the dorsal root ganglion situated in intervertebral foramina and they synapse with second order neurons (also termed dorsal horn neurons) in the dorsal horn of spinal cord. The Aδ axons are myelinated and consequently transmit signals at relatively high speed (15–55 m/s), they are associated with mechanosensitive nociceptors (high threshold, activation by mechanical stimuli only) or mechanothermal nociceptors (polymodal, activation by either mechanical or heat stimuli or both) (8). The Aδ fibres evoke sharp fast-response pain of a burning, pricking, stabbing and aching nature. C fibres are unmyelinated and therefore slower, conducting at about 2 m/s. They are associated with polymodal nociceptors (PMN) that respond to a variety of stimuli: chemical, thermal and mechanical. They evoke slow-response burning pain and dull aching pain.
Stimulation of nociceptors is not the only route via which perception of pain may be initiated. In neuropathic pain, a neural pathology arising anywhere from the peripheral to central nervous system can be responsible for the activation of the pain pathway. Neuropathic pain is typically perceived as a burning or shooting pain and is often chronic and difficult to treat.
Dorsal horn neurons and central sensitisation
The grey matter of the spinal cord was divided into ten separate laminae by Rexed in 1952. Laminae I to VI are located within the dorsal horn and the majority of nociceptors synapse in laminae I, II and V where they form primary synapses with either interneurons or dorsal horn neurons. The majority of C fibres terminate in lamina II which is also known as substantia gelatinosa (SG). Dorsal horn neurons, so-termed because their nuclei are located in the dorsal horn of the spinal cord, are second-order sensory neurons that transmit nociception from the spinal cord to higher centres in brain.
Nociceptor afferents may also synapse with interneurons, which in turn synapse with dorsal horn neurons. Interneurons are present exclusively in the central nervous system and can be either excitatory or inhibitory. A dorsal horn neuron may be excited or inhibited depending on the type of interneuron that it configures with. This primary synapse with interneuron is central to the mechanism for modulating the sensitivity of the pain system. Excitatory interneurons release glutamate, the chief excitatory neurotransmitter, which binds with a variety of receptors including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA) and G-protein-coupled metabotropic receptors. Inhibitory interneurons release γ-aminobutyric acid (GABA) and glycine as neurotransmitters. GABA binds to inotropic GABAA receptor (ligand-gated receptor) or G-protein-coupled metabotropic GABAB receptors (receptor). Glycine binds to inotropic glycine receptors. The glycine receptors are closely related to GABAA receptors, as they both open ion channels selectively permeable to the anion chloride which hyperpolarise the neuronal cell membranes. Inhibitory interneurons can be stimulated by primary afferents, the descending modulation system or Aβ fibres.
In addition, higher centres may influence pain transmissions at dorsal horn via descending modulatory systems. This modulatory input can have either inhibitory—via reducing transmission at the primary synapse; or excitatory effects—via facilitating transmission at the primary synapse and leading to central sensitisation.
With repeated or prolonged nociceptive stimulation, central sensitisation occurs at the spinal cord. This is an important process in the development and maintenance of chronic pain with the nervous system going through a process called ‘wind-up’ resulting in a persistent state of pain hypersensitivity (15). The inactive NMDA receptor is normally tightly bound to Magnesium plug, however, continuous nociceptive stimulation leads to prolonged depolarisation of dorsal horn neurons and the subsequent influx of calcium ions remove the magnesium plug. The NMDA receptor is then available for glutamate binding, hence the dorsal horn neuron produces progressively greater output with lower excitatory threshold (12). This is further facilitated by the increased release of excitatory neurotransmitter glutamate through nociceptor sensitisation. Following central sensitisation, the previously dormant WDR neurons in lamina V become active (8). Activation of postsynaptic NMDA receptors on the WDR neurons induce and potentiate the neuronal response to each stimulus. Glial cells (non-neuronal cells that synthesize myeline) are also activated in central sensitisation releasing interleukins, tumour necrosis factor (TNF), chemokines, prostaglandin and excitatory amino acid. This further up-regulates AMPA and NMDA receptors and down-regulates GABA receptors. All the above processes result in a scenario where pain is no longer correlated with the noxious peripheral stimuli; a state of potentiated, augmented and amplified pain response is observed. Once begun central sensitisation is difficult to manage due to multiple activated pathways and receptors. Hence, early prevention of sensitisation with optimal analgesia in the immediate post-surgery period is crucial in reducing the development of CPSP.
The ascending tracts—sensory-discriminative pathways and affective pathways
Dorsal horn neurons transmit pain signals from the spinal cord or trigeminal nuclei to the cerebral sensory cortex via two main ascending tracts: sensory-discriminative pathways and affective pathways. The sensory-discriminative pathway enables a nociceptive stimulus to be localised and its different qualities such as character or severity to be distinguished. The affective pathway relays the nociceptive signals to visceral, neuroendocrine and affective centres in the brain to produce the multidimensional nature of the pain response.
The sensory-discriminative route travels via the spinothalamic tract (STT) and (for the face and head) the trigemino-thalamic tracts. They are formed by dorsal horn neurons (second order neurons) which cross the midline at the spinal level and ascend within the contralateral STT or trigemino-thalamic tract. They terminate at the ventral posterior nucleus (VPN) of the thalamus, from which the third order neurons project to the somatosensory cortex.
The affective pathway travels principally via the spinoreticular tracts. It is multisynaptic and sometimes referred as paleospinothalamic projection. The affective pathway projects to brain stem areas, including the parabrachial nuclei, the periaqueductal grey (PAG) matter, the reticular formation and the catecholamine cell groups. The affective pathway transmits pain signals to visceral nuclei (e.g., nucleus tractus solitarius), the limbic system and the descending inhibitory system. These mediate the emotional and autonomic responses. Also included in the affective pathway is the spinohypothalamic tract which relays nociception to the prefrontal cortex via hypothalamus. Pain can therefore affect the hypothalamus’s homeostatic control over the body.
The cortical pain matrix and the psychological components of pain
The ascending tracts first project to subcortical structures such as the thalamus and brainstem nuclei, from there their signals are relayed on to the cortical pain matrix. This is a network of cortical sites that have been shown in imaging studies to be consistently activated in both acute and chronic pain states (12). The sites include: the primary somatosensory cortex (postcentral gyrus, S1), the secondary somatosensory cortex (S2), the insular cortex (IC) and the anterior cingulate cortex (ACC) (8). The somatosensory areas S1 and S2 are involved with perceiving the location and duration of pain. The IC and ACC are thought to be important in the emotional, affective and motivational aspects of pain due to their close association with the limbic system. In addition to these the prefrontal cortex, which is responsible for higher cortical functions such as anticipation, prediction and modelling based on previous experience, is also activated by noxious stimuli. It can also exert an enhancing or supressing effect on the intensity of pain experience. This may be the mechanism behind the ‘pain catastrophizing’ phenomenon—where the tendency to be anxious and stressed during painful stimulation contributes to a more intense pain experience and increased emotional distress (16).
The number of cerebral structures involved in the cortical pain matrix illustrates how the perception of pain is far more complex than just a simple sensory input and in fact includes an array of affective and adaptive components. There is a well-known association between psychiatric disease such as depression and anxiety with pain. Patients with chronic pain are more likely to suffer depression and anxiety disorders, while patients with pre-existing depression or anxiety are predisposed to have more pain after similar procedures. Clinicians are therefore more likely to fail in pain management if they do not address the affective components. Clinicians should always consider psychological and psychiatric therapies such as cognitive behaviour management in challenging pain management scenario and approach it simultaneously with the treatment of anxiety and depression (17).
Modulation of pain—gate control, the descending modulatory system and neuromodulators
Pain is an important protective mechanism. From an evolutionary standpoint, the ability to perceive pain ensures that an organism not only removes itself from a harmful situation but takes action to avoid it in the future. Sensitisation and the prolongation of the pain response beyond the immediate tissue injury encourages the individual to take appropriate actions to recuperate and promotes healing. However, there are times when survival may be advantaged by transiently supressing the pain response in order to allow the completion of life saving tasks such as escaping from or fighting off a threat. The ability to modulate the pain response (either enhance or suppress it) is crucial and pain modulation may occur at a cortical, brainstem and spinal level. Three mechanisms by which the pain response may be modulated have been identified: gate control, the descending modulatory system and neuromodulators (8).
Gate control
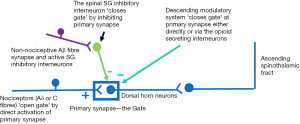
The gate control theory was first proposed by Wall and Melzack in 1965. Figure 1 (gate control theory) illustrates how nociception transmission at the primary synapse between nociceptors (primary nociceptive fibres) and dorsal horn neurons can be modulated, or ‘gated’. The ‘gate’ is open when the nociceptor activates the primary synapse, pain transmission propagates along the dorsal horn neuron and then the ascending STT. Non-nociceptive neurons such as Aβ mechanoreceptor fibres can activate inhibitory interneurons in the SG. Those inhibitory interneurons can exert their inhibitory effects both pre-synaptically and post-synaptically, thus closing the ‘gate’ and decrease pain transmission. The ‘gate’ can also be closed by the descending modulation pathway directly or via opioid secreting interneurons. This explains how pain may be relieved by rubbing, pressure or distraction, as the activated pain fibres are gated out by the other sensations via Aβ fibres or higher cortical input via descending modulation system. Clinical applications of the gate theory include transcutaneous electrical nerve stimulation (TENS), dorsal column stimulators or innovative virtual reality technology (18,19).
The descending modulatory system
The descending modulatory system is principally centred around the PAG of the midbrain, the rostral ventromedial medulla (RVM) and the spinal cord (Figure 2; PAG-RVM-Spinal axis). PAG, believed to be the main inhibitory centre for nociception, is the grey matter structure surrounding the aqueduct of sylvius in the midbrain. It has been observed that electrical stimulation or injection of morphine into the PAG produces profound analgesia and conversely, the suppression of the PAG is a major component in chronic pain states such as complex regional pain syndrome and is associated with further disease such as depression and anxiety (20,21). As a result, the role of receptors within the PAG in pain modulation has been the subject of a great deal of research, and the PAG is targeted in deep brain stimulation (DBS) therapy for the treatment of intractable pain (22).
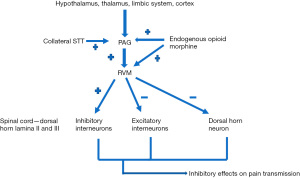
The activation of the PAG-RVM axis by collaterals from the ascending STTs forms part of negative feedback loop. The PAG is subject to complex modulation via the integration of multiple cortical higher functions such as prediction, anticipation, affect and emotions. It also incorporates the neuroendocrine axes and autonomic function in gauge the pain intensity from its hypothalamus and thalamus inputs. This model can guide us in the assessment of pain in individuals who cannot communicate, there have been trials of using additional parameters such as heart rate variability, fluctuation of skin conductance and pupil reflex to objectively assess pain (23).
The PAG delivers its main excitatory projection to the RVM. The RVM is a group of neurons with their nuclei located close to the midline on the floor of the medulla oblongata. The RVM sends descending inhibitory and excitatory fibres (the off and on cells) to the dorsal horn neurons in the spinal cord.
The RVM descending neuron exerts its effects by (24):
- Direct action on dorsal horn neurons;
- Inhibition of excitatory interneurons at the dorsal horn;
- Excitation of inhibitory interneurons at the dorsal horn.
The two most well studied RVM neurons are the nucleus raphe magnus (NRM) and the locus coeruleus (LC) and these form the two main descending modulation pathways. The serotonin containing NRM exerts its inhibitory effect through synapses in lamina II (SG) and lamina III of the dorsal horn, whereas the norepinephrine containing LC exerts an antinociceptive effects in the dorsal horn via presynaptic α-2 receptors. These α-2 receptors are the therapeutic target of α-2 receptor agonists such as clonidine and dexmedetomidine, which are used widely clinically for their analgesic, anxiolytic and sedative effects (25).
Neuromodulators
Neuromodulators are a group of molecules that modulate the effectiveness of neurotransmitter signal transmission at the synapse. They can be excitatory or inhibitory. The excitatory neuromodulators that enhance nociception include: cholecystolkinin 8 (an endogenous anti-opioid), prostaglandins (reduce glycine’s inhibitory effects and cause hyperalgesia), dynorphine 1–17, orphanin FQ and nociceptin (these reverse opioid analgesia at supraspinal sites). The inhibitory neuromodulators that supress nociception include: opioids, cannabinoids, acetylcholine (via muscarinic and nicotinic receptor at dorsal horn lamina I and II), ophanin FQ and nociceptin (these induce analgesia at spinal sites) (8). Neuromodulators produce their effects either directly, by acting on synapses or indirectly, by altering the excitability of neurons. At the synapse they may increase both the amount of neurotransmitter released and the expression of post-synaptic receptors (short-term synaptic plasticity). At the neuronal level, neuromodulators may alter the availability and activity of transmembrane voltage-gated ion channels and hence the excitability of the neuron (26).
Neuromodulators form the basis of the endogenous control of pain intensity. The excitatory neuromodulators (e.g., prostaglandin) or Inhibitory neuromodulators (e.g., opioids) that work directly on the primary synapses are responsible for creating either hyperalgesia or analgesia states at the dorsal horn level. Neuromodulators such as orphanin FQ or nociceptin, that modulate the descending pathway supra-spinally, may have opposite effects to their spinal modulation; therefore the interplay between the spinal and supra-spinal effects is important in determining the effect of some modulators. Furthermore, certain neurotransmitters such as cannabinoids need simultaneous excitation of the spinal and supra-spinal areas to produce their analgesic effects. Due to the complexities of their action the application of neuromodulators in clinical practice is challenging. For example, despite the presence of cannabinoid receptors throughout the pain pathway at the peripheral, spinal and supraspinal level, the effects of cannabinoids in the treatment of acute pain is not supported by clinical evidence and the evidence for its effectiveness in chronic pain is limited (27-29).
The gap between identification for targets on the pain pathway to the effective clinical applications in pain management is important in guiding the focus of research. There is a general shift from blocking of peripheral or central neurotransmission to the altering factors underlying the maintenance for pain such as glial activation, central sensitisation and loss of inhibition. Novel and emerging therapies are generally targeted to specific parts of the pain pathway to avoid systemic side effects that we have seen in current analgesia such as non-steroidal anti-inflammatory drugs or opioids. Antibodies, nociceptor-specific small molecules at the afferent nerve fibres; gene therapy or cell transplantation at the spinal cord all show some efficacy in long term pain (30).
Conclusions
Pain has been the focus of research for it represents a major clinical, social and economic problems (31). A large amount of research has been undertaken in the further understanding of the pain pathway and causes of pain pathology such as in chronic pain. The pain pathway is a complex process with multiple interdependent processes and under multiple modulation systems (neuronal, endocrine, inflammatory systems). This leads to two major challenging for pain, firstly, they represent pain being a highly heterogeneous disorder; secondly, systemic treatment of pain is likely to cause significant side effects. The primary treatment aim for pain management should be prevention of acute severe pain rather than rescuing as sensitisation of the pain pathway leads to positive feedback loop and pathological changes at multiple sites of the pain pathway-peripheral, spinal and supra-spinal levels. In established pain, we should target a specific part of pain pathway or aim to remove the sustaining factors for peripheral and/or central sensitisations as in disease modifying therapies. As clinicians, we should stratification our patients for their individual pain pathogenesis and formulate a multiple modal approach based on our improved knowledge of pain physiology and best evidence based medicine. Furthermore, we should always recognise that pain should not be perceived solely as a sensory neuronal activity; the revised pain definition with newly added six keynotes emphasize the importance of acknowledging pain as a personal experience and a learnt process (32). In summary, we have gained significant further understanding in the physiology of pain, however, we still have not identified any specific target sites that allow us to manage severe pain without significant side effects. This narrative review is limited for pain is such a heterogenous condition, we focus mainly on the post-surgery pain. Moreover, in most pain related studies reviewed, the pain measurement relies on the subjective feedbacks. Pain is difficult to quantify, and can be influenced by culture, personal experience, personalities and expectations. Future study should explore objective ways of assessment pain, the identification of biomarker for pain in aid of subjective feedbacks would allow more direct comparison. The complexity of neuromodulations and cortical pain matrix are still not fully understood, further research in those topics would allow us to develop therapy in targeting the affective components of pain.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research, for the series “Current Issues in Analgesia for Major Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-100/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-100/coif). The series “Current Issues in Analgesia for Major Surgery” was commissioned by the editorial office without any funding or sponsorship. LK served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. [Crossref] [PubMed]
- Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res 2017;10:2287-98. [Crossref] [PubMed]
- Fletcher D, Stamer UM, Pogatzki-Zahn E, et al. Chronic postsurgical pain in Europe: An observational study. Eur J Anaesthesiol 2015;32:725-34. [Crossref] [PubMed]
- Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am 2015;95:301-18. [Crossref] [PubMed]
- Phillips CJ. The Cost and Burden of Chronic Pain. Rev Pain 2009;3:2-5. [Crossref] [PubMed]
- Wu CL, Naqibuddin M, Rowlingson AJ, et al. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg 2003;97:1078-85. [Crossref] [PubMed]
- Beverly A, Kaye AD, Ljungqvist O, et al. Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines. Anesthesiol Clin 2017;35:e115-43. [Crossref] [PubMed]
- Lin ES. Physiology of pain. In: Smith T, Pinnock C, Lin T. editors. Fundamentals of anaesthesia. 3rd ed. Cambridge: Cambridge University Press, 2009:412-32.
- Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019;160:19-27. [Crossref] [PubMed]
- IASP. Terminology. Available online: https://www.iasp-pain.org/resources/terminology/
- He Y, Kim PY. Allodynia. 2021. In: StatPearls. Treasure Island: StatPearls Publishing, 2022.
- Feizerfan A, Sheh G. Transition from acute to chronic pain. Continuing Education in Anaesthesia, Critical Care & Pain 2015;15:98-102. [Crossref]
- Souza Monteiro de Araujo D, Nassini R, Geppetti P, et al. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets 2020;24:997-1008. [Crossref] [PubMed]
- Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain 2008;9:122-45. [Crossref] [PubMed]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895-926. [Crossref] [PubMed]
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg 2011;201:122-31. [Crossref] [PubMed]
- Gilliam WP, Schumann ME, Cunningham JL, et al. Pain catastrophizing as a treatment process variable in cognitive behavioural therapy for adults with chronic pain. Eur J Pain 2021;25:339-47. [Crossref] [PubMed]
- Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. Eur J Pain 2019;23:652-9. [Crossref] [PubMed]
- Gupta A, Scott K, Dukewich M. Innovative Technology Using Virtual Reality in the Treatment of Pain: Does It Reduce Pain via Distraction, or Is There More to It? Pain Med 2018;19:151-9. [Crossref] [PubMed]
- Freund W, Wunderlich AP, Stuber G, et al. The role of periaqueductal gray and cingulate cortex during suppression of pain in complex regional pain syndrome. Clin J Pain 2011;27:796-804. [Crossref] [PubMed]
- Mokhtar M, Singh P. Neuroanatomy, Periaqueductal Gray. 2021. In: StatPearls. Treasure Island: StatPearls Publishing, 2022.
- Farrell SM, Green A, Aziz T. The Current State of Deep Brain Stimulation for Chronic Pain and Its Context in Other Forms of Neuromodulation. Brain Sci 2018;8:158. [Crossref] [PubMed]
- Cowen R, Stasiowska MK, Laycock H, et al. Assessing pain objectively: the use of physiological markers. Anaesthesia 2015;70:828-47. [Crossref] [PubMed]
- Steeds CE. The anatomy and physiology of pain. Surgery (Oxford) 2009;27:507-11. [Crossref]
- Ju JY, Kim KM, Lee S. Effect of preoperative administration of systemic alpha-2 agonists on postoperative pain: a systematic review and meta-analysis. Anesth Pain Med (Seoul) 2020;15:157-66. [Crossref] [PubMed]
- Nadim F, Bucher D. Neuromodulation of neurons and synapses. Curr Opin Neurobiol 2014;29:48-56. [Crossref] [PubMed]
- Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol Ther 2002;95:127-35. [Crossref] [PubMed]
- Hosking RD, Zajicek JP. Therapeutic potential of cannabis in pain medicine. Br J Anaesth 2008;101:59-68. [Crossref] [PubMed]
- Meng H, Deshpande A. Cannabinoids in chronic non-cancer pain medicine: moving from the bench to the bedside. BJA Educ 2020;20:305-11. [Crossref] [PubMed]
- Manion J, Waller MA, Clark T, et al. Developing Modern Pain Therapies. Front Neurosci 2019;13:1370. [Crossref] [PubMed]
- Henschke N, Kamper SJ, Maher CG. The epidemiology and economic consequences of pain. Mayo Clin Proc 2015;90:139-47. [Crossref] [PubMed]
- Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020;161:1976-82. [Crossref] [PubMed]
Cite this article as: Liu S, Kelliher L. Physiology of pain—a narrative review on the pain pathway and its application in the pain management. Dig Med Res 2022;5:56.


