
“The pathogenesis of hepatic fibrosis: basic facts and clinical challenges”—assessment of liver fibrosis: a narrative review
Introduction
The progression of chronic liver disease (CLD), irrespective of etiology, encompasses parenchymal injury, inflammatory processes and liver fibrogenesis (1). Liver fibrosis, which is defined as excessive accumulation of extracellular matrix (ECM) proteins, ranges from mild pericellular fibrosis in early stages to cirrhosis, which is considered to be the common end-stage of any liver disease (2,3). Liver fibrosis is the determinant of disease progression, major liver-related adverse events and the risk of hepatocellular carcinoma (4). The underlying pathogenic mechanisms differ among etiologies (4). The main cell type involved in fibrogenesis are hepatic stellate cells (HSC), storing vitamin-A in a quiescent state in healthy livers and transform into a proliferative, fibrogenic activated state during liver injury (5). While in chronic viral hepatitis damage-associated molecular patterns and host’s antiviral response are triggering HSC activation, apoptosis-related interleukin and chemokine release mainly provokes HSC activation in alcoholic liver disease (4,6-9). On the other hand, in non-alcoholic steatohepatitis (NASH), lipotoxicity caused by metabolites of saturated fatty acids, damages hepatocytes and result in oxidative stress (10). The latter generates an inflammatory trigger, resulting again in HSC activation. Furthermore, insulin resistance, nutritional factors and genetic factors contribute to liver injury, which is summarized in the so-called “multiple hit”-theory (11).
Thus far, there is no Food and Drug Association (FDA)-approved antifibrotic agent, so prevention is the key strategy. Prevention of liver fibrosis is mostly achieved by an early diagnosis and treatment of the underlying liver disease. When patients are aware of their chronic liver disease, it can either be treated (e.g., metabolic or cholestatic liver disease) or halted by lifestyle changes which mainly applies for alcoholic liver disease. The clinical relevance of fibrosis prevention is explained by its consequences, which is portal hypertension, a condition caused by fibrosis-dependent increased intrahepatic resistance (12). After exceeding a specific pressure gradient between the portal vein and systemic venous circulation, usually 10 mmHg, we speak of clinically significant portal hypertension, which is associated with an increased risk of complications like ascites, varices and hypersplenism (12,13).
Liver fibrosis is usually classified into five stages depending on the quantity: F0—no fibrosis; F1—mild fibrosis, pericellular collagen deposits; F2—moderate fibrosis, beginning bridging fibrosis; F3—severe fibrosis, defined as presence of numerous bridges and septa; F4—cirrhosis according to the most used scores, which will be discussed more detailed in the histology section (14,15). In this review, significant fibrosis is considered as F2 and above (F ≥2), whereas advanced fibrosis is defined as at least strong fibrosis (F ≥3). This is of high importance when evaluating assessment methods for liver fibrosis.
In order to estimate the individual risk of CLD patients, assessment of liver fibrosis needs to be performed in routine clinical practice, what remains challenging. This review aims to summarize and evaluate available diagnostic procedures to assess liver fibrosis and its surrogates. The different methods will be critically discussed with regard to their rationale, availability, technical considerations and accuracy in the following paragraphs. This review may help hepatologists to choose to appropriate diagnostic tool and informs about their advantages and disadvantages. Liver biopsy is the gold standard of liver fibrosis assessment. We therefore discuss different histological scoring systems, which need to be attentive to the underlying etiology of CLD. Later, we will first outline biomarkers, which are commonly used for detection and grading of liver fibrosis. Many attempts were dared to calculate non-invasive liver fibrosis scores, to predict fibrosis based on standard laboratory findings. These scores will be reviewed in the third paragraph. And finally, in the previous decade, assessment of liver fibrosis through radiological examinations has markedly improved due to technical progress, which even challenges liver biopsy as reference method. Elastographic methods have already decreased the requirement of a liver biopsy, and cross-sectional imaging techniques are establishing themselves in the field of liver fibrosis assessment. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-9/rc).
Methods
In order to review the available techniques for assessment of liver fibrosis, a literature search using the NIH National Library of Medicine PubMed was performed. All literature published until December 31st, 2022 was eligible for further consideration. Open and closed access publication were both included in this review. Literature which was published in languages other than English were excluded. The search strategy summery is displayed in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | November 15th, 2021, May 1st 2022 for review |
| Databases and other sources searched | PubMed |
| Search terms used | “Assessment of liver fibrosis”, “liver fibrosis” and “non-invasive tests for liver fibrosis” |
| Timeframe | From origin to December 31st, 2021 |
| Inclusion and exclusion criteria | All published studies in English were eligible |
| Selection process | Selection was performed by the authors and conducted independently |
Histological assessment of liver fibrosis
Systematic histopathological studies of morphological aspects of liver fibrosis have been contributing to our today’s knowledge of the evolution of liver fibrosis in various chronic inflammatory conditions of the liver. Fibrosis develops in a situation of ongoing inflammation with the imbalance of extracellular matrix production (ECM) and reduction (16). Activation of hepatic stellate cells and portal fibroblasts induces their myofibroblastic proliferation accompanied by the deposition of connective tissue, which results in the picture of parenchymal and portal fibrosis (6). While in the normal liver the space of Disse consists of collagens type IV and laminin, these are replaced by collagens type I and III during the process of parenchymal fibrosis (17).
In portal tract fibrosis activated myofibroblasts and smooth muscle cells are capable of ECM production in the setting of chronic hepatitis. In chronic bile duct inflammation the source of ECM production are peribiliary fibroblasts, inducing the pattern of biliary fibrosis. The fibrosis contains abundant type I and III collagen and reticulin fibers, elastic fibers are indicators of a several months duration of the process.
For the precise histological assessment of liver fibrosis, stains for connective tissue like Masson trichrome, Elastica van Gieson, Sirius red, Orcein (18), Victoria blue, and Reticulin stains are applied, which demarcate the accumulation of connective tissue. The semiquantitative analysis of fibrosis (staging) is reported within a fibrosis score, as a reproducible predictor for disease progression and clinical outcome.
The Knodell Score was one of the early reported scoring systems for chronic hepatitis which was published in 1981 (19). In contrast to the traditional nomenclature of that time, portal based fibrosis was reported in a numerical scoring system from 0 to 4 in a reproducible and objective form. Other scoring systems and modifications followed (Ishak Score, Desmet Score, Metavir) (20-22). These scoring systems have been shown to be a prerequisite for histology-based treatment stratification, monitoring of the clinical course and outcome in chronic viral and non-viral hepatitis (23).
Taking into account, that non-alcoholic fatty liver disease (NAFLD) displays different fibrosis patterns than chronic hepatitis, special scoring systems for NAFLD were developed by Kleiner et al. (24) and Bedossa et al. in 2014 (25). The early stages of fibrosis describe mild and moderate pericellular in the absence of portal fibrosis. Histopathological staging of fibrosis in NAFLD, which is still the gold standard, revealed to be a strong predictor of mortality and time to develop severe liver disease in biopsy proven NAFLD (26).
As in NAFLD, histology of alcoholic fatty liver disease (AFLD) is characterized by steatosis, ballooning, inflammation and fibrosis. However, in contrast to NAFLD, most patients have severe fibrosis or cirrhosis at the first presentation (27). A specific scoring system for AFLD has been published by Lackner et al. (28). Liver fibrosis is staged into stage 0–3, while stage 4 (cirrhosis) is subdivided into stage 4a, 4b and 4c (cirrhosis with thin septa, broad septa and very broad septa). It was shown, that the scoring system is a prognostically relevant method for the histological assessment of fibrosis in AFLD (28).
For chronic biliary diseases different histopathological scoring systems are available like Ludwig Score (29) and Nakanuma Score (30). For primary biliary cholangitis, which are applicable for primary sclerosing cholangitis with a prognostic value (31).
With upcoming treatment options for chronic liver diseases, the histopathological assessment of liver fibrosis regression is an important issue. Histopathological criteria of fibrosis regression have been described and can be employed for the monitoring of treatment effects like in chronic hepatitis C (32).
Non-invasive tests for liver fibrosis
Serum biomarker-based non-invasive tests for liver fibrosis
Rationale
Serum biomarker-based non-invasive liver fibrosis tests (sNITs) are calculated based on serum biomarker levels which are correlating with the degree of fibrosis. Due to its easy and low risk access, many studies investigated the ability in fibrosis assessment. Since many sNITs are based on standard laboratory values, they can be assessed repetitively in fibrosis assessment, if necessary. Therefore, sNITs are well accepted and widely used in the community, especially those with high accuracy.
Indications
The most investigated, commercially available tests are the FibroTest™ (LabCorp, Burlington, NC, USA) and the Enhanced Liver Fibrosis (ELF™) test (Siemens, Munich, Germany). FibroTest™ was validated against liver biopsy in patients with chronic hepatitis C, indicating similarity in prediction of 5-year survival compared to liver biopsy (33). For hepatitis B, it showed moderate results in assessment of significant fibrosis (AUROC =0.77) but this could be improved if serum AST-levels and HIV-status were combined with the score (AUROC =0.9) (34). For NAFLD and alcoholic liver disease (ALD), a meta-analysis showed that FibroTest™ had a good accuracy adjusted for etiology specific fibrosis stage spectrum (NAFLD: AUROC =0.84, ALD: AUROC =0.85) (35).
The ELF™ test was validated in numerous studies. A meta-analysis of nine studies revealed that independent of etiology, ELF™ tests accuracy for significant and advanced fibrosis as well as cirrhosis was good (0.87≤ AUROC ≤0.88). The ELF™ test is excellent in identifying patients with advanced liver fibrosis in ALD patients (AUROC =0.92), but not showing superiority to the FibroTest™ (36).
AST-to platelet index (APRI) is a non-patented sNIT and was firstly introduced by Wai et al. in 2003 who constructed a simple algorithm to predict significant fibrosis (ISHAK ≥3). With this ratio, significant fibrosis was predicted correctly in 51% and cirrhosis in 81% of patients (AUROC =0.88 for significant fibrosis, AUROC =0.94 for cirrhosis respectively) (37). This index was later validated in other cohorts with various etiologies and is mainly considered for quickly ruling out cirrhosis, but not valuable for predicting earlier stages of fibrosis (38).
Moreover, Sterling et al. developed another easy to calculate score which comprises two more variables (age and ALT beside AST and platelet count) and was named Fibrosis-4 Index (FIB-4) (39). It was initially designed in hepatitis C/HIV coinfected patients, but later validated for hepatitis B, NAFLD and ALD (40,41). A small study by Miyata et al. demonstrated that FIB-4 could be used as a monitoring parameter for patients treated with methotrexate (42). However, in elderly people (age ≥65 years) with NAFLD, specificity of FIB-4 was very low, with the need of adjusted thresholds (43). Overall, FIB-4 showed adequate accuracies for NAFLD patients according to a meta-analysis by Xiao et al. (44). Here, summary AUROCs for detection of significant fibrosis, advanced fibrosis and cirrhosis were 0.73, 0.84 and 0.85 respectively, which was superior to other NITs except for significant fibrosis in which APRI performed better (AUROC =0.76).
Beside these two NITs which are most frequently used in daily clinical practice, many others have been developed for NAFLD, like NAFLD Fibrosis Score (NFS) (45) and BARD score (46), which overall only show, if any, minor superiority to APRI and FIB-4 (44). Very recently, novel scores are emerging, including new biomarkers with standard laboratory finding, which can further improve AUROC. Daniels et al. developed a new algorithm including age, presence of diabetes, platelet count and Pro-Collagen 3 (“ADAPT” algorithm) with an AUROC of 0.86 for predicting advanced fibrosis in NAFLD patients and 0.88 for advanced fibrosis in ALD patients, respectively (47,48). More models with both standard laboratory findings and novel biomarkers will likely come up in the near future to better address specific clinical scenarios and better predict fibrosis in order to stratify patients and further reduce the need of liver biopsies. The calculation algorithms for the above-mentioned scores can be found in Table 2. The cutoffs for the two most abundant etiologies of chronic liver disease, ALD and NAFLD can be obtained in Table 3.
Table 2
| NIT | Pro | Con | Ref. |
|---|---|---|---|
| ELF: 2.278 + 0.851 ln(HA) + 0.751 ln(PIIINP) + 0.394 ln(TIMP-1) | Best validation among sNITs | Can be obtained in validated laboratories only | (49) |
| FibroTest: Calculation based on alpha-2-macroglobulin, haptoglobin, apolipoprotein A1, gamma-glutamyl transpeptidase, total bilirubin, and ALT | Good accuracy in alcoholic and non-alcoholic fatty liver disease | Can be obtained in validated laboratories only | (33) |
| APRI: [(AST level/ULN)]/Platelet counts (109/L) | Very easy calculation algorithm | Poor performance for intermediate fibrosis stages | (37) |
| FIB-4: (Age × AST)/[Platelets × √(ALT)] | Best accuracy among non-commercial sNITs | Less reliable in patients below 35 and above 65 years | (39) |
| VCTE: It relies on a probe that includes both the vibrator and the transducer. Low frequency vibration of small amplitude is transmitted to the tissue what induces an elastic shear wave. Simultaneously, pulse-echo ultrasonic acquisitions are performed to detect the propagation of the shear wave and measure its velocity, which is directly related to liver stiffness | most validation studies performed, steatosis assessment included | No simultaneous control picture in B-mode, Disturbed by obesity and/or ascites |
(50,51) |
| P-SWE: It can be performed with standard ultrasound machines. It uses acoustic radiation force impulse which generates shear waves which velocity can be determined and translated in tissue stiffness | No extra machine needed, B-mode guided |
One-dimensional, pending validation due to various commercially available machines | (52,53) |
| 2D-SWE: Acoustic radiation force impulses at different depths generate a mechanical impulse that causes shear waves to propagate vertical to the incident ultrasound axis. The propagation can be reconstructed into a real-time color map of the shear wave front in m/s or kPa | Additional to PSWE: Larger region of interest, not hindered by visceral fat or ascites | Pending validation due to various commercially available machines | (54,55) |
| MRE: It combines a phase-contrast pulse sequence with motion-encoding gradients to encode tissue motion, which is applied by low frequency vibrations of an external driver. With postprocessing, color wave images and stiffness maps, also known as elastograms, are generated that show stiffness of liver tissue in kPa | Whole liver measurement, accurate in early stages of liver fibrosis | Time consuming, expensive, radiological expertise required | (56) |
Description of serum-derived and imaging-derived non-invasive tests for liver fibrosis assessment alongside with main pros and cons. NIT, non-invasive test; Ref, references; sNITs, serum biomarker-based non-invasive tests; ELF, enhanced liver fibrosis; HA, hyaluronic adic; PIINP, amino terminal propeptide of type III procollagen; TIMP, tissue inhibitor of matrix metalloproteinase; VCTE, vibration-controlled transient elastography; APRI, AST-to-platelet-ratio index; AST, aspartate aminotransferase; ULN, upper limit of the normal; P-SWE, point shear-wave elastography; 2D-SWE, two-dimensional shear-wave elastography; FIB-4, fibrosis-4 test; ALT, alanine aminotransferase; MRE, magnetic resonance elastography.
Table 3
| Test | Etiology | Significant fibrosis | Advanced fibrosis | Cirrhosis | Ref |
|---|---|---|---|---|---|
| APRI | Hepatitis C | >0.7 | >1.0 | >1.0 | (57) |
| APRI | NAFLD | >0.42 | >0.98 | n.a. | (58,59) |
| FIB-4 | Hepatitis C | >1.45 | >3.25 | n.a. | (60) |
| FIB-4 | NAFLD | >1.12 | >2.67 | n.a. | (58,61) |
Validated cutoffs for fibrosis classification for most commonly used sNITs. For alcoholic liver disease, the same cutoffs are used as for NAFLD. APRI, AST-to-platelet-ratio index; NAFLD, non-alcoholic fatty liver disease; FIB-4, fibrosis 4 test; n.a., not applicable; Ref, references.
Technical considerations
SNITs can be divided in commercially available test, which can be obtained only in validated laboratories resulting in very well reliable and validated results, but are therefore intricate and costly (62,63). Contrary to these tests, non-patented NITs can be calculated by standard laboratory findings and are therefore an easy, cheap and helpful diagnostic tool.
Limitations
Commercially available NITs perform well in fibrosis prediction, but pricing and logistics hinder its wide use in routine practice.
Imaging-based non-invasive tests for liver fibrosis
Rationale
B-mode abdominal ultrasound is a widely-available frequently used diagnostic tool, which can be used for detection of advanced liver fibrosis or cirrhosis but it fails in detection of liver fibrosis. However, the Vibration-controlled transient elastography (VCTE), mostly performed with FibroScan® (Echosens, Paris, France) is currently the most abundantly investigated non-invasive tool for liver fibrosis measurement which is approved by the FDA. Cross-sectional imaging, like computed tomography (CT) or magnetic resonance imaging (MRI), allows for morphological assessment of liver parenchyma and has been applied for a long time for the detection of cirrhotic liver features in clinical routine. Morphological signs of liver cirrhosis on CT or MRI includes an increased nodularity of liver surface, heterogeneous liver parenchyma, a caudate lobe enlargement, alterations in right-to-left-lobe-volume ratio, an expanded gallbladder fossa or a posterior notch sign (64,65). Other hallmarks and complications of liver cirrhosis, like portosystemic collaterals, splenomegaly and ascites can also be visualized using standard cross-sectional imaging.
Indications
VCTE was firstly introduced for fibrosis staging in chronic hepatitis C, meta-analyses have demonstrated that TE can measure the degree of fibrosis irrespective of etiology (66,67). However, excellent diagnostic accuracy (AUROC >90%) is reached only if cirrhosis is of interest. For detection of significant fibrosis, defined by METAVIR F2 or F3 since most studies were performed in viral hepatitis patients, diagnostic accuracy is good ranging between 84–89% (68). Moreover, TE can be used for longitudinal studies, as demonstrated in two studies which investigated fibrosis reversal in hepatitis C patients after successful treatment (69,70).
More recently, techniques were developed that only use ultrasound waves to detect fibrosis and are included in conventional ultrasound devices: point shear wave elastography (P-SWE) and 2D-shear wave elastography (2D-SWE). Several studies compared P-SWE to TE, and showed accurate results for the diagnosis of cirrhosis, but worse result for earlier stages of fibrosis (AUC =0.76 for significant fibrosis) (71,72). P-SWE and 2D-SWE are reliable and reproducible techniques. This was demonstrated in healthy volunteers, where similar results were observed between different examiners. However, absolute values must not be used interchangeably (73). In contrast to P-SWE, 2D-SWE provides a larger region of interest, adjustable by the examiner and allows real-time measurement (68). Due to the adjustable region of interest, in specific conditions like post-hepatectomy patients, 2D-SWE might be more accurate which was shown in a recent study (74). A large, multicenter study with 1,827 patients included, could demonstrate that patients with a liver 2D-SWE ≥20 kPa and a Model of End-Stage Liver Disease (MELD)-score ≥10 have a higher 2-year mortality and a risk of hepatic decompensation, requiring a tighter follow-up visit intervals (75). Finally, there is evidence emerging 2D-SWE seem to be superior compared with TE, with an increase of AUROC between 1.4% and 12.8% depending on fibrosis stage and underlying etiology, according to a large multi-center analysis with 1,134 patients (76). Taken this together, further studies are needed to better validate shear wave elastography, which is challenged through the numerous different commercially available machines, which renders inter-device comparability difficult. However, the algorithm predicting decompensation and outcome based on liver stiffness ≥20 kPa and MELD ≥10 holds true for different devices and etiologies (75). Example images of ultrasound-based non-invasive tests (TE, P-SWE and 2D-SWE) are provided in Figure 1.
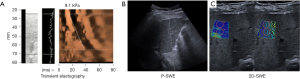
The diagnostic accuracy of standard cross-sectional imagining for the detection liver cirrhosis is moderate ranging from 68% to 72% (65). For the detection pre-cirrhotic fibrosis and fibrosis stage classification morphological assessment alone is not useful. Nevertheless, different more advanced CT and MRI techniques are capable to quantify hepatic fibrosis in a pre-cirrhotic stage and provide, in contrast to TE, SWE, or biopsy, information of fibrosis distribution in the entire liver parenchyma (56). The wider spatial resolution of cross-sectional imaging techniques could help to identify most appropriate sites for liver biopsy or lead to a disease prognostication that is more accurate (77).
Especially, delayed dual-energy CT, deep learning algorithms, MR elastography (MRE), diffusion-weighted MRI, and MRI mapping techniques can detect liver fibrosis in a pre-cirrhotic stage with a good diagnostic performance (78-80).
Dual-energy CT separates two x-ray energy spectra, allowing the differentiation of materials that have different attenuation properties at different energy levels. Dual-energy CT enables assessment of the severity of liver fibrosis by means of contrast-enhanced delayed acquisition with calculation of a normalized iodine concentration of liver parenchyma. In a study of 107 participants with chronic liver disease, dual-energy CT derived normalized iodine concentration of the right liver lobe achieved an AUC of 0.86 to differentiate F0 from F1–3 and 0.96 to differentiate F0-3 from F4 fibrosis (79).
Deep learning techniques, especially convolutional neural networks (CNNs), have an enormous potential for image segmentation and classification, which also includes the detection of liver fibrosis. With deep learning algorithms, liver cirrhosis detection, even on standard T2-weighted images, can achieve a classification accuracy of up 0.99 (81). Reported AUC values of different CNNs to differentiate between F0 and F1-4 stages range from 0.77 to 0.96, depending on applied imaging modality and technique (82).
Among cross-sectional imaging methods, MRE (Figure 2) is the best studied MRI-based imaging technique for liver fibrosis assessment and shows a high diagnostic performance for classification of liver fibrosis stages with AUCs of 0.84–0.95 (F ≥1), 0.88–0.98 (F ≥2), 0.93–0.98 (F ≥3), and 0.92–0.99 (F ≥4) (80,83-85). MRE is also useful to differentiate NAFLD from individuals with steatohepatitis, even before onset of fibrosis development (86). However, diagnostic performance of MRE might be limited in inflammatory hepatic disease, iron overload or cardiac congestion (56).
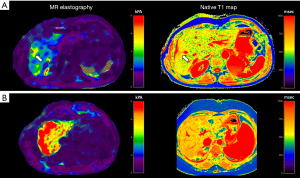
Diffusion-weighted MRI is based upon measuring the random Brownian motion of water molecules. Diffusion of water molecules is restricted in fibrosis and leads to lower apparent diffusion coefficient (ADC) values, which are calculated from at least two b-values using a monoexponential model. Although MRE has a significantly higher accuracy than diffusion-weighted MRI for differentiation of different fibrosis stages, future developments in diffusion-weighted MRI including perfusion models might further elevate the diagnostic performance of this technique (80,87).
T1rho elongation was demonstrated to be an effective biomarker for collagen deposition and therefore liver fibrosis in animal models (88). Significant differences of T1rho values were demonstrated between Child-Pugh cirrhosis stages, with AUCs of 0.95–0.98, indicating the ability of MRI techniques in differentiating different stages of cirrhosis (89). However, it should be noted that women show a physiologically decrease of liver T1rho value with increasing age (90).
Hepatic T1 relaxation times and T1-derived extracellular volume fraction (ECV) are also promising quantitative MRI techniques to diagnose liver fibrosis, which can be obtained with T1 mapping sequences (91,92). Hepatic T1 relaxations times are increased in liver fibrosis and allow the calculation of hepatic ECV, which dichotomizes the liver parenchyma into an extracellular and cellular compartment due to an additional measurement of contrast-enhanced hepatic T1 relaxation times (93). Hepatic ECV already showed promising results for the detection of clinically significant fibrosis (F ≥2) in patients with primary sclerosing cholangitis or autoimmune hepatitis (94,95).
Technical considerations
Technical details of the discussed methods are provided in Table 2. VCTE is based on both ultrasound and low-frequency waves whose motion is directly correlated with liver elasticity (66). In contrast to VCTE, PSWE and 2D-SWE are not limited to obesity or presence of ascites and provide comparable results to TE (96). Here, an acoustic radiofrequency impulse is produced, which leads to transversely-oriented waves. Propagation velocity can be determined and tissue elasticity can be calculated based on these parameters (97). It has been demonstrated that intra-operator variability is increasing at higher liver stiffness measurements (LSM) and obese patients. It is therefore recommended to perform multiple LSM if body mass index is above 25 kg/m² and the median liver stiffness is measured above 7.1 kPa (98).
Limitations
For VCTE, the measurement depth is between 25–65 mm with the standard probe (68). Thus, in obese patients, TE needs to be performed with a larger probe permitting deeper tissue penetration with comparable results (99,100). As mentioned above, standard cross-sectional imaging techniques have low accuracy in fibrosis determination. Diffusion weighed MRI can detect liver cirrhosis due to its association with the ADC, which is lower in cirrhosis, but fails in differentiating individual stages of fibrosis (101). This can be improved via novel techniques as MRE and diffusion-weighted MRI.
Further tests for liver fibrosis
Rationale
Since performance of liver biopsies is associated with a periprocedural risk of complications, and sNITs and iNITs still comprise a portion of false-negative and false-positive classifications, further research is urgently needed in order to find novel biomarkers for fibrosis assessment. It stands to reason that components of hepatic ECM, which is modified during fibrogenesis, may serve as biomarkers. Furthermore, altered micro RNAs (miR) patterns have been identified during liver fibrosis and are therefore promising biomarkers (102). Giving recent technological advances, including high throughput screening and Omics techniques, new biomarkers are likely to emerge in the near future.
Indications
To be suitable as biomarkers, the molecules of interest should be well-detectable in peripheral blood samples. It was hypothesized that miR, that are downregulated in chronic diseased liver tissue, might be released via exocytotic pathways (103). Importantly, many miR are therefore detectable in peripheral blood samples of patients with fibrosis and are therefore suggested to function as biomarkers (102). As one of the first miRs, miR-122 was proposed to be marker of liver injury, given its down-regulation in liver fibrosis in hepatitis C, NASH and drug-induced liver damage (104-106). Indeed, circulating miR-122 levels are inversely correlated with chronic inflammation in patients with chronic hepatitis C, but are not capable to correctly predict liver fibrosis (107-109). However, in HIV/hepatitis C-coinfected patients, miR-122 correlated negatively with the hepatic venous pressure gradient, rendering it suitable for identifying portal hypertension (109). In the same cohort, miR-122 and miR-22 were correlating with significant fibrosis according to sNITs (110). In a microarray-based approach in a cohort of 130 chronic hepatitis C patients, the let-7 miR-family showed the best ability to predict liver fibrosis but were not superior to sNITs (111). For liver cirrhosis, two miRs, miR-571 and miR-652, were demonstrated to be differentially altered in serum samples. While miR-571 showed stage-dependent values according to the Child-Pugh classification, miR-652 was dysregulated independent of cirrhosis stage (112).
Beside miRs, components of the ECM have been identified as circulating biomarkers. Circulating collagen fragments are generated during fibrosis-related ECM remodeling and were checked for their ability to assess liver fibrosis (113-115). In particular, collagen peptides are suggested as biomarkers (116-118). Matrix metalloproteinase (MMP) degraded n-terminal propeptide of type III collagen, PRO-C3, was demonstrated to strongly correlate with FIB-4 values (sNIT, see above) and could serve as a fibrosis biomarker, which was proved in a cohort of HIV/Hepatitis C-coinfected patients (116). ECM components are emerging as biomarkers, which are not only assessing fibrosis but can predict patients’ outcome. A significant correlation between PRO-C3 and Hepatic venous pressure gradient (HVPG) was shown, providing additional predictive value for portal hypertension (116). Elastin is processed during fibrotic remodeling and its fragments can be detected in hepatic venous blood. It has been demonstrated that elastin fragments are more abundant in Child-Pugh C cirrhotic patients compared to Child-Pugh A and B. Moreover, circulating elastin fragments levels in the hepatic vein might reflect fibrosis remodeling and predict survival, what was investigated in patients receiving transjugular intrahepatic portosystemic stent shunting (TIPS) (119). MMP degraded elastin further might predict fibrosis progression, which was demonstrated in HIV infected patients (120). A model including MMP degraded elastin, PRO C3 and type VI collagen was demonstrated a high correlation with HVPG, which can be used to detect the degree of portal hypertension (121). Indeed, elastin is the most relevant biomarker in terms of prognosis either progression of fibrosis or outcome in CLD patients, probably since it reflects the crosslinking of ECM and therefore a more difficult to reverse process (122). More recently, microfibrillar-associated protein 4 (MFAP-4) was identified as novel biomarker, and validated in patients with liver fibrosis due to chronic hepatitis C and ALD (123,124). Combining MFAP-4 with other sNITs was able to improve the diagnostic accuracy in prediction of advanced fibrosis (123).
Technical considerations
Micro RNAs are small non-coding RNAs which are involved in posttrancriptional gene expression regulation. Micro RNAs have emerged as putative biomarkers due to their high abundance in various body fluids and their molecular stability (125,126). Since there is a lack of an endogenous normalizing control, which can be used for normalization of quantitative miR levels, spiked in of foreign RNA such as Simian virus-40 RNA (106,109), C. elegans short RNA, or to a short synthetic oligonucleotide (127) are frequently used as technical control and reference for quantification. Studies in patients undergoing TIPS are of great value because the access to blood of the portal vein. In portal vein analysis, hepatic derivation of biomarkers can be proven (128). Advanced sequencing methods (e.g., small RNA sequencing) will allow deeper insights in miR profiles of extracellular vesicles providing further evidence for these novel biomarkers (129).
Limitations
Due to its experimental nature, novel biomarkers are currently not used in the clinics and require further validation. Liver fibrosis is a dynamic process in chronic liver disease. It is noteworthy, that miR abundance can be perturbated because of the injury-dependent replacement of hepatocytes during fibrogenesis, which are the main source of miRs (107). Therefore, the combination of different approaches such as miR analyses and collagen peptide monitoring, and future options such as exosomal classification and non-coding RNA patterns might provide efficient non-invasive diagnostic strategies.
Conclusions
Assessment of liver fibrosis is key in order to stratify patients and plan management and surveillance. Liver biopsy and histological assessment still remains the gold-standard in determining fibrosis despite its inherent limitations. The value of NITs is increasingly acknowledged in daily clinical practice. Among them elastography methods were extensively investigated and are with AUROCs >0.9 very useful. However, NITs based on serum markers perform similar and do not consist inter-operator variability. Finally, cross-sectional imaging techniques are emerging and provide information on fibrosis distribution since the whole liver is examined. Combination of sNIT and iNIT is probably the best way to stratify patients’ risk as outlined in the current Baveno VII guidelines (130) and demonstrated in recent studies (75). Further research is urgently needed to convey between these methods and provide clear clinical algorithms for patients with CLD and suspected liver fibrosis.
Acknowledgments
Funding: This work was supported by German Research Foundation (DFG) [project ID 403224013 – SFB 1382 (A09) to JT], by the German Federal Ministry of Education and Research (BMBF) for the DEEP-HCC project and by the Hessian Ministry of Higher Education to JT, Research and the Arts (HMWK) for the ENABLE and ACLF-I cluster projects to JT. The MICROB-PREDICT (project ID 825694 to JT), DECISION (project ID 847949 to JT), GALAXY (project ID 668031 to JT), LIVERHOPE (project ID 731875 to JT), and IHMCSA (project ID 964590 to JT) projects have received funding from the European Union’s Horizon 2020 research and innovation program. The manuscript reflects only the authors’ views, and the European Commission is not responsible for any use that may be made of the information it contains. The funders had no influence on study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ralf Weiskirchen) for the series “The Pathogenesis of Hepatic Fibrosis: Basic Facts and Clinical Challenges” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-9/rc
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-9/coif). The series “The Pathogenesis of Hepatic Fibrosis: Basic Facts and Clinical Challenges” was commissioned by the editorial office without any funding or sponsorship. JAL received speaker fees from Philips Healthcare. Received fees related to the scientific advisory board of Bayer Healthcare. JT received Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Versantis, Gore, Boehringer-Ingelheim, Alexion, Falk, Grifols and CSL Behring. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 2019;65:37-55. [Crossref] [PubMed]
- Böttcher K, Pinzani M. Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Adv Drug Deliv Rev 2017;121:3-8. [Crossref] [PubMed]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18. [Crossref] [PubMed]
- Roehlen N, Crouchet E, Baumert TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020;9:875. [Crossref] [PubMed]
- Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14:397-411. [Crossref] [PubMed]
- Li J, Zeng C, Zheng B, et al. HMGB1-induced autophagy facilitates hepatic stellate cells activation: a new pathway in liver fibrosis. Clin Sci (Lond) 2018;132:1645-67. [Crossref] [PubMed]
- Nishitsuji H, Funami K, Shimizu Y, et al. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J Virol 2013;87:8169-78. [Crossref] [PubMed]
- Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 2014;147:765-783.e4. [Crossref] [PubMed]
- Natori S, Rust C, Stadheim LM, et al. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol 2001;34:248-53. [Crossref] [PubMed]
- Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci 2014;15:8591-638. [Crossref] [PubMed]
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016;65:1038-48. [Crossref] [PubMed]
- Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy. JHEP Rep 2021;3:100316. [Crossref] [PubMed]
- Bosch J, Abraldes JG, Berzigotti A, et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 2009;6:573-82. [Crossref] [PubMed]
- Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994;19:1513-20. [Crossref] [PubMed]
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47:598-607. [Crossref] [PubMed]
- Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut 2015;64:830-41. [Crossref] [PubMed]
- Nakatsukasa H, Nagy P, Evarts RP, et al. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest 1990;85:1833-43. [Crossref] [PubMed]
- Scheuer PJ, Maggi G. Hepatic fibrosis and collapse: histological distinction by orecin staining. Histopathology 1980;4:487-90. [Crossref] [PubMed]
- Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431-5. [Crossref] [PubMed]
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9. [Crossref] [PubMed]
- Desmet VJ. Histological classification of chronic hepatitis. Acta Gastroenterol Belg 1997;60:259-67. [PubMed]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15-20. [Crossref] [PubMed]
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825-32. [Crossref] [PubMed]
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [Crossref] [PubMed]
- Bedossa PFLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565-75. [Crossref] [PubMed]
- Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265-73. [Crossref] [PubMed]
- Lackner C, Spindelboeck W, Haybaeck J, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol 2017;66:610-8. [Crossref] [PubMed]
- Lackner C, Stauber RE, Davies S, et al. Development and prognostic relevance of a histologic grading and staging system for alcohol-related liver disease. J Hepatol 2021;75:810-9. [Crossref] [PubMed]
- Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol 1978;379:103-12. [Crossref] [PubMed]
- Nakanuma Y, Zen Y, Harada K, et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int 2010;60:167-74. [Crossref] [PubMed]
- de Vries EM, de Krijger M, Färkkilä M, et al. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: An international cohort study. Hepatology 2017;65:907-19. [Crossref] [PubMed]
- Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med 2000;124:1599-607. [Crossref] [PubMed]
- Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem 2006;52:1887-96. [Crossref] [PubMed]
- Zeremski M, Dimova RB, Benjamin S, et al. FibroSURE as a noninvasive marker of liver fibrosis and inflammation in chronic hepatitis B. BMC Gastroenterol 2014;14:118. [Crossref] [PubMed]
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007;7:40. [Crossref] [PubMed]
- Thiele M, Madsen BS, Hansen JF, et al. Accuracy of the Enhanced Liver Fibrosis Test vs FibroTest, Elastography, and Indirect Markers in Detection of Advanced Fibrosis in Patients With Alcoholic Liver Disease. Gastroenterology 2018;154:1369-79. [Crossref] [PubMed]
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-26. [Crossref] [PubMed]
- Abd El Rihim AY, Omar RF, Fathalah W, et al. Role of fibroscan and APRI in detection of liver fibrosis: a systematic review and meta-analysis. Arab J Gastroenterol 2013;14:44-50. [Crossref] [PubMed]
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. [Crossref] [PubMed]
- Li Q, Ren X, Lu C, et al. Evaluation of APRI and FIB-4 for noninvasive assessment of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN: A retrospective cohort study. Medicine (Baltimore) 2017;96:e6336. [Crossref] [PubMed]
- Xu XL, Jiang LS, Wu CS, et al. The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: A diagnostic or screening tool? J Formos Med Assoc 2022;121:454-66. [Crossref] [PubMed]
- Miyata M, Kuroda M, Unakami M, et al. Validation of the fibrosis-4 (FIB-4) index in the diagnosis of liver disease of rheumatoid arthritis patients treated with methotrexate. Mod Rheumatol 2019;29:936-42. [Crossref] [PubMed]
- McPherson S, Hardy T, Dufour JF, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol 2017;112:740-51. [Crossref] [PubMed]
- Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017;66:1486-501. [Crossref] [PubMed]
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-54. [Crossref] [PubMed]
- Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441-7. [Crossref] [PubMed]
- Daniels SJ, Leeming DJ, Eslam M, et al. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology 2019;69:1075-86. [Crossref] [PubMed]
- Madsen BS, Thiele M, Detlefsen S, et al. PRO-C3 and ADAPT algorithm accurately identify patients with advanced fibrosis due to alcohol-related liver disease. Aliment Pharmacol Ther 2021;54:699-708. [Crossref] [PubMed]
- Gunes Yegin E, Durusoy SS, Ture Ozdemir F, et al. Appraising diagnostic performance of ELF test by pathological staging and digital quantification of liver fibrosis. Ann Hepatol 2019;18:833-40. [Crossref] [PubMed]
- Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8. [Crossref] [PubMed]
- Sandrin L, Tanter M, Gennisson JL, et al. Shear elasticity probe for soft tissues with 1-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control 2002;49:436-46. [Crossref] [PubMed]
- Kishimoto R, Suga M, Koyama A, et al. Measuring shear-wave speed with point shear-wave elastography and MR elastography: a phantom study. BMJ Open 2017;7:e013925. [Crossref] [PubMed]
- Palmeri ML, Wang MH, Dahl JJ, et al. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol 2008;34:546-58. [Crossref] [PubMed]
- Bouchet P, Gennisson JL, Podda A, et al. Artifacts and Technical Restrictions in 2D Shear Wave Elastography. Ultraschall Med 2020;41:267-77. [Crossref] [PubMed]
- Bercoff J, Tanter M, Muller M, et al. The role of viscosity in the impulse diffraction field of elastic waves induced by the acoustic radiation force. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:1523-36. [Crossref] [PubMed]
- Guglielmo FF, Venkatesh SK, Mitchell DG, Liver MR. Elastography Technique and Image Interpretation: Pearls and Pitfalls. Radiographics 2019;39:1983-2002. [Crossref] [PubMed]
- Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726-36. [Crossref] [PubMed]
- Nielsen MJ, Leeming DJ, Goodman Z, et al. Comparison of ADAPT, FIB-4 and APRI as non-invasive predictors of liver fibrosis and NASH within the CENTAUR screening population. J Hepatol 2021;75:1292-300. [Crossref] [PubMed]
- Kruger FC, Daniels CR, Kidd M, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J 2011;101:477-80. [PubMed]
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32-6. [Crossref] [PubMed]
- Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104-12. [Crossref] [PubMed]
- Imbert-Bismut F, Messous D, Thibault V, et al. Intra-laboratory analytical variability of biochemical markers of fibrosis (Fibrotest) and activity (Actitest) and reference ranges in healthy blood donors. Clin Chem Lab Med 2004;42:323-33. [Crossref] [PubMed]
- Xie Q, Zhou X, Huang P, et al. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: a meta-analysis. PLoS One 2014;9:e92772. [Crossref] [PubMed]
- Ito K, Mitchell DG, Gabata T, et al. Expanded gallbladder fossa: simple MR imaging sign of cirrhosis. Radiology 1999;211:723-6. [Crossref] [PubMed]
- Kudo M, Zheng RQ, Kim SR, et al. Diagnostic accuracy of imaging for liver cirrhosis compared to histologically proven liver cirrhosis. A multicenter collaborative study. Intervirology 2008;51:17-26. [Crossref] [PubMed]
- Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705-13. [Crossref] [PubMed]
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960-74. [Crossref] [PubMed]
- Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol 2016;13:402-11. [Crossref] [PubMed]
- Qiu LX, Liu YL, Lin W, et al. Liver stiffness measurement is a potent predictor of histological fibrosis regression after hepatitis C virus clearance. Eur J Gastroenterol Hepatol 2021;33:547-54. [Crossref] [PubMed]
- Malin JJ, Boesecke C, Schwarze-Zander C, et al. Liver stiffness regression after successful Hepatitis C treatment is independent of HIV coinfection. HIV Med 2019;20:230-6. [Crossref] [PubMed]
- Deffieux T, Gennisson JL, Bousquet L, et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J Hepatol 2015;62:317-24. [Crossref] [PubMed]
- Guibal A, Renosi G, Rode A, et al. Shear wave elastography: An accurate technique to stage liver fibrosis in chronic liver diseases. Diagn Interv Imaging 2016;97:91-9. [Crossref] [PubMed]
- Fang C, Konstantatou E, Romanos O, et al. Reproducibility of 2-Dimensional Shear Wave Elastography Assessment of the Liver: A Direct Comparison With Point Shear Wave Elastography in Healthy Volunteers. J Ultrasound Med 2017;36:1563-9. [Crossref] [PubMed]
- Lee DH, Lee ES, Bae JS, et al. 2D shear wave elastography is better than transient elastography in predicting post-hepatectomy complication after resection. Eur Radiol 2021;31:5802-11. [Crossref] [PubMed]
- Trebicka J, Gu W, de Ledinghen V, et al. Two-dimensional shear wave elastography predicts survival in advanced chronic liver disease. Gut 2022;71:402-14. [Crossref] [PubMed]
- Herrmann E, de Lédinghen V, Cassinotto C, et al. Assessment of biopsy‐proven liver fibrosis by two‐dimensional shear wave elastography: An individual patient data‐based meta‐analysis. Hepatology 2018;67:260-72. [Crossref] [PubMed]
- Lamb P, Sahani DV, Fuentes-Orrego JM, et al. Stratification of patients with liver fibrosis using dual-energy CT. IEEE Trans Med Imaging 2015;34:807-15. [Crossref] [PubMed]
- Kupczyk PA, Mesropyan N, Isaak A, et al. Quantitative MRI of the liver: Evaluation of extracellular volume fraction and other quantitative parameters in comparison to MR elastography for the assessment of hepatopathy. Magn Reson Imaging 2021;77:7-13. [Crossref] [PubMed]
- Marri UK, Das P. Noninvasive Staging of Liver Fibrosis Using 5-Minute Delayed Dual-Energy CT: Comparison with US Elastography and Correlation with Histologic Findings. Radiology 2021;298:600-8. [Crossref] [PubMed]
- Wang QB, Zhu H, Liu HL, et al. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology 2012;56:239-47. [Crossref] [PubMed]
- Nowak S, Mesropyan N, Faron A, et al. Detection of liver cirrhosis in standard T2-weighted MRI using deep transfer learning. Eur Radiol 2021;31:8807-15. [Crossref] [PubMed]
- Anteby R, Klang E, Horesh N, et al. Deep learning for noninvasive liver fibrosis classification: A systematic review. Liver Int 2021;41:2269-78. [Crossref] [PubMed]
- Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2016;26:1431-40. [Crossref] [PubMed]
- Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015;13:440-451.e6. [Crossref] [PubMed]
- Guo Y, Parthasarathy S, Goyal P, et al. Magnetic resonance elastography and acoustic radiation force impulse for staging hepatic fibrosis: a meta-analysis. Abdom Imaging 2015;40:818-34. [Crossref] [PubMed]
- Chen J, Talwalkar JA, Yin M, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 2011;259:749-56. [Crossref] [PubMed]
- Petitclerc L, Sebastiani G, Gilbert G, et al. Liver fibrosis: Review of current imaging and MRI quantification techniques. J Magn Reson Imaging 2017;45:1276-95. [Crossref] [PubMed]
- Zhao F, Zhou N, Wang JL, et al. Collagen deposition in the liver is strongly and positively associated with T1rho elongation while fat deposition is associated with T1rho shortening: an experimental study of methionine and choline-deficient (MCD) diet rat model. Quant Imaging Med Surg 2020;10:2307-21. [Crossref] [PubMed]
- Allkemper T, Sagmeister F, Cicinnati V, et al. Evaluation of fibrotic liver disease with whole-liver T1ρ MR imaging: a feasibility study at 1.5 T. Radiology 2014;271:408-15. [Crossref] [PubMed]
- Wáng YXJ, Deng M, Lin J, et al. Age- and Gender-Associated Liver Physiological T1rho Dynamics Demonstrated with a Clinically Applicable Single-Breathhold Acquisition. SLAS Technol 2018;23:179-87. [Crossref] [PubMed]
- Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014;60:69-77. [Crossref] [PubMed]
- Luetkens JA, Klein S, Traeber F, et al. Quantitative liver MRI including extracellular volume fraction for non-invasive quantification of liver fibrosis: a prospective proof-of-concept study. Gut 2018;67:593-4. [Crossref] [PubMed]
- Luetkens JA, Klein S, Träber F, et al. Quantification of Liver Fibrosis at T1 and T2 Mapping with Extracellular Volume Fraction MRI: Preclinical Results. Radiology 2018;288:748-54. [Crossref] [PubMed]
- Mesropyan N, Kupczyk P, Kukuk GM, et al. Diagnostic value of magnetic resonance parametric mapping for non-invasive assessment of liver fibrosis in patients with primary sclerosing cholangitis. BMC Med Imaging 2021;21:65. [Crossref] [PubMed]
- Mesropyan N, Kupczyk P, Dold L, et al. Non-invasive assessment of liver fibrosis in autoimmune hepatitis: Diagnostic value of liver magnetic resonance parametric mapping including extracellular volume fraction. Abdom Radiol (NY) 2021;46:2458-66. [Crossref] [PubMed]
- Thiele M, Detlefsen S, Sevelsted Møller L, et al. Transient and 2-Dimensional Shear-Wave Elastography Provide Comparable Assessment of Alcoholic Liver Fibrosis and Cirrhosis. Gastroenterology 2016;150:123-33. [Crossref] [PubMed]
- Davis LC, Baumer TG, Bey MJ, et al. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2019;38:2-12. [Crossref] [PubMed]
- Dioguardi Burgio M, Grégory J, Ronot M, et al. 2D-shear wave elastography: number of acquisitions can be reduced according to clinical setting. Insights Imaging 2021;12:145. [Crossref] [PubMed]
- de Lédinghen V, Wong VW, Vergniol J, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J Hepatol 2012;56:833-9. [Crossref] [PubMed]
- Cardoso AC, Cravo C, Calçado FL, et al. The performance of M and XL probes of FibroScan for the diagnosis of steatosis and fibrosis on a Brazilian nonalcoholic fatty liver disease cohort. Eur J Gastroenterol Hepatol 2020;32:231-8. [Crossref] [PubMed]
- Wang YXJ, Huang H, Zheng CJ, et al. Diffusion-weighted MRI of the liver: challenges and some solutions for the quantification of apparent diffusion coefficient and intravoxel incoherent motion. Am J Nucl Med Mol Imaging 2021;11:107-42. [PubMed]
- Huang J, Yu X, Fries JW, et al. MicroRNA function in the profibrogenic interplay upon chronic liver disease. Int J Mol Sci 2014;15:9360-71. [Crossref] [PubMed]
- Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther 2017;174:63-78. [Crossref] [PubMed]
- Morita K, Taketomi A, Shirabe K, et al. Clinical significance and potential of hepatic microRNA-122 expression in hepatitis C. Liver Int 2011;31:474-84. [Crossref] [PubMed]
- Cheung O, Puri P, Eicken C, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 2008;48:1810-20. [Crossref] [PubMed]
- Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A 2009;106:4402-7. [Crossref] [PubMed]
- Trebicka J, Anadol E, Elfimova N, et al. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol 2013;58:234-9. [Crossref] [PubMed]
- Bihrer V, Friedrich-Rust M, Kronenberger B, et al. Serum miR-122 as a biomarker of necroinflammation in patients with chronic hepatitis C virus infection. Am J Gastroenterol 2011;106:1663-9. [Crossref] [PubMed]
- Jansen C, Reiberger T, Huang J, et al. Circulating miRNA-122 levels are associated with hepatic necroinflammation and portal hypertension in HIV/HCV coinfection. PLoS One 2015;10:e0116768. [Crossref] [PubMed]
- Anadol E, Schierwagen R, Elfimova N, et al. Circulating microRNAs as a marker for liver injury in human immunodeficiency virus patients. Hepatology 2015;61:46-55. [Crossref] [PubMed]
- Matsuura K, De Giorgi V, Schechterly C, et al. Circulating let-7 levels in plasma and extracellular vesicles correlate with hepatic fibrosis progression in chronic hepatitis C. Hepatology 2016;64:732-45. [Crossref] [PubMed]
- Roderburg C, Mollnow T, Bongaerts B, et al. Micro-RNA profiling in human serum reveals compartment-specific roles of miR-571 and miR-652 in liver cirrhosis. PLoS One 2012;7:e32999. [Crossref] [PubMed]
- Karsdal MA, Daniels SJ, Holm Nielsen S, et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int 2020;40:736-50. [Crossref] [PubMed]
- Schuppan D, Myneni S, Surabattula R. Liquid biomarkers for fibrotic NASH - progress in a complex field. J Hepatol 2022;76:5-7. [Crossref] [PubMed]
- Karsdal MA, Genovese F, Madsen EA, et al. Collagen and tissue turnover as a function of age: Implications for fibrosis. J Hepatol 2016;64:103-9. [Crossref] [PubMed]
- Jansen C, Leeming DJ, Mandorfer M, et al. PRO-C3-levels in patients with HIV/HCV-Co-infection reflect fibrosis stage and degree of portal hypertension. PLoS One 2014;9:e108544. [Crossref] [PubMed]
- Luo Y, Oseini A, Gagnon R, et al. An Evaluation of the Collagen Fragments Related to Fibrogenesis and Fibrolysis in Nonalcoholic Steatohepatitis. Sci Rep 2018;8:12414. [Crossref] [PubMed]
- Leeming DJ, Nielsen SH, Vongsuvanh R, et al. Endotrophin, a pro-peptide of Type VI collagen, is a biomarker of survival in cirrhotic patients with hepatocellular carcinoma. Hepat Oncol 2020;8:HEP32. [Crossref] [PubMed]
- Nielsen MJ, Lehmann J, Leeming DJ, et al. Circulating Elastin Fragments Are Not Affected by Hepatic, Renal and Hemodynamic Changes, But Reflect Survival in Cirrhosis with TIPS. Dig Dis Sci 2015;60:3456-64. [Crossref] [PubMed]
- Leeming DJ, Anadol E, Schierwagen R, et al. Combined antiretroviral therapy attenuates hepatic extracellular matrix remodeling in HIV patients assessed by novel protein fingerprint markers. AIDS 2014;28:2081-90. [Crossref] [PubMed]
- Leeming DJ, Karsdal MA, Byrjalsen I, et al. Novel serological neo-epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment Pharmacol Ther 2013;38:1086-96. [Crossref] [PubMed]
- Ortiz C, Schierwagen R, Schaefer L, et al. Extracellular Matrix Remodeling in Chronic Liver Disease. Curr Tissue Microenviron Rep 2021;2:41-52. [Crossref] [PubMed]
- Bracht T, Mölleken C, Ahrens M, et al. Evaluation of the biomarker candidate MFAP4 for non-invasive assessment of hepatic fibrosis in hepatitis C patients. J Transl Med 2016;14:201. [Crossref] [PubMed]
- Madsen BS, Thiele M, Detlefsen S, et al. Prediction of liver fibrosis severity in alcoholic liver disease by human microfibrillar-associated protein 4. Liver Int 2020;40:1701-12. [Crossref] [PubMed]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011;12:861-74. [Crossref] [PubMed]
- Beermann J, Piccoli MT, Viereck J, et al. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev 2016;96:1297-325. [Crossref] [PubMed]
- Roberts TC, Coenen-Stass AM, Wood MJ. Assessment of RT-qPCR normalization strategies for accurate quantification of extracellular microRNAs in murine serum. PLoS One 2014;9:e89237. [Crossref] [PubMed]
- Jansen C, Eischeid H, Goertzen J, et al. The role of miRNA-34a as a prognostic biomarker for cirrhotic patients with portal hypertension receiving TIPS. PLoS One 2014;9:e103779. [Crossref] [PubMed]
- Wang X, He Y, Mackowiak B, et al. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 2021;70:784-95. [Crossref] [PubMed]
- de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022;76:959-74. [Crossref] [PubMed]
Cite this article as: Brol MJ, Drebber U, Luetkens JA, Odenthal M, Trebicka J. “The pathogenesis of hepatic fibrosis: basic facts and clinical challenges”—assessment of liver fibrosis: a narrative review. Dig Med Res 2022;5:24.


