Moving from laparoscopic to robotic pancreatoduodenectomy: same to be a natural evolution
“The intuitive mind is a sacred gift and the rational mind is a faithful servant. We have created a society that honours the servant and has forgotten the gift…”—Albert Einstein.
Although in the current state of the Evidence Based Medicine (EBM), this article has no scientific basis, we will build on this reflection of Albert Einstein and share with you our thoughts on what is currently just an intuition about: “Moving from laparoscopic to robotic pancreaticoduodenectomy (RPD) same to be a natural evolution.”.
Of course, it is not as forecasters but rather as sensitizers that we write this article, and for this, we will use the most recent literature and our long experience of the minimally invasive surgery (MIS) started in 1989 and the minimally invasive pancreatic surgery (MIPS) that we have realized since 1995.
This is not the first time that the question has been asked and published about whether RPD is superior to laparoscopic pancreaticoduodenectomy (LPD). F Köckerling already concluded in 2014 that the oncological accuracy of robotic resection for pancreatic resection is seen to be adequate. Only the operating time is generally longer than for standard laparoscopic and open procedures, but the blood loss is less, conversion rates are lower and hospital stay is shorter (1). Of course, Köckerling concluded that randomized prospective studies were needed to be able to draw factual conclusions, and unfortunately, apart from the recent multicentre training program LEALAPS-3 published in 2021, which concluded that the RPD is feasible and safe in centres where at least 20 of these procedures are carried out per year (2), no other quality prospective randomized studies are available to us to date.
This lack of factual data is not surprising; it is difficult to launch such studies because of the hassle of not only selecting patients but also surgeon, as indirectly, the complexity of minimally invasive pancreatoduodenectomy (MIPD) and its serious post-operative complications have not spared teams that define themselves as “high volume”, as shown by the premature interruption of the LEOPARD-2 study (3).
We will then start by describing our opinion about MIPD, whose story begins in 1994, where Gagner and Pomp published the first minimally invasive laparoscopic pancreaticoduodenectomy (MILPD) (4). Since then, some teams around the world have clearly defined the key elements for achieving the MILPD, which are summarized in Figure 1.
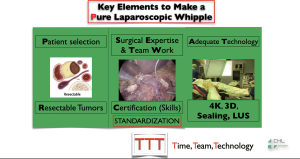
Of course, in most cases, it is called “basic” MILPD:
- Patients with no tumour involvement of the vessels based on the expertise of the tumour multidisciplinary board;
- No vascular anatomical variants present between 10 and 20 patients (5);
- Patients undergoing a “standard resection” (6) without any total mesopancreas excision (TMpE).
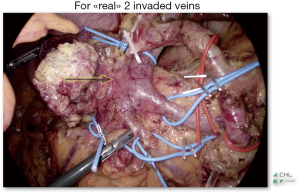
Because, what about the majority of patients who are immediately borderline, operated after neoadjuvant chemotherapy, with veins affected by the tumor (Figure 2) and to whom the resection and the reconstruction does heavily complicate the laparoscopic gesture, being responsible of the majority of unplanned laparotomic conversions during MILPD, which are published to be around 24% (7,8). It is easy to understand why the trial LEOPARD-2 was prematurely interrupted.
Additionally, 30 years after the first MILPD, we note that its implementation remains dramatically low. Probably still below 5% of pancreatic surgeons have implemented it, and we know that innovations come out of experimentation when the “bar” of more than 5% of users is reached (9,10).
Finally, we were very sensitized by the conclusions of R Ciría, who, after a comprehensive review of the state of the art of the MILPD, presented that up to date it has no advantage compared to the laparotomic PD, during the Hepato-Bilio-Pancreatic (HPB) Webinar of Spanish Surgeons Association (AEC) in 2021 (11). Thus, we are free to think, and we have also communicated at various meetings since 2019 that laparoscopy is more than questionable as a recommendable way to achieve a MIPD (12).
About minimally invasive robotic PD (MIRPD), it is also not very recent, and we owe the first to PC Giulianotti in 2003 (13). Its current implementation is still very weak but with increasing interest in the world of pancreatic surgeons.
Of course, the implementation of MIRPD will first meet financial criteria of accessibility to the robot, but there are also other reasons to be considered, for example, how to get out of the “comfort zone”, or in other words, how to learn it in order to not be overwhelmed by the impact of unplanned laparotomic conversions, almost always hemorrhagic and its serious consequences in terms of postoperative morbidity, doubling mortality, which have already been shown during laparoscopic approaches (7,8).
The challenge is big but not insurmountable; the MIRPD is aimed to pancreatic surgeons who have a “Minimally-Invasive Mind” and who “own” the robot, which means who know how to manipulate it. Therefore, robotic simulation is crucial, as it will allow to put the instrument in the surgeon’s hands and, especially, in the surgeon’s brain.
Then, it is recommended to do a “case observation” for a surgeon who does MIRPD. We recommend doing this step in a team involving the surgeon, who will be around the patient, the robotic scrub nurses and the console surgeon. The 3rd step is optional and consists of inviting an expert surgeon as advisor (proctor) during your first patients. The most important step is the standardization process of the surgical procedure and, of course, it should be started with simple cases at the beginning.
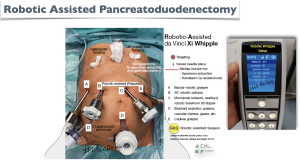
We use a robot Da Vinci Xi with an operating table connected to the robot and two operating consoles.
These 5 photos (Figures 3-7) show:
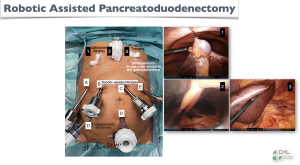

- Figure 3: our setup;
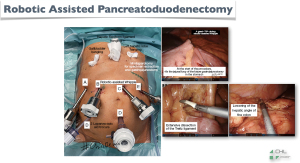
- Figure 4: three surgical steps made laparoscopically through the robotic ports, in order to reduce the operating time of some steps, which are simple but paradoxically difficult to make via robotics;
- Figure 5: have a very good surgical exposure by percutaneous suspension of the gallbladder, falciform ligament and left liver;
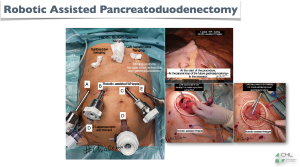
- Figure 6: the exteriorization of the specimen that can be done by a left sub-costal incision, which will allow us, at the same time, to carry out the extracorporeal gastro-jejunal anastomosis in a safe and very fast way;
- Figure 7: cosmetic results.
After a learning curve of three cases where standard resections were carried out, we extended MIRPD to patients with vascular anatomical variants and to borderline patients requiring venous resection.
We are currently performing all MIRPD with artery [superior mesenteric artery (SMA)] first approach and TMpE for all patients affected by pancreatic ductal adenocarcinoma (14).
To date, there is no conversion to laparotomy or laparoscopy. These 3 mini-videos illustrate two demonstrative examples of MIRPD, which we’ve published about our standardized technique:
- The Video 1: tips to make RPD;
- The Video 2: robotic pancreatojejunostomy;
- The Video 3: extended dissection with TMpE.
From our practice, we consider that if we have a robotic platform, the MIPD should be performed only by robotic approach, as the surgery is extremely precise; with excellent oncological radicality, pancreaticojejunostomy and hepaticojejunostomy are easier to achieve than by laparoscopy. and the comfort of surgeons is remarkable. Thus, the postoperative evolution is not inferior to those observed by laparoscopy in our group.
With two consoles—teaching and learning—proctoring is excellent, and the learning curve is shorter. We also recommend a pre-operative and post-operative briefing for all procedures in presence of all the members of the team (surgeons and nurses), in order to optimize the procedure by studying the difficult steps. In this way, a preventive solution can be provided, which can save operative time and reduce complications.
Here are our keys to make a MIRPD (Figure 8): we strongly point the attention on the fact that, in our opinion, robotic surgery is the result of team work, in which the surgeon leader is supported by the assistants during all the surgery without a real technical dependence because he is the only one manipulating the operating instruments. In others words, if laparoscopy is many hands and many minds, robotics is many minds but one hand, that of the surgeon leader. Laparoscopic surgery is the result of a team work, in which the surgeon leader is depending on the assistants’ minds and skills in order to achieve good results. In recent literature, it has been published that MIRPD is feasible, safe and not inferior, with a conversion rate of 6.5%, a pancreatic fistula rate of 23.6% and a 90-day mortality of 2%. However, the same study reports that, during the same period, laparoscopic approach for PD has decreased from 15% to 1%, and the robotic approach increased from 0 to 25% for the same procedure (2).
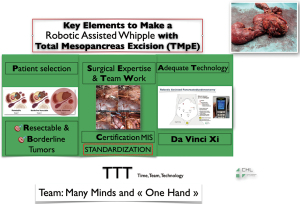
Conclusions
We intuitively think that MIRPD is superior to MILPD because the “surgical gesture is simple”. Although PD is still not a simple procedure, the implementation of the MIPD is better and more efficient via robotic approach, and the best way to compare is to perform a mandatory benchmarking and publish it, and make it factual that “moving from laparoscopy to robotic PD is a natural evolution…”.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Focus on Technical Advancement in Mini-invasive HPB Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-6/coif). The series “Focus on Technical Advancement in Mini-invasive HPB Surgery” was commissioned by the editorial office without any funding or sponsorship. ER and JSA served as the unpaid Guest Editors of the series. ER serves as an unpaid editorial board member of Digestive Medicine Research from September 2020 to August 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Köckerling F. Robotic vs. Standard Laparoscopic Technique - What is Better? Front Surg 2014;1:15. [PubMed]
- van Hilst J, de Rooij T, Bosscha K, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019;4:199-207. [Crossref] [PubMed]
- Zwart MJW, Nota CLM, de Rooij T, et al. Outcomes of a Multicenter Training Program in Robotic Pancreatoduodenectomy (LAELAPS-3). Ann Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Kim JH, Gonzalez-Heredia R, Daskalaki D, et al. Totally replaced right hepatic artery in pancreaticoduodenectomy: is this anatomical condition a contraindication to minimally invasive surgery? HPB (Oxford) 2016;18:580-5. [Crossref] [PubMed]
- Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156:591-600. [Crossref] [PubMed]
- Stiles ZE, Dickson PV, Deneve JL, et al. The impact of unplanned conversion to an open procedure during minimally invasive pancreatectomy. J Surg Res 2018;227:168-77. [Crossref] [PubMed]
- Lof S, Vissers FL, Klompmaker S, et al. Risk of conversion to open surgery during robotic and laparoscopic pancreatoduodenectomy and effect on outcomes: international propensity score-matched comparison study. Br J Surg 2021;108:80-7. [Crossref] [PubMed]
- Hughes-Hallett A, Mayer EK, Pratt PJ, et al. Quantitative analysis of technological innovation in minimally invasive surgery. Br J Surg 2015;102:e151-7. [Crossref] [PubMed]
- de Rooij T, Besselink MG, Shamali A, et al. Pan-European survey on the implementation of minimally invasive pancreatic surgery with emphasis on cancer. HPB (Oxford) 2016;18:170-6. [Crossref] [PubMed]
- Ciría R. Minimally Invasive Pancreaticoduodenectomy: State of the Art. Webinar AEC 2021. Available online: https://youtu.be/600F4oKEDhg
- Azagra S. Laparoscopy, but what happens to you? In: Advances in Fluorescence Imaging – ICG 2.0. International Workshop. Intraoperative ICG Fluorescence Imaging in Abdominal Surgery: Prevention of complications and oncological perspectives. Milano, 27-28 September 2018. Available online: https://cecongressi.it/wp-content/uploads/2020/09/ICG20_2018_09_20_DEF.pdf
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Azagra JS, Rosso E, Pascotto B, et al. Real robotic total mesopancreas excision (TMpE) assisted by hanging manoeuver (HM): Standardised technique. Int J Med Robot 2021;17:e2259. [Crossref] [PubMed]
Cite this article as: Azagra JS, Pascotto B, Gonzalez LG, De Blasi V, Rosso E, Goergen M; #ChirDeLux. Moving from laparoscopic to robotic pancreatoduodenectomy: same to be a natural evolution. Dig Med Res 2022;5:21.