
Rare histologies in peritoneal carcinomatosis: a narrative review
Introduction
Background
Peritoneal carcinomatosis (PC) usually occurs in advanced abdominal cancer. Historically, management of PC emphasized palliative care (1,2). In the 1990s, this was challenged for select cancers by surgical resection termed cytoreductive surgery (CRS) and intraperitoneal (IP) chemotherapy including hyperthermic intraperitoneal chemotherapy (HIPEC) (1-6). CRS is used to remove visible disease while HIPEC is used to remove microscopic disease during the same surgical procedure. HIPEC is the use of heated IP chemotherapy intra-operatively via the use the surgically placed catheters.
CRS and HIPEC are common for PC of colorectal, gastric, appendiceal, ovarian, pseudomyxoma peritonei, and mesothelioma origins (1,3,7,8). However, the role of CRS and HIPEC is undefined for other cancers which are considered rare and/or rarely treated histologies based on prior literature and delineated in the Appendix 1 (3,7-9). In addition, other techniques may improve survival in patients with PC including: novel IP chemotherapy (immunotherapy, hydrogel, electrostatic, oncolytic virus, and others), pressurized intraperitoneal aerosolized chemotherapy (PIPAC), photothermal therapy, and electrochemotherapy (2,10-18). PIPAC is the use of aerosolized chemotherapy infused intraabdominally with a surgically placed catheter. This review aims to identify cancers uncommonly treated for PC that may benefit from additional surgical or regional interventions.
Objectives
This review will summarize current incidence, prevalence, and management of untreated PC from 2016 to date. We present the following article in accordance with Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-4/rc).
Methods
A literature search was conducted for published case reports, clinical trials, or observational/retrospective reviews using PubMed for articles in all languages from 2016 to October 13, 2021. Figure 1 and Table 1 summarize the narrative review (19). A full search strategy is in Table 2 and the Appendix 1.
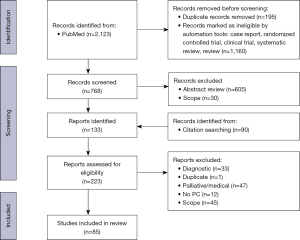
Table 1
| Author | Year | Type | Primary site | Malignancy | Sample size | CRS or surgery | IP C | Other treatment | OS (M) | RFS (M) | PFS (M) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yamada (20) | 2020 | Phase I/II clinical trial | Pancreas | PDAC | 38 | No1 | Yes | Ge/Nab-Px/IP Px | 12.43 | – | – |
| 8 | Yes1 | Yes | Ge/Nab-Px/IP Px | 16.53 | – | – | |||||
| Satoi (21) | 2017 | Phase II clinical trial | Pancreas | PDAC | 25 | No1 | Yes | Px/TS-1/IP Px | 14.23 | – | – |
| 8 | Yes1 | Yes | Px/TS-1/IP Px | 27.83 | – | – | |||||
| Takahara (22) | 2016 | Phase II clinical trial | Pancreas | PDAC | 35 | No1 | Yes | Px/TS-1/IP Px | 4.83 | – | 2.83 |
| Schwarz (23) | 2018 | Retrospective | Pancreas | PDAC | 21 | Yes1 | Yes | HIPEC | 4–243,8 | 13.2 | – |
| Tentes (24) | 2018 | Retrospective | Pancreas | PDAC | 6 | Yes1 | Yes | HIPEC | 83 | – | – |
| Graversen (25) | 2017 | Observational | Pancreas | PDAC | 5 | – | Yes | Ge/TS-1/PIPAC | 143 | – | – |
| Khosrawipour (26) | 2017 | Observational | Pancreas | PDAC | 20 | – | Yes | PIPAC | 9.154 | – | – |
| Rotolo (27) | 2020 | Case report | Pancreas | PDAC | 1 | No1 | No | FOLFIRINOX | 33 | – | 11 |
| No1 | Yes | FOLFIRI/PIPAC | – | 22 | |||||||
| Dumont (28) | 2020 | Phase I clinical trial | Mixed | Gastric | 3 | – | Yes | PIPAC | – | – | – |
| Small bowel | 2 | – | Yes | PIPAC | – | – | – | ||||
| Colorectal | 5 | – | Yes | PIPAC | – | – | – | ||||
| Liu (29) | 2018 | Observational | Small bowel | Adenocarcinoma | 152 | Yes1 | Yes | HIPEC | 325 | – | – |
| Liu (30) | 2016 | Observational | Small bowel | Mixed | 21 | Yes1 | Yes | HIPEC/C | 485 | – | – |
| 10 | Yes1 | No | C | 155 | – | – | |||||
| Seomangal (31) | 2019 | Case report | Jejunum | Adenocarcinoma | 1 | No1 | No | FOLFOX + bevacizumab | 2 | – | 6 |
| Yes | No | – | – | – | |||||||
| Sawatsubashi (32) | 2018 | Case report | Duodenum | Adenocarcinoma | 1 | Yes | No | Cisplatin + TS-1 | 33 | 24 | – |
| Yes1 | No | CAPOX + bevacizumab | – | 6 | |||||||
| Yes1 | No | R | – | – | |||||||
| Takemoto (33) | 2016 | Case report | Jejunum | Adenocarcinoma | 1 | Yes1 | No | TS-1 | 2 | 22 | – |
| Yes | No | Paclitaxel + doxifluridine | 55 | – | |||||||
| Achard (34) | 2020 | Case report | Prostate | Adenocarcinoma | 1 | Yes | No | – | 2 | 120 | – |
| No | No | Degarelix/R | 24 | – | |||||||
| Yes1 | No | Degarelix | – | – | |||||||
| 1 | Yes | No | – | 2 | 10 | – | |||||
| No | No | ADT/R | 131 | – | |||||||
| Yes1 | No | ADT/docetaxel | – | – | |||||||
| Motterle (35) | 2020 | Case report | Prostate | Adenocarcinoma | 1 | Yes | No | – | 2 | 20 | – |
| Yes1 | No | – | 24 | – | |||||||
| No1 | No | ADT | 18 | – | |||||||
| 1 | Yes | No | – | 2 | 48 | – | |||||
| Yes1 | No | Docetaxel/ADT | 60 | – | |||||||
| 1 | Yes | No | – | 2 | 34 | – | |||||
| Yes1 | No | Docetaxel/ADT | – | 34 | |||||||
| Caño-Velasco (36) | 2019 | Case report | Prostate | Adenocarcinoma | 1 | Yes | No | – | 2 | 6 | – |
| No | No | R/H | 24 | – | |||||||
| Yes | No | – | 18 | – | |||||||
| Yes1 | No | H/C | – | – | |||||||
| Le Thiec (37) | 2019 | Case report | Prostate | Adenocarcinoma | 1 | Yes | No | – | 2 | 24 | – |
| No | No | R | 36 | – | |||||||
| Yes | No | – | 30† | – | |||||||
| Yes1 | No | US | 6 | – | |||||||
| Li (38) | 2020 | Case report | Bladder | Urothelial carcinoma | 1 | Yes | No | – | 41 | <1 | – |
| Yes | No | – | 10 | – | |||||||
| No1 | No | Carboplatin/gemcitabine | – | 12 | |||||||
| Yes | No | Nivolumab | – | 6 | |||||||
| No | No | Carboplatin/gemcitabine | – | 6 | |||||||
| No | No | – | 41 | – | 6† | ||||||
| Pandey (39) | 2018 | Case report | Kidney | RCC | 1 | Yes | No | – | 16 | 6 | – |
| Yes1 | No | Sunitinib | – | 8 | |||||||
| No1 | No | Sorafenib | – | 2 | |||||||
| Kimura (40) | 2020 | Case report | Ileum | GIST | 1 | Yes1 | No | Imatinib | 2 | 144 | – |
| Yes | No | Sunitinib | 24 | – | |||||||
| Ono (41) | 2019 | Case report | Small bowel | GIST | 1 | Yes1 | No | Imatinib | 2 | – | 19 |
| Yes1 | No | Imatinib | – | 15 | |||||||
| Terada (42) | 2017 | Case report | Small bowel | GIST | 1 | Yes | No | Imatinib | 2 | 24 | – |
| Yes1 | No | Imatinib | – | – | |||||||
| Yes1 | No | Imatinib | – | – | |||||||
| Yes1 | No | Imatinib | – | – | |||||||
| Yes1 | No | Imatinib | – | – | |||||||
| Yes1 | No | Imatinib | – | 36 | |||||||
| Ishigame (43) | 2018 | Case report | Duodenum | GIST | 1 | Yes | No | Imatinib | 99 | 8 | – |
| Yes | No | Imatinib | 40 | – | |||||||
| Yes1 | No | Imatinib | 10 | – | |||||||
| Yes1 | No | Imatinib | 7 | – | |||||||
| Yes | No | Sunitinib7 | 8 | – | |||||||
| No | No | Imatinib + TACE | 4 | – | |||||||
| No | No | Imatinib + TACE | 2 | – | |||||||
| No | No | Imatinib + RFA | 5 | – | |||||||
| Yes6 | No | Sunitinib | – | – | |||||||
| Sugase (44) | 2016 | Case report | Jejunum | GIST | 1 | Yes | No | – | 2 | 24 | – |
| No1 | No | Nilotinib | – | 57 | |||||||
| Yes | No | Nilotinib | 21 | – | |||||||
| Jejunum | GIST | 1 | Yes | No | Imatinib | 2 | 48 | – | |||
| No | No | Nilotinib | – | 41 | |||||||
| Yes | No | – | 16 | – | |||||||
| No1 | No | Imatinib | – | – | |||||||
| Monobe (45) | 2017 | Case report | Jejunum | GIST | 1 | No | No | Imatinib | 2 | – | 36 |
| Yes1 | No | – | – | 17 | |||||||
| No1 | No | Imatinib | 24 | – | |||||||
| Modak (46) | 2020 | Phase I clinical trial | Various | DSRCT | 48 | Yes1 | No | C/IP 131I-omburtamab9 | – | – | – |
| RMS | 3 | Yes1 | No | C/IP 131I-omburtamab9 | – | – | – | ||||
| ES | 1 | Yes1 | No | C/IP 131I-omburtamab9 | – | – | – | ||||
| Hayes-Jordan (47) | 2018 | Phase II clinical trial | Various | DSRCT | 14 | Yes1 | Yes | C/R/HIPEC | 44.3§ | 14.9§ | – |
| RMS | 2 | Yes1 | Yes | C/HIPEC | 12.5§ | 4.5§ | – | ||||
| UDS | 2 | Yes1 | Yes | C/R/HIPEC | 12.5§ | 4.5§ | – | ||||
| ES | 1 | Yes1 | Yes | C/R/HIPEC | 12.5§ | 4.5§ | – | ||||
| Wang (48) | 2021 | Retrospective | Peritoneum | DSRCT | 6 | Yes1 | Yes | HIPEC | – | 14.43 | – |
| 2 | Yes1 | No | – | – | – | 7.5–22.6 | |||||
| Stiles (49) | 2020 | Retrospective | Peritoneum | DSRCT | 10 | Yes1 | Yes | HIPEC | 453 | – | – |
| Gani (50) | 2019 | Retrospective | Peritoneum | DSRCT | 200 | Yes1 | – | – | 25.93 | – | – |
| 285 | No1 | – | – | 25.93 | – | – | |||||
| Honoré (51) | 2019 | Retrospective | Peritoneum | DSRCT | 17 | Yes1 | Yes | HIPEC or EPIC | 253 | – | – |
| 54 | Yes1 | No | – | 253 | – | – | |||||
| 29 | No1 | No | – | 253 | – | – | |||||
| Scheer (52) | 2019 | Observational | Various | DSRCT | 5 | Yes1 | Yes | HIPEC | – | – | – |
| 35 | Yes1 | No | – | – | – | – | |||||
| 20f | No1 | No | – | 19.23 | – | – | |||||
| Stiles (53) | 2018 | Retrospective | Various | DSRCT | 74 | Yes1 | No | – | 31.33 | – | – |
| 48 | No1 | No | – | 18.33 | – | – | |||||
| Subbiah (54) | 2018 | Retrospective | Peritoneum | DSRCT | 82 | Yes1 | Yes | HIPEC | 353 | – | – |
| 32 | Yes1 | No | – | 353 | – | – | |||||
| 73 | No1 | No | – | 353 | – | – | |||||
| Zmora (55) | 2018 | Retrospective | Various | DSRCT | 1 | Yes1 | Yes | V/I/D/E/HIPEC | 21 | – | – |
| RMS | 2 | Yes1 | Yes | C/R/HIPEC | – | 28–29 | – | ||||
| RMS | 1 | Yes1 | Yes | C/R/HIPEC | 7 | – | – | ||||
| NB | 1 | Yes1 | Yes | C/R/HIPEC | – | 3 | – | ||||
| Angarita (56) | 2017 | Retrospective | Peritoneum | DSRCT | 5 | Yes1 | No | – | 513 | – | – |
| 15 | No1 | No | – | 213 | – | – | |||||
| Honoré (57) | 2017 | Retrospective | Peritoneum | DSRCT | 37 | Yes1 | No | – | 423 | – | – |
| 11 | Yes1 | Yes | HIPEC or EPIC | 423 | – | – | |||||
| Frank (58) | 2017 | Retrospective | Various | DSRCT | 1 | Yes1 | No | V/IF/D/E | 20.4 | – | – |
| Scalabre (59) | 2018 | Retrospective | Various | DSRCT | 7 | Yes1 | Yes | C/HIPEC | 16.46‡ | – | – |
| ES | 1 | Yes1 | Yes | C/R/HIPEC | 2 | – | 12.6 | ||||
| RMS | 1 | Yes1 | Yes | C/R/HIPEC | 17.5 | – | – | ||||
| Atallah (60) | 2016 | Retrospective | Peritoneum | DSRCT | 27 | Yes1 | No | R/C | 40.33 | – | – |
| 22 | Yes1 | No | C | 28.33 | – | – | |||||
| Osborne (61) | 2016 | Retrospective | Peritoneum | DSRCT | 32 | Yes1 | Yes | HIPEC/C/R | 603 | – | – |
| Somashekhar (62) | 2016 | Retrospective | Various | DSRCT | 1 | Yes1 | Yes | HIPEC | – | – | – |
| Sjoberg Bexelius (63) | 2021 | Case report | Peritoneum | DSRCT | 1 | No1 | No | V/D/Cy & IF/E | 2 | – | 8 |
| Yes1 | Yes | R/Vi/Cy/HIPEC | – | 18 | |||||||
| Gill (64) | 2021 | Case report | Peritoneum | DSRCT | 1 | No1 | No | V/D/Cy/IF/E | 2 | – | 2 |
| Yes1 | Yes | V/Da/I & V/IF/Te/HIPEC | 11 | – | |||||||
| No | No | 90Yittrium Radioembolization | 18 | – | |||||||
| Xiao (65) | 2021 | Case report | Peritoneum | DSRCT | 1 | No1 | No | V/D/Cy & IF/E | 2 | – | 3.5 |
| Yes1 | Yes | HIPEC | 0.5 | – | |||||||
| Yes | No | D/Cy & B/M/T/R/aH | 72 | – | |||||||
| Tsoukalas (66) | 2020 | Case report | Peritoneum | DSRCT | 1 | Yes1 | Yes | HIPEC/R seed | 6 | 1¶ | – |
| No1 | No | Cy/A/V/I/E | – | 5 | |||||||
| Nacef (67) | 2019 | Case report | Peritoneum | DSRCT | 1 | Yes1 | No | – | 2 | 1.25 | – |
| No1 | No | Cy/D/V | – | 1 | |||||||
| No1 | No | IF/E | – | – | |||||||
| Cracco (68) | 2017 | Case report | Peritoneum | DSRCT | 1 | Yes1 | Yes | 90Yittrium Radioembolization/C/HIPEC | 2 | – | 3 |
| Yes | No | Do/IF | 24 | – | |||||||
| Gesche (69) | 2019 | Observational | Various | RMS | 6 | Yes1 | Yes | – | 2 | 7–41 | – |
| Karamveri (70) | 2019 | Retrospective | Various | Liposarcoma | 7 | Yes1 | Yes | HIPEC | 553 | 95 | – |
| Leiomyosarcoma | 4 | Yes1 | Yes | HIPEC | 553 | 95 | – | ||||
| RMS | 5 | Yes1 | Yes | HIPEC | 553 | 95 | – | ||||
| Ovarian sarcoma | 4 | Yes1 | Yes | HIPEC | 553 | 95 | – | ||||
| Naffouje (71) | 2018 | Retrospective | Various | Liposarcoma | 15 | Yes1 | Yes | HIPEC | – | – | – |
| Leiomyosarcoma | 4 | Yes1 | Yes | HIPEC | – | – | – | ||||
| DSRCT | 2 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Angiosarcoma | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| PEComa | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Histiocytoma | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Carcinosarcoma | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Abu-Zaid (72) | 2016 | Retrospective | Various | Liposarcoma | 7 | Yes1 | Yes | HIPEC | 28.33 | 185 | – |
| Leiomyosarcoma | 1 | Yes1 | Yes | HIPEC | 28.33 | 185 | – | ||||
| ES | 1 | Yes1 | Yes | HIPEC | 28.33 | 185 | – | ||||
| GIST | 2 | Yes1 | Yes | HIPEC | 28.33 | 185 | – | ||||
| Spiliotis (73) | 2016 | Retrospective | Various | Liposarcoma | 4 | Yes1 | Yes | HIPEC | 123 | 105 | – |
| Leiomyosarcoma | 2 | Yes1 | Yes | HIPEC | 28–33 | 0–16 | – | ||||
| Fibrosarcoma | 1 | Yes1 | Yes | HIPEC | 4 | 0 | – | ||||
| RMS | 1 | Yes1 | Yes | HIPEC | 12 | 0 | – | ||||
| Kawamura (74) | 2019 | Case report | Abdominal | RMS | 1 | Yes1 | No | C/R | 2 | 36 | – |
| Pleština (75) | 2019 | Case report | Lung | UPS | 1 | Yes | No | – | 5 | 3 | – |
| Yes1 | No | – | – | 2 | |||||||
| Jun-long (76) | 2017 | Case report | Epididymus | Liposarcoma | 1 | Yes | No | – | 180 | 120 | – |
| Yes | No | – | 48 | – | |||||||
| Yes1 | No | – | – | 12 | |||||||
| Lin (77) | 2016 | Case report | Pancreas | Stromal tumor & leiomyosarcoma | 1 | Yes | No | 125I/microwave coagulation/imatinib | 2 | 13 | – |
| Yes1 | No | Microwave coagulation | – | – | |||||||
| Mehta (78) | 2018 | Observational | Liver | HCC | 21 | Yes1 | Yes | HIPEC | 46.7 3 | – | – |
| Berger (79) | 2016 | Retrospective | Liver | HCC | 17 | Yes1 | No | – | 19.53 | – | – |
| 5 | Yes1 | Yes | HIPEC | 29.73 | – | – | |||||
| Ji (80) | 2019 | Case report | Liver | HCC | 1 | Yes1 | No | Sorafenib | 2 | – | 3 |
| Yes1 | Yes | HIPEC/IP C/IV C | 22 | – | |||||||
| Takase (81) | 2019 | Case report | Liver | HCC | 1 | Yes | No | – | 2 | 33 | – |
| Yes | No | – | 25 | – | |||||||
| Yes1 | No | – | – | 2 | |||||||
| No1 | No | Sorafenib | – | 3 | |||||||
| Yes1 | No | Sorafenib | – | 11 | |||||||
| Yes1 | No | – | – | 5 | |||||||
| Yes1 | No | Sorafenib | – | 4 | |||||||
| Takiuchi (82) | 2018 | Case report | Liver | HCC | 1 | Yes | No | No | 30 | 7 | – |
| No | No | TACE/RFA | 8 | – | |||||||
| Yes1 | No | Sorafenib | – | 2.5 | |||||||
| Yes1 | No | TACE/TS-1/IAC/R/CK | – | 12.5 | |||||||
| Spiliotis (83) | 2017 | Case report | Liver | HCC | 3 | Yes | No | – | 2 | 8–24 | – |
| Yes1 | Yes | HIPEC | – | 2–28 | |||||||
| 1 | Yes | No | – | 14 | 6 | – | |||||
| Yes1 | Yes | HIPEC | – | 8 | |||||||
| Kyziridis (84) | 2020 | Case report | Liver | FHCC | 1 | Yes | No | – | 2 | 12 | – |
| Yes | No | – | 4 | – | |||||||
| Yes1 | No | – | 84 | – | |||||||
| Yes1 | No | – | 12 | – | |||||||
| Yes1 | Yes | HIPEC | 12 | – | |||||||
| Amblard (85) | 2018 | Retrospective | Various | CCA | 34 | Yes1 | Yes | HIPEC/C | 21.43 | – | 8.83 |
| 21 | No1 | No | C | 9.33 | – | 9.33 | |||||
| Falkenstein (86) | 2018 | Phase I clinical trial | Abdominal | GC | 5 | – | Yes | PIPAC | 2.364 | – | – |
| CCA | 8 | – | Yes | PIPAC | 44 | – | – | ||||
| Stefano (87) | 2021 | Case report | IH | CCA | 1 | Yes | No | Ge/Ci | 2 | 11 | – |
| Yes | No | Ce/FOLFIRI | 8 | – | |||||||
| No | No | Derazantinib | – | 14 | |||||||
| No1 | No | No | – | 3 | |||||||
| Yes1 | Yes | REP/Bl/HIPEC | – | 3 | |||||||
| No | No | FOLFOX | – | 5 | |||||||
| No | No | RE | – | 2 | |||||||
| No | No | TACE | – | 12 | |||||||
| Hernandez (88) | 2020 | Case report | IH | CCA | 1 | Yes1 | No | Ge/Ci | 2 | – | 1a |
| Yes1 | Yes | HIPEC | 12 | – | |||||||
| Mikuriya (89) | 2020 | Case report | Gallbladder | Adenocarcinoma | 1 | Yes1 | No | Ge/Ci | 2 | – | 12 |
| Yes | No | TS-1 then UFT | – | 15 | |||||||
| No | No | No | – | 5 | |||||||
| Hughes (90) | 2018 | Phase II clinical trial | Adrenal | ACC | 10 | Yes1 | Yes | HIPEC | 2 | – | 193 |
| Sugarbaker (91) | 2016 | Case report | Adrenal | ACC | 1 | Yes | No | R/C | 2 | – | 13 |
| No1 | No | – | – | 2 | |||||||
| Yes1 | Yes | HIPEC | – | 5 | |||||||
| Yes1 | Yes | HIPEC | 4 | – | |||||||
| Yes | Yes | HIPEC | – | – | |||||||
| Di Giorgio (92) | 2020 | Retrospective | Mixed | PDAC | 14 | No1 | Yes | PIPAC/C/other | 16.23 | – | – |
| CCA | 6 | No1 | Yes | PIPAC/C/other | 12.33 | – | – | ||||
| Leigh (93) | 2020 | Retrospective | Mixed | HCC | 9 | Yes1 | Yes | HIPEC | 423 | – | 73 |
| CCA | 4 | Yes1 | Yes | HIPEC | 193 | – | 103 | ||||
| GC | 3 | Yes1 | Yes | HIPEC | 83 | – | 23 | ||||
| PDAC | 1 | Yes1 | Yes | HIPEC | 153 | – | 153 | ||||
| Graversen (94) | 2018 | Phase I clinical trial | Mixed | Small bowel | 2 | – | Yes | PIPAC | – | – | – |
| CCA | 2 | – | Yes | PIPAC | – | – | – | ||||
| Pancreas | 3 | – | Yes | PIPAC | – | – | – | ||||
| Unknown | 1 | – | Yes | PIPAC | – | – | – | ||||
| Honoré (95) | 2016 | Retrospective | Mixed | DSRCT | 4 | Yes1 | Yes | HIPEC or EPIC | – | – | – |
| ACC | 4 | Yes1 | Yes | HIPEC or EPIC | – | – | – | ||||
| UC | 3 | Yes1 | Yes | HIPEC or EPIC | – | – | – | ||||
| FHCC | 3 | Yes1 | Yes | HIPEC or EPIC | – | – | – | ||||
| SPN | 2 | Yes1 | Yes | HIPEC or EPIC | – | – | – | ||||
| NB | 1 | Yes1 | Yes | HIPEC or EPIC | – | – | – | ||||
| RMS | 1 | Yes1 | Yes | HIPEC or EPIC | – | – | – | ||||
| Goéré (9) | 2017 | Retrospective | Mixed | Breast | 17 | Yes1 | Yes | HIPEC | – | – | – |
| CCA | 39 | Yes1 | Yes | HIPEC | – | – | – | ||||
| DSRCT | 34 | Yes1 | Yes | HIPEC | – | – | – | ||||
| GIST | 47 | Yes1 | Yes | HIPEC | – | – | – | ||||
| HCC | 19 | Yes1 | Yes | HIPEC | – | – | – | ||||
| NET | 114 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Pancreas | 30 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Sarcoma | 166 | Yes1 | Yes | HIPEC | – | – | – | ||||
| UC | 35 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Horvath (96) | 2018 | Retrospective | Mixed | PDAC | 6 | – | Yes | PIPAC | 12.74 | – | – |
| CCA | 6 | – | Yes | PIPAC | 15.14 | – | – | ||||
| Kurtz (97) | 2018 | Retrospective | Mixed | Hepatobiliary | 9 | – | Yes | PIPAC | – | – | – |
| Prostate | 1 | – | Yes | PIPAC | – | – | – | ||||
| Brandl (98) | 2017 | Retrospective | Mixed | Small bowel | 3 | Yes1 | Yes | HIPEC | – | – | – |
| Sarcoma | 3 | Yes1 | Yes | HIPEC | – | – | – | ||||
| CCA | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| NET | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Unknown | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| MPNST | 1 | Yes1 | Yes | HIPEC | – | – | – | ||||
| Teixeira Farinha (99) | 2017 | Retrospective | Mixed | Small bowel | 1 | – | Yes | PIPAC | – | – | – |
| Graziosi (100) | 2016 | Retrospective | Mixed | Small bowel | 2 | Yes1 | Yes | HIPEC | – | – | – |
| Breast | 1 | No1 | Yes | HIPEC | – | – | – | ||||
| Hamilton (101) | 2016 | Retrospective | Mixed | Small bowel | 1 | Yes1 | Yes | HIPEC | – | – | – |
| Pastrián (102) | 2019 | Case report | Liver | NET | 1 | Yes1 | No | Ci/E | 30 | – | – |
| Yes1 | No | Ce/Te | – | – | |||||||
| Yes1 | No | Everolimus | – | – | |||||||
| Yes1 | No | Sunitinib | – | – | |||||||
| Nagaro (103) | 2019 | Case report | Pancreas | NET | 1 | Yes | No | – | 2 | 120 | – |
| Yes | No | – | 96 | – | |||||||
| No | No | RFA | – | 6 | |||||||
| Yes | No | – | 24 | – | |||||||
| Yes1 | No | – | 24 | – |
1peritoneal metastases; 2alive at time of publication; 3mOS; 4median survival after 1st PIPAC; 5median survival from surgery; 6aborted surgery; 7discontinued due to side-effects; 8complete cytoreduction vs. incomplete cytoreduction; 9IP 131I-omburtamab; †not specific number of months in case report; ‡mOS; §median survival from the time of treatment; ¶subsequent imaging. CRS, cytoreductive surgery; OS, overall survival from diagnosis; RFS, recurrence-free survival from treatment; PFS, progression-free survival from treatment; M, months; PDAC, pancreatic ductal adenocarcinoma; NET, neuroendocrine tumor; SPN, solid pseudopapillary neoplasm; RCC, renal cell carcinoma; GIST, gastrointestinal stromal tumor; PEComa, perivascular epithelial cell tumor; FHCC, fibrolamellar hepatocellular carcinoma; CCA, cholangiocarcinoma; GC, gallbladder carcinoma; HCC, hepatocellular carcinoma; MPNST, malignant peripheral nerve sheath tumor; ACC, adrenal cortical carcinoma; DSRCT, desmoplastic small round cell tumor; RMS, rhabdomyosarcoma; UDS, undifferentiated sarcoma; ES, Ewing’s sarcoma; UPS, undifferentiated pleomorphic sarcoma; NB, nephroblastoma; UC, urachal carcinoma; C, chemotherapy; Ci, cisplatin; R, radiation; Ge, gemcitabine; Nab-Px, nab-paclitaxel; FOLFIRINOX, Fluorouracil, leucovorin, irinotecan, and oxaliplatin; FOLFOX, folinic acid, 5-fluorouracil, oxaliplatin; Ce, capecitabine; D, doxorubicin; FOLFIRI, folinic acid, 5-fluorouracil, irinotecan; S-1, tegafur, gimeracil, oteracil; CAPOX, Capecitabine and Oxaliplatin; V, vincristine; Vi, vinorelbine; Da, dactinomycin; Cy, cyclophosphamide; I, ifosfamide; B, busulfan; M, melphalan; T, thiotepa; A, adriamycin; E, etoposide; Te, temozolomide; PIPAC, pressurized intraperitoneal aerosolized chemotherapy; HIPEC, hyperthermic intraperitoneal chemotherapy; EPIC, early postoperative intraperitoneal chemotherapy; TACE, transarterial chemoembolization; Px, paclitaxel; IH, intrahepatic; IP, intraperitoneal; RFA, radiofrequency ablation; REP, reversible electroporation; US, ultrasound ablation; ADT, androgen deprivation therapy; H, hormone therapy; aH, autologous hematopoietic cell transplant; UFT, tegafur/uracil; IAC, intraarterial chemotherapy with epirubicin, cisplatin, & capecitabine or 5-fluorouracil or other chemotherapy; CK, cyberknife; Bl, bleomycin; mOS, median overall survival.
Table 2
| Items | Specification |
|---|---|
| Date of search | 13-Oct-21 |
| Databases & other sources | PubMed was used as primary database. A review of references was used to find additional relevant publications |
| Search terms | Please see Appendix 1 (Section “Iterative searches”) |
| Timeframe | All articles from January 1, 2016 to October 13, 2021 |
| Inclusion & exclusion criteria | Including all published case reports, clinical trials, or observational/retrospective reviews |
| Excluding only systemic therapy, diagnostic, duplicate data, non-PC, or otherwise outside of scope. See specific histology in Appendix 1 (Section “Search criteria”) | |
| Selection process | Primary author conducted initial search and selection of articles based on specific criteria. Expert review of included studies was conducted by senior author for discrepancy |
PC, peritoneal carcinomatosis.
Discussion
Narrative
One case report, four reviews and 3 trials were specific to pancreas (20-27). Three case reports, two reviews, and one clinical trial were specific to small intestine (28-33). Six case reports covered urologic malignancy (34-39). Ten case reports, five reviews, and two clinical trials covered sarcomas (40-47,59,69-71,73-77). Six case reports and 13 reviews were specific to desmoplastic small round cell tumor (DSRCT) (48-54,56-58,60-68). Eight case reports and three reviews covered hepatobiliary (78-85,87-89). One case report and one clinical trial were specific to adrenocortical carcinoma (ACC) (90,91). Two case reports were specific to neuroendocrine tumor (NET) (102,103). Twelve reviews and two clinical trials covered various cancer types (9,55,72,86,92-101). Overall, 37 case reports, 39 observational or retrospective reviews, and nine clinical trials were found as summarized in Figure 1 and Table 1.
Pancreas
Pancreatic ductal adenocarcinoma (PDAC)
In the United States (US) pancreatic cancer is common with 5-year survival of 9% (104). Globally, pancreatic cancer will be the second cause of cancer death by 2030 (105). PDAC accounts for 85% of all pancreatic cancers (106). Ninety percent appear sporadically, while others may be inherited (107,108). Inherited risk includes family history (80%) and known genetic factors (20%) (108). Fifty percent present with advanced disease with 1-year survival of 12% (106). Forty-two percent of advanced patients have PC (109).
Treatment
The National Comprehensive Cancer Network (NCCN) for advanced PDAC depends on performance status. Good performance status is treated with systemic therapy or clinical trials, while poor performance status is offered palliative care, radiation, and single agent therapy (110). The Japan Pancreas Society (JPS) similarly advises systemic therapy (111). Radiation may be used for metastatic disease (111). The role of CRS and HIPEC in PC of PDAC is unclear (3).
Literature review
Eight publications were found while five publications overlap with other cancer types as summarized in Table 1 (9,20-27,92-94,96). Three studies evaluated the use of IP chemotherapy (20-22). Takahara et al. (n=35) conducted a Phase II trial in patients with gemcitabine resistance and distant metastases using intravenous (IV) chemotherapy and IP paclitaxel with median overall survival (mOS) of 4.8 months (M) (22). Satoi et al. used the same treatment, however, patients had no resistance and only PC with mOS of 14.2 M (21). Eight patients who received curative resection resulted in mOS of 27.8 M (21). Yamada et al. used an alternative IV regimen with IP chemotherapy with mOS of 12.4 M (20). Eight patients underwent curative surgery and had mOS of 16.5 M (20). Four studies evaluated CRS and HIPEC (9,23,24,93). Schwarz et al. (n=21) found improvement with complete vs. incomplete CRS (mOS, 23 vs. 4 M, P<0.001) (23). Tentes et al. (n=6) achieved mOS of 8 M (24). Goéré et al. (n=30) reported hazard ratio (HR) in a mixed cohort including pancreas (HR: 3.23, 1.61–6.45), indicating relative low benefit (9). Leigh et al. (n=1) achieved OS of 15 M (93). Six studies evaluated PIPAC (25-27,92,94,96). Graversen et al. (n=5) reported mOS of 14 M (25). Khosrawipour et al. (n=20) reported mOS of 9.15 M after 1st PIPAC (26). Di Giorgio et al. (n=14) reported mOS of 16.2 M and mOS from 1st PIPAC of 10.9 (92). Horvath et al. (n=6) reported mOS of 12.7 M after 1st PIPAC (96). Graversen et al. (n=3) reported feasibility (94). A case report of IV chemotherapy with PIPAC reported 33 M OS (27). Neoadjuvant/adjuvant IP chemotherapy and complete CRS seem to offer benefit while HIPEC and PIPAC require further evaluation.
Breast
Breast cancer
Breast cancer may be the most frequent extra-abdominal tumor with PC (112). In the US, 1 in 8 women have breast cancer, representing 30% of all cancers and 15% of all cancer deaths (104). Breast cancer risk is multifactorial including: genetics, family history, hormonal therapy, and other factors (113). Estimates predict increases in advanced disease (114). One percent of all advanced patients have PC with increased incidence in lobular type (112,115).
Treatment
Molecular markers, not histological subtype is used to determine treatment e.g., ER, PR, HER2 (116). In NCCN guidelines, treatment of advanced disease may include: ovarian ablation (surgical or medical) and/or systemic therapies (117). Surgery or radiation may be considered for symptom management (117). European Society for Medical Oncology (ESMO) guidelines are similar but include additional markers including: HER2, BRCA, PIK3CA, PD-L1, and others (118). Surgery is reserved for downstaging or curative resection (118). CRS and HIPEC may provide survival benefit (3).
Literature review
Two publications overlap with other cancer types as summarized in Table 1 (9,100). Goéré et al. (n=17) evaluated CRS and HIPEC in a mixed cohort reporting HR including breast cancer (2.26, 1.01–5.05) indicating a relative benefit (9). Graziosi et al. reported laparoscopic palliative HIPEC that did not qualify for CRS (100). HIPEC and CRS appeared to show relative benefit in a single study. Given increasing burden, modern studies including CRS, HIPEC, PIPAC, and IP chemotherapy are needed.
Small intestine
Small bowel adenocarcinoma (SBA)
Small bowel malignancy is rare accounting for 3% of gastrointestinal tract malignancies in the US (104,119). Histological types include: adenocarcinoma (34–36%), carcinoid (26–28%), lymphoma (19–22%), and others (119). SBA occurs in 0.57–0.7 per 100,000 (120,121). Thirty percent will present with advanced disease with 5-year survival rate of 10% (122,123). PC occurs in 25–50% of advanced disease (124).
Treatment
French intergroup guidelines for advanced disease have no standard recommendation, however, fluoropyrimidine combination with a platinum agent may be used (125). NCCN guidelines are similar with selective addition of antiangiogenic, PD-1 and/or CTLA-4 drugs (126). Metastasectomy may be considered for select patients (126). CRS and HIPEC for PC may be beneficial in spite of limited data and heterogeneity (3).
Literature review
Six publications were found while five overlap with other cancer types as summarized in Table 1 (28-33,94,98-101). Five studies evaluated CRS with HIPEC in patients with small bowel malignancy (29,30,98,100,101). Liu et al. (n=152) achieved mOS 32 M (29). Multivariable regression showed survival benefit with: peritoneal cancer index (PCI) <15, well differentiated tumor, negative lymph nodes, and treatment within 6 M of detection (29). Liu et al. (n=31) evaluated CRS ± HIPEC (mOS, 48 vs. 15 M, P=0.019) (30). Brandl et al. (n=3) achieved a median tumor free interval of 9.4 M (98). Graziosi et al. (n=1) did not report individual outcomes (100). Hamilton et al. (n=1) reported a major complication, but no outcomes (101). Three studies evaluated PIPAC (28,94,99). Dumont et al. (n=2) conducted a Phase I study in a mixed cohort where two unidentified patients underwent complete CRS with HIPEC after PIPAC (28). Teixeira Farinha et al. (n=1) validated acceptable quality of life in mixed cohort (99). Graversen et al. (n=2) evaluated a mixed cohort with no individual results (94). Three cases were reported (31-33). Seomangal et al. treated PC with chemotherapy and curative resection with no recurrence (31). Takemoto et al. treated PC with initial resection, adjuvant chemotherapy, and repeat resection with OS 77 M (33). Sawatsubashi et al. treated PC complicated by bleeding with radiation, with OS 33 M (32). CRS and HIPEC may be beneficial. PIPAC requires larger trials.
Urologic
Prostate
Globally, prostate cancer is the 2nd cause of cancer death in the US and 5th globally with 5% presenting with advanced disease (104,127). Globally, black race has doubled mortality attributed to biological risk and other factors (128-130). Ninety percent presents as adenocarcinoma (131,132). Ten-year survival was 18.5% in advanced disease (133). PC may occur in 4–9% of advanced patients (134-136).
Treatment
NCCN guidelines differ for advanced disease in hormone naïve prostate cancer (HNPC) compared to castration resistant prostate cancer (CRPC) treated primarily with variations of systemic therapies and radiation (137). Surgery is reserved for biopsy or bone metastases (137). ESMO guidelines are similar to NCCN (138). Surgery is reserved for bone lesions and palliative care (138). There is no clear role for CRS or HIPEC in PC (3).
Literature review
Four publications were found while one study overlaps with other cancer types as summarized in Table 1 (34-37,97). Kurtz et al. (n=1) evaluated PIPAC in a mixed cohort, no outcomes were reported (97). Four case reports evaluated PC (34-37). Achard et al. (n=2) treated with CRS and systemic therapy, with no survival data (34). Motterle et al. (n=3) treated with CRS and systemic therapy with continued survival 34–60 M (35). Caño-Velasco et al. (n=1) treated with CRS and systemic therapy with no evidence of disease (36). Le Thiec et al. excised peritoneal nodules and ablated an anastomotic recurrence with ultrasound with biochemical response (37). HNPC and CRPC require different strategies. CRS, PIPAC, and ultrasound ablation should be evaluated in larger trials.
Bladder
In the US, bladder cancer is the 4th most common and 8th cause of cancer death in men (104). Ninety percent of bladder cancers of the urothelial cell carcinoma (UCC) or transitional cell carcinoma (TCC) type (139-141). Others (5–10%) include: squamous cell carcinoma, adenocarcinoma, and others (140,141). Adenocarcinoma of the bladder has a urachal carcinoma (UC) and non-urachal subtype (worse prognosis) (140). Five percent of patients present with advanced disease, while 35% will advance after initial cystectomy with a 5-year survival of 10% (142,143). PC may occur in up to 24% of patients with bladder cancer with higher risk in non UCC (6.4% vs. 32%, P<0.0002) (144).
Treatment
According to NCCN guidelines, treatment for advanced disease is based on histology (142). Advanced UCC may be treated with metastasectomy, intraoperative radiation therapy (IORT), or systemic therapy (142). ESMO guidelines recommend systemic therapy alone (145). Non UCC is treated per guidelines of similar histology. For example, UC is treated as gastrointestinal adenocarcinoma (142). Advanced bladder cancer has poor outcomes and new treatment is needed (146). CRS and HIPEC may have a role in PC for UC (3).
Literature review
One publication was found while two studies overlap with other cancer types as summarized in Table 1 (9,38,95). Two studies treated UC with CRS and HIPEC (9,95). Honoré et al. (n=3) treated patients with median PCI of 11, median follow-up of 20 M, and one death at 14 M (95). Two patients are disease free at 20 and 37 M (95). Goéré et al. (n=35) reported HR by cancer type including UC (1.00, Ref) indicating a relative benefit (9). Li et al. treated UCC with systemic therapy and local resection with continued progression (38). HIPEC and CRS may be beneficial in UC, while the benefit in UCC is unknown, larger trials are needed.
Kidney
In the US, kidney cancer affects 1 in 47–82 with 16–30% presenting with advanced disease (104,147). Clear cell accounts for 75% of renal cell carcinoma (RCC) (148). PC is rare and only described in case reports (149).
Treatment
NCCN guidelines for advanced disease are based on histology of RCC (150). Clear cell is treated with surgical metastasectomy, radiation, ablative, and systemic therapies (150). ESMO guidelines are similar but only recommend systemic therapy for uncontrolled disease (151). Systemic treatment is determined by risk stratification (151). Overall, non clear cell treatment for advanced disease is similar to clear cell (152). The role of CRS and HIPEC with PC is unknown.
Literature review
One publication was found while two studies overlap with other cancer types as summarized in Table 1 (39,55,95). A nephroblastoma (NB) patient was treated with CRS and HIPEC with PCI of 4 and death at 5 M (95). A second NB was treated with complete CRS and HIPEC with recurrence at 3 M and alive at 8 M (55). Pandey et al. (n=1) treated RCC with CRS and systemic therapy with progression-free survival (PFS) 8 M with OS 16 M (39). More data is required for CRS and HIPEC in kidney malignancy.
Sarcoma
General sarcoma
Sarcomas can be divided into soft tissue sarcoma (STS) and bone sarcoma (153). In the US, 1% of cancers are STS with 15% with advanced disease and 5-year survival of 16% (154). There are more than 50 histologic subtypes including: leiomyosarcoma or gastrointestinal stromal tumor (GIST) (23–24%), liposarcoma (11–20%), rhabdomyosarcoma (RMS) (5%), Ewing’s sarcoma (ES) (2–3%), and DSRCT (<1%) (155,156). PC occurs in 2–19% (157,158).
Treatment
According to NCCN guidelines advanced disease is treated with: radiation, chemotherapy, surgical resection, embolization, ablation and/or other systemic therapies (159). Special considerations are given to GIST, desmoid tumors, ES, and RMS. RMS has pleomorphic and non-pleomorphic subtypes. Non-pleomorphic RMS should be treated under specialist care (159). ESMO guidelines for advanced disease recommend treatment with surgery and chemotherapy (histology specific) (160). Special considerations are given to retroperitoneal sarcomas, uterine sarcomas, desmoid tumors, and breast sarcomas (160). Complete CRS in PC may be beneficial in leiomyosarcoma and liposarcoma (3). The role of CRS in PC in other malignancies is unknown, while HIPEC requires further evaluation (3).
Literature review
Seven publications were found while nine studies overlapped with other cancer types as summarized in Table 1 (9,46,47,55,59,69-77,95,98). 11 studies reported the use of CRS and HIPEC in various sarcomas (9,47,55,59,69-73,95,98). Hayes-Jordan et al. reported a mixed cohort of sarcoma: RMS (n=2), undifferentiated (n=2), and ES (n=1) (47). All had complete CRS with mOS 12.52 M and median recurrence-free survival (mRFS) of 4.5 M (47). Karamveri et al. reported a mixed cohort: liposarcoma (n=7), leiomyosarcoma (n=4), RMS (n=5), ovarian sarcoma (n=4) (70). Overall patients had mOS 55 M and mRFS 9 M (70). Naffouje et al. reported a mixed cohort: liposarcoma (n=15), leiomyosarcoma (n=4), DSRCT (n=2), angiosarcoma (n=1), PEComa (n=1), histiocytoma (n=1), and carcinosarcoma (n=1) (71). Improved survival was associated with low vs. high simplified peritoneal sarcomatosis score (SPSS) (mOS, 36 vs. 16 M, P=0.021) (71). Abu-Zaid et al. reported a mixed cohort: liposarcoma (n=7), leiomyosarcoma (n=1), ES (n=1), GIST (n=2) (72). Overall patients had mOS 28.3 M and mRFS 18 M (72). Spiliotis et al. reported a mixed cohort: liposarcoma (n=4, mOS 12 M, mRFS 10 M), leiomyosarcoma (n=2, OS 28–33 M, RFS 0–16 M), fibrosarcoma (n=1, OS 4 M, RFS 0 M), and RMS (n=1, OS 12 M, RFS 0 M) (73). Zmora et al. (n=3) treated RMS with mRFS 28–29 M (n=2) and OS 7 M (n=1) (55). Scalabre et al. treated RMS (n=1) reporting OS 17.5 M and ES (n=1) reporting PFS of 12.6 M (59). Honoré et al. treated RMS with PCI of 6 and OS after HIPEC 15 M (95). Brandl et al. (n=1) treated malignant peripheral nerve sheath tumor with PFS 5.3 M with OS 10 M (98). Gesche et al. (n=6) treated RMS with RFS 7–41 M (69). Goéré et al. (n=166) treated a mixed cohort reporting HR by tumor type including: sarcoma (1.81, 1.01–3.25) and ovarian with (0.82, 0.45–1.49) amongst others (9). IP radioimmunotherapy was evaluated after CRS in a mixed cohort including RMS (2) and ES (1) in a Phase I study (46). Four cases were reported (74-77). Kawamura et al. treated RMS with CRS and whole abdominopelvic radiation (WART) with RFS 3 years (74). Pleština et al. planned CRS for lung undifferentiated sarcoma but treatment was complicated by emergent bowel obstruction and resection and survival of 2 M (75). Li et al. treated epididymal liposarcoma with CRS with death at 1 year after treatment and OS 15 years (76). Lin et al. treated a pancreatic stromal tumor transdifferentiation into leiomyosarcoma after imatinib treatment and PC with resection and microwave coagulation showing evolution of malignancy due to treatment (77). Given tumor heterogeneity, multidisciplinary therapy can provide tailored treatment including: CRS, HIPEC, regional radiation, and/or systemic therapy. More research is needed on IP radioimmunotherapy.
GIST
GIST is the most common gastrointestinal mesenchymal malignancy. In the US, incidence is 0.70 per 100,000 (161). Most GISTs are sporadic, however, risk can be inherited or genetic (162). Primary site includes: gastric (65%), small intestine (30%), colorectal (5%) (161). The focus of this review with be small bowel GIST. 11% had PC in a population where 43% had small bowel GIST (163).
Treatment
NCCN guidelines do not differentiate treatment for advanced GIST by primary site. Tyrosine kinase inhibitor (TKI) therapy followed by resection, ablation, radiation, or changes to TKI therapy is recommended (164). ESMO guidelines are similar (165). Non--gastric GIST may not respond to TKIs compared to gastric GIST (166). Other emerging modalities of treatment include: various forms of radiation, immunotherapy, and other systemic therapies (167-169).There does not appear to be a role for HIPEC for GIST with PC (3,170).
Literature review
Six publications were found while two studies overlap with other cancer types as summarized in Table 1 (9,40-45,72). Goéré et al. treated mixed cohort with CRS and HIPEC reporting HR including: GIST (1.43, 0.72–2.84), ovarian (0.82, 0.45–1.49), and sarcoma (1.81, 1.01–3.25) amongst others (9). Abu-Zaid et al. (n=2 of 11) treated a mixed cohort with CRS and HIPEC with no individual results (72). Six case reports were found (40-45). Kimura et al. treated bowel perforation with PC with CRS and imatinib with RFS of 12 years followed by resection and use of sunitinib with RFS 24 M (40). Two other cases were similar (41,42). Ishigame et al. treated PC 40 M after primary resection, requiring multiple surgeries (43). Ultimately the patient progressed with liver disease with OS >99 M (43). Sugase et al. reported recurrence after primary resection at 24 M with PC, treated with nilotinib with PFS 57 M, followed surgical resection (44). Monobe et al. treated with CRS without resecting lesions <5 mm followed by imatinib with RFS 24 M (45). The role of CRS appears to be beneficial, while CRS with HIPEC requires more data.
DSRCT
DSRCT is a rare STS affecting young patients occurring in 0.3 per million (171). Five-year survival is <35% with worse outcomes for black patients (171). The primary site of DSRCT is most commonly the abdomen/pelvis but can include many other areas (171). PC occurs in 50% (172).
Treatment
NCCN and ESMO do not have specific guidelines for DSRCT (159,160). Treatment of DSRCT is multimodal with neoadjuvant chemotherapy with or without consolidation therapy including radiation or myeloablation prior to surgical resection or CRS (172,173). HIPEC remains to be evaluated, however, CRS appears beneficial with no maximum PCI score (3).
Literature review
Nineteen publications were found as summarized in Table 1 (48-54,56-58,60-68). Seven additional studies overlapped with other cancer types also summarized in Table 1 (9,46,47,55,59,71,95). Fourteen publications evaluated HIPEC in various contexts. Wang et al. evaluated CRS and HIPEC (n=6) vs. CRS (n=2) with patients currently alive with disease (8.4–20.3 vs. 7.5–22.6 M) (48). Stiles et al. (n=10) evaluated CRS and HIPEC with mOS of 45 M, however, there was heterogeneity in multimodal treatment (49). Honoré et al. (n=100) evaluated outcomes in DSRCT by treatment: chemotherapy (n=80), surgery (n=71), HIPEC or early postoperative intraperitoneal chemotherapy (EPIC) (n=17), radiation (n=26), and postoperative chemotherapy (n=54) (51). Cure was associated with female gender, PCI, stage, complete CRS, and radiation (51). Scheer et al. (n=60) evaluated treatment by: chemotherapy (n=60), stem cell (n=9), HIPEC (n=5), and surgery (n=40) (52). HIPEC could not be evaluated while complete vs. incomplete CRS showed benefit (mOS, 50.4 vs. 31.2 M) (52). Subbiah et al. (n=187) evaluated treatment by: chemotherapy (n=183), surgery (n=114), HIPEC (n=82), and radiation (n=91) (54). Survival benefit was associated with surgery and radiation, but not HIPEC (54). Honoré et al. (n=48) evaluated outcomes after complete CRS also treated by: chemotherapy (n=38), radiation (n=23), HIPEC or EPIC (n=11) (57). Only WART was associated with improved survival (57). Somashekhar et al. (n=1) evaluated CRS and HIPEC in a mixed cohort, with no individual results (62). Honoré et al. (n=4) evaluated CRS and HIPEC in a mixed cohort with median PCI of 21, RFS 11-16 M, median follow-up after HIPEC of 29 M, and 3 deaths (95). Goéré et al. (n-34) evaluated CRS and HIPEC in a mixed cohort reporting HR by etiology including: DSRCT (2.29, 1.13–4.65), sarcoma (1.81, 1.01–3.25), and ovarian cancer (0.82, 0.45–1.49) (9). Hayes-Jordan et al. (n=14) conducted a Phase II trial with CRS and HIPEC with mOS 58.44 M (47). Zmora et al. (n=1) evaluated CRS and HIPEC in a mixed cohort with OS of 21 M (55). Naffouje et al. (n=4 of 25) evaluated CRS and HIPEC in a mixed cohort with benefit of low vs. high SPSS (mOS, 36 vs. 16 M, P=0.021) (71). Scalabre et al. (n=7) evaluated CRS and HIPEC with chemotherapy with mean OS of 16.46 M (59). Osborne et al. (n=32) evaluated CRS, HIPEC, WART, and chemotherapy with mOS 60 M (61). Five studies evaluated CRS, chemotherapy, and radiation (50,53,56,58,60). Gani et al. (n=485) evaluated outcomes by treatment including: surgery (n=200), chemotherapy (n=415), and radiation (n=63) (50). Regression showed improved OS with surgery, chemotherapy, and radiation (50). Stiles et al. (n=125) evaluated the benefit of CRS (mOS, 31.3 vs. 18.3 M, no significance) (53). Multimodal treatment was effective with CRS, chemotherapy, and radiation vs. no treatment (mOS, 28.8 vs. 8.4 M, P<0.001) (53). Angarita et al. (n=20) evaluated outcomes after CRS (n=5), radiation (n=3), and chemotherapy (n=20) with significantly improved OS after CRS with HR (0.1, 0.3–0.7, P<0.02) (56). Frank et al. (n=1) evaluated a mixed cohort treated with CRS and chemotherapy with OS 20.4 M (58). Atallah et al. (n=49) evaluated complete CRS with and without radiation (mOS, 40.3 vs. 28.3 M, no significance) (60). Modak et al. (n=48) evaluated the safety of CRS followed by IP radioimmunotherapy in a mixed cohort in a Phase I trial (46). Five case reports followed patients after CRS, HIPEC, chemotherapy, radiation, and/or radioembolization with four patients alive at publication (63-66,68). Recurrence after CRS and HIPEC was treated with CRS, radioembolization, chemotherapy, and/or autologous hematopoietic cell transplant (64-66,68). Nacef et al. did not use HIPEC as part of therapy with continued progression on systemic chemotherapy (67). Overall, there is a consistent benefit of CRS, chemotherapy, and radiation while the benefit of HIPEC is unclear. IP radioimmunotherapy may offer a significant tool in the future radiosensitivity.
Hepatobiliary
Hepatocellular carcinoma (HCC)
In the US, liver cancer is a common cause of cancer death (11.4–12.5 per 100,000) with 3% presenting with advanced disease (104). HCC constitutes over 75% of liver malignancies (174). Increased risk is associated with: male gender, race, geographic location, hepatitis B and C virus, aflatoxins, smoking, alcohol, and non-alcoholic fatty liver disease (NAFLD) (174,175). In meta-analyses, PNPLA3 gene variant is associated with Hispanic race, alcoholic liver cirrhosis, NAFLD, and HCC (176-178). Five-year survival for HCC is 18.2% (179). Four percent of advanced patients have PC (180).
Treatment
According to NCCN guidelines, advanced HCC is treated with systemic therapy, clinical trials, or supportive care (181). ESMO guidelines are similar (182). Other options include targeted therapies and immunotherapies (183). The role of CRS and HIPEC for PC is unclear (3).
Literature review
Six publications were found while two studies overlap with other cancer types as summarized in Table 1 (9,78-83,93). Six studies evaluated CRS and HIPEC in various contexts (9,78-80,83,93). Goéré et al. (n=19) evaluated a mixed cohort reporting HR including: HCC (0.77, 0.29–2.03), sarcoma (1.81, 1.01–3.25), and ovarian cancer (0.82, 0.45–1.49) (9). Leigh et al. (n=9) evaluated a mixed cohort with mOS 42 M (93). Mehta et al. (n=21) compared complete and incomplete CRS with HIPEC (mOS, 46.7 vs. 5.9 M) (78). Berger et al. (n=22) evaluated CRS with and without HIPEC (mOS, 29.7 vs. 19.5 M, p=0.32) (79). Ji et al. reported a case treated with CRS and HIPEC followed by IP and IV chemotherapy with RFS 22 M (80). Spiliotis et al. (n=4) achieved PFS of 2–28 M (n=3) and one death with OS of 14 M (83). Two other cases were reported. Takase et al reported five surgical resections of PC followed by systemic therapy extending survival 2 years (81). Takiuchi et al. reported use of CRS, systemic therapy, radiotherapy, and chemoembolization with death at 15 M after PC (82). CRS with HIPEC appears to be beneficial with the possibility of additional benefit with adjuvant IP and IV chemotherapy, however, larger studies are needed.
Fibrolamellar hepatocellular carcinoma (FHCC)
FHCC is a variant of HCC with incidence of 0.02 per 100,000 in the US (184). Patients are typically young, have a single lesion, and normal alpha fetoprotein (185). Patients have mOS of 32.9 M with more than 20% with advanced disease (184). Eighteen percent have PC with advanced disease (186,187).
Treatment
NCCN does not provide specific recommendations for FHCC given its rarity (181). ESMO guidelines are also lacking (182). Aggressive surgical therapy, liver transplant, radiation, and investigational therapies may be beneficial while chemotherapy has no benefit (185,188). CRS and HIPEC may not improve survival in FHCC with PC, although data is limited at this time (3).
Literature review
One publication was found while one study overlaps with other cancer types as summarized in Table 1 (84,95). Honoré et al. (n=3) evaluated CRS and HIPEC in a mixed cohort with median PCI of 7, median follow-up after HIPEC of 37 M, and one deceased patient (95). Distant metastatic disease was reported at 13 M (95). Kyziridis et al. (n=1) reported treatment with CRS and HIPEC with RFS 12 M (84). CRS and HIPEC needs further evaluation in larger studies. Distant recurrence remains an issue.
Cholangiocarcinoma (CCA)
Globally, CCA is the second most common liver malignancy (15%) classified by anatomy into intrahepatic, hilar, and extrahepatic types (174,189,190). In the US, intrahepatic (65%) is more common (189). Up to 28% of patients may present with advanced disease with mOS of 4.5 M (191). PC occurs in more than 44% (192).
Treatment
According to NCCN Guidelines, CCA management depends on anatomic location of the primary tumor (181). Advanced disease may be treated with biliary drainage, systemic therapy, radiation, or clinical trials (181). ESMO has similar recommendations (193). The role of CRS and HIPEC for PC is unclear (3).
Literature review
Four publications were found through review while seven studies overlap with other cancer types as summarized in Table 1 (9,85-88,92-94,96-98). Four studies evaluated CRS and HIPEC in CCA (9,85,93,98). Amblard et al. (n=55) evaluated CRS and HIPEC vs. chemotherapy alone with mOS (21.4 vs. 9.3, P<0.007) (85). Leigh et al. (n=4) achieved mOS of 19 M (93). Goéré et al. (n=39) evaluated a mixed cohort reporting HR including: CCA (2.85, 1.45–5.6), HCC (0.77, 0.29–2.03), and DSRCT (2.29, 1.13–5.6) (9). Brandl et al. (n=1) recorded survival after treatment of 12.7 M (98). Five studies evaluated PIPAC (86,92,94,96,97). Di Giorgio et al. (n=6) achieved mOS from 1st PIPAC of 10.9 M and mOS of 12.3 M (92). Graversen et al. (n=2) evaluated safety in mixed cohort in a Phase I study (94). Horvath et al. (n=6) evaluated a mixed cohort achieving mOS 15.1 M from 1st PIPAC (96). Falkenstein et al. (n=8) achieved mOS from 1st PIPAC of 4 M (86). Kurtz et al. (n=9) reported feasibility of PIPAC in a mixed cohort (97). Two cases were reported. Hernandez et al. reported treatment with CRS and HIPEC with RFS 12 M (88). Stefano et al. reported unresectable CCA with PC treated with CRS, HIPEC, and intraoperative reversible electroporation with chemotherapy (87). Patient recurred in new lesions treated with radioembolization or chemoembolization, with PFS of 12 M (87). Current evidence supports CRS and HIPEC while other therapies such as PIPAC and electrochemotherapy require further evaluation.
Gallbladder carcinoma (GC)
In the US, GC occurs in 1.13 per 100,000 and deaths in 0.62 per 100,000 with female predominance globally (194,195). Native American and Hispanic background has worse survival (194). Subtypes include: adenocarcinoma (88%), others (10%), and squamous cell carcinoma (1%) (194). Overall, patients have mOS of 10 M (196). Five-year survival in advanced GC is 2% (195). PC occurs in 26% of advanced patients (197).
Treatment
According to NCCN guidelines, advanced GC may be treated with systemic therapy, clinical trials, and supportive care (181). ESMO guidelines do not differentiate between GC and CCA in advanced disease (193). The role of CRS and HIPEC for PC remains controversial (3).
Literature review
One publication was found while two studies overlap with other cancer types as summarized in Table 1 (86,89,93). Leigh et al. (n=3) evaluated CRS and HIPEC with mOS 8 M and 13 M OS with complete CRS (n=1) (93). Falkenstein et al. (n=5) achieved mOS from 1st PIPAC of 2.36 M (86). Poor survival may be due to lack of strict exclusion criteria. Mikuriya et al. reported treatment with palliative hepatectomy, bile duct resection, lymph node dissection, and cholangiojejunostomy to improve chemotherapy adherence with reduced risk of cholangitis (89). Patient continues to survive over 24 M (89). CRS, HIPEC, and PIPAC require further evaluation.
Adrenal
ACC
In the US, ACC occurs in 2.92 per million with 5-year survival of 30% and 40% of patients with advanced disease (198). ACC may present with three histologic subtypes: oncocytic, myxoid, and a very aggressive sarcomotoid subtype (199). PC occurs in 6–19% of patients with advanced disease (200).
Treatment
NCCN guidelines recommend the following in advanced ACC: resection if >90% removable, local therapy (radiation, ablation, or liver-directed therapy), and systemic therapy (201). ESMO guidelines are similar (202). In PC, CRS offers benefit in select patients while HIPEC is controversial (3).
Literature review
Two publications were found while one study overlaps with other cancer types as summarized in Table 1 (90,91,95). Honoré et al. (n=4) evaluated CRS and HIPEC or EPIC in a mixed cohort with median PCI 11, mRFS 12 M, and median follow-up after HIPEC of 40 M with 3 patients deceased (95). Hughes et al. (n=9) evaluated CRS and HIPEC achieving median IP PFS 19 M (90). Sugarbaker et al. reported the treatment of ACC with CRS and HIPEC twice with RFS of 4 M, however, this represented a new lesion in the contralateral adrenal gland treated with resection and HIPEC with no PC upon surgery (91). CRS and HIPEC should be evaluated in larger studies to verify benefit.
NET of any primary site
NET
In the US, NETs occur in 6.98 per 100,000 (203). Primary site and grade affect 5-year survival rates: cecum (61%), colon (29–64%), lung (32–60%), pancreas (48–50%), rectum (28–87%), small intestine (69–73%), and stomach (32–67%) (203,204). Gastric and large intestine NETs are not covered in this review. Advanced disease occurs in 1.5 per 100,000 (203). PC occurs in 18% of patients with advanced disease (205,206).
Treatment
According to NCCN guidelines, initial treatment of NETs is determined by primary site. Advanced disease treatment includes: surgical resection, somatostatin analogues, peptide receptor radionuclide therapy (PRRT), liver directed therapy, radiation, or other systemic therapies (201). ESMO guidelines are similar, however, include liver transplant as an option (207). In PC, CRS may only benefit patients with grade I and II well-differentiated tumors, while the role of HIPEC remains controversial (3,208).
Literature review
Two publications were found while two studies overlap with other cancer types as summarized in Table 1 (9,98,102,103). Brandl et al. (n=1) evaluated CRS and HIPEC in a mixed cohort with RFS of 13.5 M and continued survival of 48.9 M (98). Goéré et al. (n=114) evaluated CRS and HIPEC in a mixed cohort reporting HR including: NETs (1.41, 0.77–2.58), sarcoma (1.81, 1.01–3.25), and ovarian (0.82, 0.45–1.49) (9). Pastrián et al. reported CRS liver NET with PC with recurrence and OS of 30 M (102). Nagaro et al. reported pancreatic NET treated with CRS with no evidence of disease for 2 years (103). CRS offers benefit in select patients while CRS and HIPEC may have some benefit, however, larger trials are required.
Summary
PC commonly occurs (>20%) with treatment limited to palliation for cancers including: PDAC, SBA, UC, UCC, Sarcoma, DSRCT, CCA, and GC. PC may occur (>10%) with unclear management for cancers including: GIST, FHCC, ACC, and NET. PC rarely occurs in some common cancers with unclear management for: breast, prostate, kidney, and HCC.
PC from uncommonly treated malignancies require further consideration. CRS with multidisciplinary treatment (TKIs, radiation, chemotherapy, radio-immunotherapy, or other systemic therapies) appears to offer benefit with cancers: GIST, DSRCT, FHCC, and NETs. CRS and HIPEC may offer benefit but require larger validation with cancers: breast, SBA, UC, sarcoma, HCC, CCA, and ACC. There is not enough information for cancers: prostate, UCC, kidney, and GC. Causes of PC are heterogeneous and require tailored multidisciplinary treatment based on tumor histology and response to treatment which may include CRS and various forms of IP therapy.
In the future, clinical practice may be altered based on preliminary results. PDAC may benefit from staged IP chemotherapy and/or PIPAC. IP radioimmunotherapy or IORT may be useful for patients with radio-sensitive malignancy such as prostate, kidney, sarcoma, and DSRCT but require more research. PIPAC and electrochemotherapy may be useful in chemosensitive and/or chemoresistant malignancy but will require continued research.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrew M. Blakely and Oliver S. Eng) for the series “Peritoneal Carcinomatosis: History and Future” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-4/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-4/coif). The series “Peritoneal Carcinomatosis: History and Future” was commissioned by the editorial office without any funding or sponsorship. MUA has received support from Stanford to attend conferences for education. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lambert LA. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin 2015;65:284-98. [Crossref] [PubMed]
- Van Oudheusden TR, Grull H, Dankers PY, et al. Targeting the peritoneum with novel drug delivery systems in peritoneal carcinomatosis: a review of the literature. Anticancer Res 2015;35:627-34. [PubMed]
- Bhatt A. Management of peritoneal metastases-cytoreductive surgery, HIPEC and beyond. Singapore: Springer, 2018.
- Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1-11. [PubMed]
- Gilly FN, Beaujard A, Glehen O, et al. Peritonectomy combined with intraperitoneal chemohyperthermia in abdominal cancer with peritoneal carcinomatosis: phase I-II study. Anticancer Res 1999;19:2317-21. [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Sibio S, Atta JMF, Impagnatiello A, et al. Epidemiology: Extent of the Problem. In: Di Giorgio A, Pinto E. editors. Treatment of Peritoneal Surface Malignancies. Milano: Springer, 2015:5-14.
- Kitai T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: a systematic review including evidence from Japan. Surg Today 2021;51:1085-98. [Crossref] [PubMed]
- Goéré D, Passot G, Gelli M, et al. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: results from a worldwide analysis issue of the Peritoneal Surface Oncology Group International (PSOGI). Int J Hyperthermia 2017;33:520-7. [Crossref] [PubMed]
- Miller AM, Lemke-Miltner CD, Blackwell S, et al. Intraperitoneal CMP-001: A Novel Immunotherapy for Treating Peritoneal Carcinomatosis of Gastrointestinal and Pancreaticobiliary Cancer. Ann Surg Oncol 2021;28:1187-97. [Crossref] [PubMed]
- Valle SJ, Akhter J, Mekkawy AH, et al. A novel treatment of bromelain and acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: A phase I first in man study. Eur J Surg Oncol 2021;47:115-22. [Crossref] [PubMed]
- Wang X, Sun K, Tan Y, et al. Efficacy and safety of selenium nanoparticles administered intraperitoneally for the prevention of growth of cancer cells in the peritoneal cavity. Free Radic Biol Med 2014;72:1-10. [Crossref] [PubMed]
- Willaert W, Van de Sande L, Van Daele E, et al. Safety and preliminary efficacy of electrostatic precipitation during pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable carcinomatosis. Eur J Surg Oncol 2019;45:2302-9. [Crossref] [PubMed]
- Braet H, Rahimi-Gorji M, Debbaut C, et al. Exploring high pressure nebulization of Pluronic F127 hydrogels for intraperitoneal drug delivery. Eur J Pharm Biopharm 2021;169:134-43. [Crossref] [PubMed]
- Ploug M, Graversen M, Pfeiffer P, et al. Bidirectional treatment of peritoneal metastasis with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) and systemic chemotherapy: a systematic review. BMC Cancer 2020;20:105. [Crossref] [PubMed]
- Pinto A, Pocard M. Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: a systematic review. Pleura Peritoneum 2018;3:20180124. [Crossref] [PubMed]
- Josserand V, Kéramidas M, Lavaud J, et al. Electrochemotherapy guided by intraoperative fluorescence imaging for the treatment of inoperable peritoneal micro-metastases. J Control Release 2016;233:81-7. [Crossref] [PubMed]
- Lauer UM, Schell M, Beil J, et al. Phase I Study of Oncolytic Vaccinia Virus GL-ONC1 in Patients with Peritoneal Carcinomatosis. Clin Cancer Res 2018;24:4388-98. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Yamada S, Fujii T, Yamamoto T, et al. Phase I/II study of adding intraperitoneal paclitaxel in patients with pancreatic cancer and peritoneal metastasis. Br J Surg 2020;107:1811-7. [Crossref] [PubMed]
- Satoi S, Fujii T, Yanagimoto H, et al. Multicenter Phase II Study of Intravenous and Intraperitoneal Paclitaxel With S-1 for Pancreatic Ductal Adenocarcinoma Patients With Peritoneal Metastasis. Ann Surg 2017;265:397-401. [Crossref] [PubMed]
- Takahara N, Isayama H, Nakai Y, et al. Intravenous and intraperitoneal paclitaxel with S-1 for treatment of refractory pancreatic cancer with malignant ascites. Invest New Drugs 2016;34:636-42. [Crossref] [PubMed]
- Schwarz L, Levine EA, Morris D, et al. CRS with HIPEC for peritoneal carcinomatosis of pancreatic adenocarcinoma. What do we expect? Results of a Multicenter International Study. HPB 2018;20:S324. [Crossref]
- Tentes AA, Pallas N, Karamveri C, et al. Cytoreduction and HIPEC for peritoneal carcinomatosis of pancreatic cancer. J BUON 2018;23:482-7. [PubMed]
- Graversen M, Detlefsen S, Bjerregaard JK, et al. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Clin Exp Metastasis 2017;34:309-14. [Crossref] [PubMed]
- Khosrawipour T, Khosrawipour V, Giger-Pabst U. Pressurized Intra Peritoneal Aerosol Chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS One 2017;12:e0186709. [Crossref] [PubMed]
- Rotolo S, Ferracci F, Santullo F, et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): A case report of a multimodal treatment for peritoneal metastases of pancreatic origin. Int J Surg Case Rep 2020;77S:S75-8. [Crossref] [PubMed]
- Dumont F, Passot C, Raoul JL, et al. A phase I dose-escalation study of oxaliplatin delivered via a laparoscopic approach using pressurised intraperitoneal aerosol chemotherapy for advanced peritoneal metastases of gastrointestinal tract cancers. Eur J Cancer 2020;140:37-44. [Crossref] [PubMed]
- Liu Y, Yonemura Y, Levine EA, et al. Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Metastases From a Small Bowel Adenocarcinoma: Multi-Institutional Experience. Ann Surg Oncol 2018;25:1184-92. [Crossref] [PubMed]
- Liu Y, Ishibashi H, Takeshita K, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Dissemination from Small Bowel Malignancy: Results from a Single Specialized Center. Ann Surg Oncol 2016;23:1625-31. [Crossref] [PubMed]
- Seomangal K, Neary P. Stage IV jejunal adenocarcinoma: a multimodal therapeutic success story. J Surg Case Rep 2019;2019:rjz239. [Crossref] [PubMed]
- Sawatsubashi T, Nihei K, Nakatsuka H, et al. A Case of Melena Caused by Peritoneal Dissemination, Treated with Interventional Radiology. Gan To Kagaku Ryoho 2018;45:1880-2. [PubMed]
- Takemoto Y, Noriyuki T, Takei D, et al. A Case of Long-Term Survival in a Patient with Small Intestinal Adenocarcinoma with Peritoneal Dissemination. Gan To Kagaku Ryoho 2016;43:121-4. [PubMed]
- Achard V, Achard G, Friedlaender A, et al. Prostate Cancer Nonascitic Peritoneal Carcinomatosis After Robot-assisted Laparoscopic Radical Prostatectomy: 3 Case Reports and Review of the Literature. Urology 2020;137:121-5. [Crossref] [PubMed]
- Motterle G, Ahmed ME, Andrews JR, et al. Tumor Seeding after Robot-Assisted Radical Prostatectomy: Literature Review and Experience from a Single Institution. J Urol 2020;203:1141-6. [Crossref] [PubMed]
- Caño-Velasco J, Herranz-Amo F, Polanco Pujol L, et al. Port site and peritoneal metastasis following laparoscopic radical prostatectomy and salvage radiotherapy for prostate adenocarcinoma. Arch Esp Urol 2019;72:974-7. [PubMed]
- Le Thiec M, Rusu D, Fleury V, et al. 68Ga-PSMA PET/CT Urachus Metastases in Recurrent Prostate Cancer With Very Low PSA Level. Clin Nucl Med 2019;44:40-1. [Crossref] [PubMed]
- Li HK, Thibodeau R, Nsouli T, et al. Peritoneal and port site seeding of an undiagnosed urothelial carcinoma of the bladder after robot-assisted laparoscopic prostatectomy. Radiol Case Rep 2020;15:2550-3. [Crossref] [PubMed]
- Pandey M, Ramasamy M, Shukla M. Unusual progression of renal cell carcinoma with carcinomatosis peritoneii and Krukenberg tumour and alopecia with sunitinib therapy in young female. World J Surg Oncol 2018;16:23. [Crossref] [PubMed]
- Kimura T, Togawa T, Onishi K, et al. Efficacy of Long-Term Adjuvant Therapy With Imatinib Mesylate After Extensive Surgical Treatment for Ruptured Gastrointestinal Stromal Tumors of the Small Intestine With Peritoneal Metastases: A Case Report. J Investig Med High Impact Case Rep 2020;8:2324709620970736. [Crossref] [PubMed]
- Ono T, Tanaka N, Suzuki H, et al. A Case of Peritoneal Metastasis Resection from GIST of the Small Intestine with Focal Resistance Against Imatinib Mesylate. Gan To Kagaku Ryoho 2019;46:2452-4. [PubMed]
- Terada Y, Sonoda H, Miyake T, et al. A Case of Small Intestinal GIST with Long-Term Survival after Tumor Resection for Repeated Peritoneal Recurrence. Gan To Kagaku Ryoho 2017;44:1521-2. [PubMed]
- Ishigame T, Kimura T, Kase K, et al. A Case of Long-Term Survival of Metastatic and Recurrent Duodenal Gastrointestinal Stromal Tumor Treated with Multimodality Managements. Gan To Kagaku Ryoho 2018;45:527-9. [PubMed]
- Sugase T, Takahashi T, Ishikawa T, et al. Surgical resection of recurrent gastrointestinal stromal tumor after interruption of long-term nilotinib therapy. Surg Case Rep 2016;2:137. [Crossref] [PubMed]
- Monobe Y, Naomoto Y, Hayashi J, et al. A case of gastrointestinal stromal tumor (GIST) with peritoneal dissemination—Imatinib re-challenged case—. Kawasaki Med J 2017;43:5-12.
- Modak S, Zanzonico P, Grkovski M, et al. B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study. J Clin Oncol 2020;38:4283-91. [Crossref] [PubMed]
- Hayes-Jordan AA, Coakley BA, Green HL, et al. Desmoplastic Small Round Cell Tumor Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial. Ann Surg Oncol 2018;25:872-7. [Crossref] [PubMed]
- Wang LL, Ji ZH, Gao Y, et al. Clinicopathological features of desmoplastic small round cell tumors: clinical series and literature review. World J Surg Oncol 2021;19:193. [Crossref] [PubMed]
- Stiles ZE, Murphy AJ, Anghelescu DL, et al. Desmoplastic Small Round Cell Tumor: Long-Term Complications After Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2020;27:171-8. [Crossref] [PubMed]
- Gani F, Goel U, Canner JK, et al. A national analysis of patterns of care and outcomes for adults diagnosed with desmoplastic small round cell tumors in the United States. J Surg Oncol 2019;119:880-6. [Crossref] [PubMed]
- Honoré C, Delhorme JB, Nassif E, et al. Can we cure patients with abdominal Desmoplastic Small Round Cell Tumor? Results of a retrospective multicentric study on 100 patients. Surg Oncol 2019;29:107-12. [Crossref] [PubMed]
- Scheer M, Vokuhl C, Blank B, et al. Desmoplastic small round cell tumors: Multimodality treatment and new risk factors. Cancer Med 2019;8:527-42. [Crossref] [PubMed]
- Stiles ZE, Dickson PV, Glazer ES, et al. Desmoplastic small round cell tumor: A nationwide study of a rare sarcoma. J Surg Oncol 2018;117:1759-67. [Crossref] [PubMed]
- Subbiah V, Lamhamedi-Cherradi SE, Cuglievan B, et al. Multimodality Treatment of Desmoplastic Small Round Cell Tumor: Chemotherapy and Complete Cytoreductive Surgery Improve Patient Survival. Clin Cancer Res 2018;24:4865-73. [Crossref] [PubMed]
- Zmora O, Hayes-Jordan A, Nissan A, et al. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for disseminated intra-abdominal malignancies in children-a single-institution experience. J Pediatr Surg 2018;53:1381-6. [Crossref] [PubMed]
- Angarita FA, Hassan S, Cannell AJ, et al. Clinical features and outcomes of 20 patients with abdominopelvic desmoplastic small round cell tumor. Eur J Surg Oncol 2017;43:423-31. [Crossref] [PubMed]
- Honoré C, Atallah V, Mir O, et al. Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS One 2017;12:e0171639. [Crossref] [PubMed]
- Frank JA, Ranft A, Paulussen M, et al. Results for patients with sarcoma not otherwise specified and other diagnoses than Ewing sarcoma treated according to the Euro-EWING 99 trial. Pediatr Blood Cancer 2017; [Crossref] [PubMed]
- Scalabre A, Philippe-Chomette P, Passot G, et al. Cytoreductive surgery and hyperthermic intraperitoneal perfusion with chemotherapy in children with peritoneal tumor spread: A French nationwide study over 14 years. Pediatr Blood Cancer 2018; [Crossref] [PubMed]
- Atallah V, Honore C, Orbach D, et al. Role of Adjuvant Radiation Therapy After Surgery for Abdominal Desmoplastic Small Round Cell Tumors. Int J Radiat Oncol Biol Phys 2016;95:1244-53. [Crossref] [PubMed]
- Osborne EM, Briere TM, Hayes-Jordan A, et al. Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol 2016;119:40-4. [Crossref] [PubMed]
- Somashekhar SP, Prasanna G, Jaka R, et al. Hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: A single institution Indian experience. Natl Med J India 2016;29:262-6. [PubMed]
- Sjoberg Bexelius T, Chisholm JC, Okoye B, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) as another treatment modality for desmoplastic round cell tumour patients: first paediatric experience from UK. BMJ Case Rep 2021;14:234876. [Crossref] [PubMed]
- Gill H, Shahbazi N, Yu Z, et al. Yttrium-90 radioembolization in desmoplastic small round cell tumor with recurrent hepatic metastasis following hyperthermic intraperitoneal chemotherapy. Radiol Case Rep 2021;16:1259-63. [Crossref] [PubMed]
- Xiao J, Browning MB, Boyd KP, et al. Multimodal Therapy Including Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Can Result in Long-term Disease-free Survival in Pediatric Desmoplastic Small Round Cell Tumor With Extraperitoneal Disease. J Pediatr Hematol Oncol 2021;43:228-31. [Crossref] [PubMed]
- Tsoukalas N, Kiakou M, Nakos G, et al. Desmoplastic small round-cell tumour of the peritoneal cavity: case report and literature review. Ann R Coll Surg Engl 2020;102:e77-81. [Crossref] [PubMed]
- Nacef K, Chaouch MA, Bouriga R, et al. A Case Report of Abdominal Desmoplastic Small Round Cell Tumor in a Young Tunisian Woman. J Gastrointest Cancer 2019;50:568-71. [Crossref] [PubMed]
- Cracco A, Roy M, Simpfendorfer CH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy combined with two-stage hepatectomy for multiple and bilobar desmoplastic small round cell tumor liver metastases. J Gastrointest Oncol 2017;8:E60-4. [Crossref] [PubMed]
- Gesche J, Beckert S, Neunhoeffer F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A safe treatment option for intraperitoneal rhabdomyosarcoma in children below 5 years of age. Pediatr Blood Cancer 2019;66:e27517. [Crossref] [PubMed]
- Karamveri C, Pallas N, Kyziridis D, et al. Cytoreductive Surgery in Combination with HIPEC in the Treatment of Peritoneal Sarcomatosis. Indian J Surg Oncol 2019;10:40-5. [Crossref] [PubMed]
- Naffouje SA, Tulla KA, Salti GI. A Simplified Peritoneal Sarcomatosis Score for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Gastrointest Oncol 2018;9:1138-43. [Crossref] [PubMed]
- Abu-Zaid A, Azzam A, Abuzaid M, et al. Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy for Management of Peritoneal Sarcomatosis: A Preliminary Single-Center Experience from Saudi Arabia. Gastroenterol Res Pract 2016;2016:6567473. [Crossref] [PubMed]
- Spiliotis J, Kopanakis N, Argyriou EO, et al. Locoregional treatment of peritoneal sarcomatosis A single-centre experience. Ann Ital Chir 2016;87:333-6. [PubMed]
- Kawamura M, Okudaira K, Itoh Y, et al. Whole abdominopelvic intensity-modulated radiation therapy for peritoneal disseminated rhabdomyosarcoma with three-year follow-up: a case report. Radiat Oncol 2019;14:127. [Crossref] [PubMed]
- Pleština S, Librenjak N, Marušić A, et al. An extremely rare primary sarcoma of the lung with peritoneal and small bowel metastases: a case report. World J Surg Oncol 2019;17:147. [Crossref] [PubMed]
- Li JL, Fan J. Epididymal liposarcoma with metastases to the retroperitoneal space and peritoneal cavity: A case report and literature review. Zhonghua Nan Ke Xue 2017;23:1103-6. [PubMed]
- Lin C, Wang L, Sheng J, et al. Transdifferentiation of pancreatic stromal tumor into leiomyosarcoma with metastases to liver and peritoneum: a case report. BMC Cancer 2016;16:947. [Crossref] [PubMed]
- Mehta S, Schwarz L, Spiliotis J, et al. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study. Eur J Surg Oncol 2018;44:1786-92. [Crossref] [PubMed]
- Berger Y, Spivack JH, Heskel M, et al. Extrahepatic metastasectomy for hepatocellular carcinoma: Predictors of long-term survival. J Surg Oncol 2016;114:469-74. [Crossref] [PubMed]
- Ji ZH, An SL, Li XB, et al. Long-term progression-free survival of hepatocellular carcinoma with synchronous diffuse peritoneal metastasis treated by CRS+HIPEC: A case report and literature review. Medicine (Baltimore) 2019;98:e14628. [Crossref] [PubMed]
- Takase K, Katsura Y, Takeda Y, et al. A Case of Peritoneal Recurrence of HCC Who Achieved Long-Term Survival after a Multidisciplinary Treatment. Gan To Kagaku Ryoho 2019;46:2476-8. [PubMed]
- Takiuchi D, Morimoto O, Hokkoku D, et al. A Case of Peritoneal Dissemination of Hepatocellular Carcinoma after Laparoscopic Hepatectomy. Gan To Kagaku Ryoho 2018;45:2174-6. [PubMed]
- Spiliotis J, Nikolaou G, Kopanakis N, et al. Hepatocellular Carcinoma Peritoneal Metastasis: Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Gulf J Oncolog 2017;1:20-3. [PubMed]
- Kyziridis D, Kalakonas A, Zarambouka K, et al. Cytoreductive Surgery and HIPEC for Recurrent Fibrolamellar Hepatocellular Carcinoma with Peritoneal Carcinomatosis. J Gastrointest Cancer 2020;51:300-3. [Crossref] [PubMed]
- Amblard I, Mercier F, Bartlett DL, et al. Cytoreductive surgery and HIPEC improve survival compared to palliative chemotherapy for biliary carcinoma with peritoneal metastasis: A multi-institutional cohort from PSOGI and BIG RENAPE groups. Eur J Surg Oncol 2018;44:1378-83. [Crossref] [PubMed]
- Falkenstein TA, Götze TO, Ouaissi M, et al. First Clinical Data of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) as Salvage Therapy for Peritoneal Metastatic Biliary Tract Cancer. Anticancer Res 2018;38:373-8. [PubMed]
- Stefano M, Prosperi E, Fugazzola P, et al. Case Report: Cytoreductive Surgery and HIPEC Associated With Liver Electrochemotherapy in a Cholangiocarcinoma Patient With Peritoneal Carcinomatosis and Liver Metastasis Case Report. Front Surg 2021;8:624817. [Crossref] [PubMed]
- Hernandez DL, Restrepo J, Garcia Mora M. Peritoneal Metastasis of Cholangiocarcinoma Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy at the Instituto Nacional de Cancerología, Colombia. Cureus 2020;12:e6697. [Crossref] [PubMed]
- Mikuriya K, Koga C, Shimizu J, et al. A Case of Unresectable Gallbladder Cancer with Relatively Good Prognosis Treated with Upfront Surgery Followed by Systemic Chemotherapy. Gan To Kagaku Ryoho 2020;47:168-70. [PubMed]
- Hughes MS, Lo WM, Beresnev T, et al. A Phase II Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Recurrent Adrenocortical Carcinoma. J Surg Res 2018;232:383-8. [Crossref] [PubMed]
- Sugarbaker PH. Peritoneal metastases from adrenal cortical carcinoma treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Tumori 2016;102:588-92. [Crossref] [PubMed]
- Di Giorgio A, Sgarbura O, Rotolo S, et al. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin or oxaliplatin for peritoneal metastasis from pancreatic adenocarcinoma and cholangiocarcinoma. Ther Adv Med Oncol 2020;12:1758835920940887. [Crossref] [PubMed]
- Leigh N, Solomon D, Pletcher E, et al. Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy indicated in hepatobiliary malignancies? World J Surg Oncol 2020;18:124. [Crossref] [PubMed]
- Graversen M, Detlefsen S, Bjerregaard JK, et al. Prospective, single-center implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol 2018;10:1758835918777036. [Crossref] [PubMed]
- Honoré C, Goéré D, Macovei R, et al. Peritoneal carcinomatosis from unusual cancer origins: Is there a role for hyperthermic intraperitoneal chemotherapy? J Visc Surg 2016;153:101-7. [Crossref] [PubMed]
- Horvath P, Beckert S, Struller F, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases of pancreas and biliary tract cancer. Clin Exp Metastasis 2018;35:635-40. [Crossref] [PubMed]
- Kurtz F, Struller F, Horvath P, et al. Feasibility, Safety, and Efficacy of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastasis: A Registry Study. Gastroenterol Res Pract 2018;2018:2743985. [Crossref] [PubMed]
- Brandl A, Zielinski CB, Raue W, et al. Peritoneal metastases of rare carcinomas treated with cytoreductive surgery and HIPEC - A single center case series. Ann Med Surg (Lond) 2017;22:7-11. [Crossref] [PubMed]
- Teixeira Farinha H, Grass F, Kefleyesus A, et al. Impact of Pressurized Intraperitoneal Aerosol Chemotherapy on Quality of Life and Symptoms in Patients with Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol Res Pract 2017;2017:4596176. [Crossref] [PubMed]
- Graziosi L, Marino E, De Angelis V, et al. Survival prognostic factors in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy treatment: analysis from a single oncological center. World J Surg Oncol 2016;14:97. [Crossref] [PubMed]
- Hamilton TD, Taylor EL, Cannell AJ, et al. Impact of Major Complications on Patients' Quality of Life After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2016;23:2946-52. [Crossref] [PubMed]
- Pastrián LG, Ruz-Caracuel I, Gonzalez RS. Giant Primary Neuroendocrine Neoplasms of the Liver: Report of 2 Cases With Molecular Characterization. Int J Surg Pathol 2019;27:893-9. [Crossref] [PubMed]
- Nagaro H, Hirose Y, Katada T, et al. A Case of Peritoneal Metastases after Radiofrequency Ablation for Liver Metastasis from a Pancreatic Neuroendocrine Tumor. Gan To Kagaku Ryoho 2019;46:2015-7. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Luo G, Fan Z, Gong Y, et al. Characteristics and Outcomes of Pancreatic Cancer by Histological Subtypes. Pancreas 2019;48:817-22. [Crossref] [PubMed]
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019;10:10-27. [Crossref] [PubMed]
- Tonini V, Zanni M. Pancreatic cancer in 2021: What you need to know to win. World J Gastroenterol 2021;27:5851-89. [Crossref] [PubMed]
- Peixoto RD, Speers C, McGahan CE, et al. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med 2015;4:1171-7. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:439-57. [Crossref] [PubMed]
- Okusaka T, Nakamura M, Yoshida M, et al. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas 2020;49:326-35. [Crossref] [PubMed]
- Flanagan M, Solon J, Chang KH, et al. Peritoneal metastases from extra-abdominal cancer - A population-based study. Eur J Surg Oncol 2018;44:1811-7. [Crossref] [PubMed]
- Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med 2019;21:1708-18. [Crossref] [PubMed]
- Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:809-15. [Crossref] [PubMed]
- Bertozzi S, Londero AP, Cedolini C, et al. Prevalence, risk factors, and prognosis of peritoneal metastasis from breast cancer. Springerplus 2015;4:688. [Crossref] [PubMed]
- Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA 2019;321:288-300. [Crossref] [PubMed]
- Gradishar WJ, Moran MS, Abraham J, et al. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 1.2022. National Comprehensive Cancer Network . 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49. [Crossref] [PubMed]
- Howe JR, Karnell LH, Menck HR, et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer 1999;86:2693-706. [Crossref] [PubMed]
- Barsouk A, Rawla P, Barsouk A, et al. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med Sci (Basel) 2019;7:46. [Crossref] [PubMed]
- Faivre J, Trama A, De Angelis R, et al. Incidence, prevalence and survival of patients with rare epithelial digestive cancers diagnosed in Europe in 1995-2002. Eur J Cancer 2012;48:1417-24. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63-71. [Crossref] [PubMed]
- Overman MJ, Hu CY, Kopetz S, et al. A population-based comparison of adenocarcinoma of the large and small intestine: insights into a rare disease. Ann Surg Oncol 2012;19:1439-45. [Crossref] [PubMed]
- Rovers KP, de Bree E, Yonemura Y, et al. Treatment of peritoneal metastases from small bowel adenocarcinoma. Int J Hyperthermia 2017;33:571-8. [Crossref] [PubMed]
- Locher C, Batumona B, Afchain P, et al. Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis 2018;50:15-9. [Crossref] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, et al. Small Bowel Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/small_bowel.pdf
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 2016;66:290-308. [Crossref] [PubMed]
- Panigrahi GK, Praharaj PP, Kittaka H, et al. Exosome proteomic analyses identify inflammatory phenotype and novel biomarkers in African American prostate cancer patients. Cancer Med 2019;8:1110-23. [Crossref] [PubMed]
- Rawla P. Epidemiology of Prostate Cancer. World J Oncol 2019;10:63-89. [Crossref] [PubMed]
- Marcus DM, Goodman M, Jani AB, et al. A comprehensive review of incidence and survival in patients with rare histological variants of prostate cancer in the United States from 1973 to 2008. Prostate Cancer Prostatic Dis 2012;15:283-8. [Crossref] [PubMed]
- Li J, Wang Z. The pathology of unusual subtypes of prostate cancer. Chin J Cancer Res 2016;28:130-43. [PubMed]
- Siegel DA, O'Neil ME, Richards TB, et al. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity - United States, 2001-2017. MMWR Morb Mortal Wkly Rep 2020;69:1473-80. [Crossref] [PubMed]
- Hanyok BT, Howard LE, Amling CL, et al. Is computed tomography a necessary part of a metastatic evaluation for castration-resistant prostate cancer? Results from the Shared Equal Access Regional Cancer Hospital Database. Cancer 2016;122:222-9. [Crossref] [PubMed]
- Shinagare AB, Keraliya A, Somarouthu B, et al. Imaging yield from 133 consecutive patients with prostate cancer and low trigger PSA from a single institution. Clin Radiol 2016;71:e143-9. [Crossref] [PubMed]
- Ganeshan D, Aparicio AM, Morani A, et al. Pattern and Distribution of Distant Metastases in Anaplastic Prostate Carcinoma: A Single-Institute Experience With 101 Patients. AJR Am J Roentgenol 2017;209:327-32. [Crossref] [PubMed]
- Schaeffer E, Srinivas S, Antonarakis ES, et al. Prostate Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119-34. [Crossref] [PubMed]
- Chalasani V, Chin JL, Izawa JI. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J 2009;3:S193-8. [Crossref] [PubMed]
- Dahm P, Gschwend JE. Malignant non-urothelial neoplasms of the urinary bladder: a review. Eur Urol 2003;44:672-81. [Crossref] [PubMed]
- Martin JW, Jefferson FA, Huang M, et al. A California Cancer Registry Analysis of Urothelial and Non-urothelial Bladder Cancer Subtypes: Epidemiology, Treatment, and Survival. Clin Genitourin Cancer 2020;18:e330-6. [Crossref] [PubMed]
- Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 5.2021, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
- National Cancer Institute. Cancer Stat Facts: Bladder Cancer. Surveillance, Epidemiology, and End Results Program. 2021 [cited 2021 Mar 12]. Available online: https://seer.cancer.gov/statfacts/html/urinb.html
- Shinagare AB, Ramaiya NH, Jagannathan JP, et al. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol 2011;196:117-22. [Crossref] [PubMed]
- Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:244-58. [Crossref] [PubMed]
- Hepp Z, Shah SN, Smoyer K, et al. Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis. J Manag Care Spec Pharm 2021;27:240-55. [Crossref] [PubMed]
- Wong MCS, Goggins WB, Yip BHK, et al. Incidence and mortality of kidney cancer: temporal patterns and global trends in 39 countries. Sci Rep 2017;7:15698. [Crossref] [PubMed]
- Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras 2015;48:166-74. [Crossref] [PubMed]
- Chandrasekar T, Klaassen Z, Goldberg H, et al. Metastatic renal cell carcinoma: Patterns and predictors of metastases-A contemporary population-based series. Urol Oncol 2017;35:661.e7-661.e14. [Crossref] [PubMed]
- Motzer RJ, Jonasch E, Agarwal N, et al. NCCN Guidelines Insights: Kidney Cancer, Version 3.2022. National Comprehensive Cancer Network. 2021 [cited 2021 Apr 12]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v58-68. [Crossref] [PubMed]
- Psutka SP, Master VA. Role of metastasis-directed treatment in kidney cancer. Cancer 2018;124:3641-55. [Crossref] [PubMed]
- Saltus CW, Calingaert B, Candrilli S, et al. Epidemiology of Adult Soft-Tissue Sarcomas in Germany. Sarcoma 2018;2018:5671926. [Crossref] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Soft Tissue including Heart Cancer. Surveillance, Epidemiology, and End Results Program. 2021 [cited 2021 Aug 12]. Available online: https://seer.cancer.gov/statfacts/html/soft.html
- Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer 2006;119:2922-30. [Crossref] [PubMed]
- Brennan MF, Antonescu CR, Moraco N, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg 2014;260:416-21; discussion 421-2. [Crossref] [PubMed]
- Vezeridis MP, Moore R, Karakousis CP. Metastatic patterns in soft-tissue sarcomas. Arch Surg 1983;118:915-8. [Crossref] [PubMed]
- Savina M, Le Cesne A, Blay JY, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med 2017;15:78. [Crossref] [PubMed]
- von Mehren M, Kane JM, Bui MM, et al. NCCN Clinical Practice Guidelines: Soft Tissue Sarcoma, Version 2.2021. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Gronchi A, Miah AB, Dei Tos AP, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol 2021;32:1348-65. [Crossref] [PubMed]
- Patel N, Benipal B. Incidence of Gastrointestinal Stromal Tumors in the United States from 2001-2015: A United States Cancer Statistics Analysis of 50 States. Cureus 2019;11:e4120. [Crossref] [PubMed]
- Wu CE, Tzen CY, Wang SY, et al. Clinical Diagnosis of Gastrointestinal Stromal Tumor (GIST): From the Molecular Genetic Point of View. Cancers (Basel) 2019;11:679. [Crossref] [PubMed]
- Iqbal N, Sharma A, Shukla N, et al. Advanced gastrointestinal stromal tumors: 10-years experience from a tertiary care centre. Trop Gastroenterol 2015;36:168-73. [Crossref] [PubMed]
- von Mehren M, Kane JM, Bui MM, et al. Gastrointestinal Stromal Tumors, Version 1.2021. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf
- Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022;33:20-33. [Crossref] [PubMed]
- Qi J, Liu HL, Ren F, et al. Preoperative adjuvant therapy for locally advanced and recurrent/metastatic gastrointestinal stromal tumors: a retrospective study. World J Surg Oncol 2020;18:70. [Crossref] [PubMed]
- Omari J, Drewes R, Matthias M, et al. Treatment of metastatic, imatinib refractory, gastrointestinal stroma tumor with image-guided high-dose-rate interstitial brachytherapy. Brachytherapy 2019;18:63-70. [Crossref] [PubMed]
- Al-Share B, Alloghbi A, Al Hallak MN, et al. Gastrointestinal stromal tumor: a review of current and emerging therapies. Cancer Metastasis Rev 2021;40:625-41. [Crossref] [PubMed]
- Loaiza-Bonilla A, Bonilla-Reyes PA. Somatostatin Receptor Avidity in Gastrointestinal Stromal Tumors: Theranostic Implications of Gallium-68 Scan and Eligibility for Peptide Receptor Radionuclide Therapy. Cureus 2017;9:e1710. [Crossref] [PubMed]
- Schrage Y, Hartgrink H, Smith M, et al. Surgical management of metastatic gastrointestinal stromal tumour. Eur J Surg Oncol 2018;44:1295-300. [Crossref] [PubMed]
- Lettieri CK, Garcia-Filion P, Hingorani P. Incidence and outcomes of desmoplastic small round cell tumor: results from the surveillance, epidemiology, and end results database. J Cancer Epidemiol 2014;2014:680126. [Crossref] [PubMed]
- Hayes-Jordan A, LaQuaglia MP, Modak S. Management of desmoplastic small round cell tumor. Semin Pediatr Surg 2016;25:299-304. [Crossref] [PubMed]
- Bulbul A, Fahy BN, Xiu J, et al. Desmoplastic Small Round Blue Cell Tumor: A Review of Treatment and Potential Therapeutic Genomic Alterations. Sarcoma 2017;2017:1278268. [Crossref] [PubMed]
- Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep 2019;6:104-11. [Crossref] [PubMed]
- Akinyemiju T, Abera S, Ahmed M, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683-91. [Crossref] [PubMed]
- Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol 2014;109:325-34. [Crossref] [PubMed]
- Walker RW, Belbin GM, Sorokin EP, et al. A common variant in PNPLA3 is associated with age at diagnosis of NAFLD in patients from a multi-ethnic biobank. J Hepatol 2020;72:1070-81. [Crossref] [PubMed]
- Tian C, Stokowski RP, Kershenobich D, et al. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet 2010;42:21-3. [Crossref] [PubMed]
- Wang S, Sun H, Xie Z, et al. Improved survival of patients with hepatocellular carcinoma and disparities by age, race, and socioeconomic status by decade, 1983-2012. Oncotarget 2016;7:59820-33. [Crossref] [PubMed]
- Sarma M, Padma S, Pavithran P, et al. Extrahepatic metastases of hepatocellular carcinoma on 18F FDG PET CT. J Egypt Natl Canc Inst 2021;33:36. [Crossref] [PubMed]
- Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 5.2021, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238-55. Erratum in: Ann Oncol 2019;30:871-3 Erratum in: Ann Oncol 2019;30:871-3. Erratum in: Ann Oncol 2022. [Epub ahead of print]. doi: 10.1016/j.annonc.2022.03.009. [Crossref] [PubMed]
- Singal AG, Gopal P, Yopp AC. Hepatocellular Carcinoma: Epidemiology, Basic Principles of Treatment, and Clinical Data. In: Meyer J, Schefter T. editors. Radiation Therapy for Liver Tumors. Cham: Springer International Publishing, 2017:149-78.
- Ramai D, Ofosu A, Lai JK, et al. Fibrolamellar Hepatocellular Carcinoma: A Population-Based Observational Study. Dig Dis Sci 2021;66:308-14. [Crossref] [PubMed]
- Kassahun WT. Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol 2016;14:151. [Crossref] [PubMed]
- Chakrabarti S, Tella SH, Kommalapati A, et al. Clinicopathological features and outcomes of fibrolamellar hepatocellular carcinoma. J Gastrointest Oncol 2019;10:554-61. [Crossref] [PubMed]
- Ganeshan D, Szklaruk J, Kaseb A, et al. Fibrolamellar hepatocellular carcinoma: multiphasic CT features of the primary tumor on pre-therapy CT and pattern of distant metastases. Abdom Radiol (NY) 2018;43:3340-8. [Crossref] [PubMed]
- Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma 2015;2:151-7. [PubMed]
- Gad MM, Saad AM, Faisaluddin M, et al. Epidemiology of Cholangiocarcinoma; United States Incidence and Mortality Trends. Clin Res Hepatol Gastroenterol 2020;44:885-93. [Crossref] [PubMed]
- Davis SL. Intrahepatic and Hilar Cholangiocarcinomas: Epidemiology, Basic Principles of Treatment, and Clinical Data. in: Meyer J, Schefter T. editors. Radiation Therapy for Liver Tumors. Cham: Springer International Publishing, 2017:201-21.
- Waseem D, Tushar P. Intrahepatic, perihilar and distal cholangiocarcinoma: Management and outcomes. Ann Hepatol 2017;16:133-9. [Crossref] [PubMed]
- Hahn F, Müller L, Mähringer-Kunz A, et al. Distant Metastases in Patients with Intrahepatic Cholangiocarcinoma: Does Location Matter? A Retrospective Analysis of 370 Patients. J Oncol 2020;2020:7195373. [Crossref] [PubMed]
- Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v28-37. [Crossref] [PubMed]
- Henley SJ, Weir HK, Jim MA, et al. Gallbladder Cancer Incidence and Mortality, United States 1999-2011. Cancer Epidemiol Biomarkers Prev 2015;24:1319-26. [Crossref] [PubMed]
- Rawla P, Sunkara T, Thandra KC, et al. Epidemiology of gallbladder cancer. Clin Exp Hepatol 2019;5:93-102. [Crossref] [PubMed]
- Jaruvongvanich V, Yang JD, Peeraphatdit T, et al. The incidence rates and survival of gallbladder cancer in the USA. Eur J Cancer Prev 2019;28:1-9. [Crossref] [PubMed]
- Sulieman I, Elmoghazy W, El Ansari W, et al. Gallbladder cancer: 7-Year experience from Qatar. Ann Med Surg (Lond) 2019;44:33-8. [Crossref] [PubMed]
- Chandrasekar T, Goldberg H, Klaassen Z, et al. The who, when, and why of primary adrenal malignancies: Insights into the epidemiology of a rare clinical entity. Cancer 2019;125:1050-9. [Crossref] [PubMed]
- Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014;35:282-326. [Crossref] [PubMed]
- Glenn JA, Else T, Hughes DT, et al. Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery 2019;165:186-95. [Crossref] [PubMed]
- Shah MH, Goldner WS, Benson AB, et al. Neuroendocrine and Adrenal Tumors, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Fassnacht M, Assie G, Baudin E, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1476-90. [Crossref] [PubMed]
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589-97. [Crossref] [PubMed]
- Lombard-Bohas C, Mitry E, O'Toole D, et al. Thirteen-month registration of patients with gastroenteropancreatic endocrine tumours in France. Neuroendocrinology 2009;89:217-22. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer 2016;139:2679-86. [Crossref] [PubMed]
- Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:844-60. [Crossref] [PubMed]
- de Mestier L, Lardière-Deguelte S, Brixi H, et al. Updating the surgical management of peritoneal carcinomatosis in patients with neuroendocrine tumors. Neuroendocrinology 2015;101:105-11. [Crossref] [PubMed]
Cite this article as: Ahmad MU, Gebregziabher B, Lee B. Rare histologies in peritoneal carcinomatosis: a narrative review. Dig Med Res 2022;5:26.


