Neuraxial anaesthesia and its role in enhanced recovery after surgery: a narrative review
Introduction
Intrathecal (spinal), epidural and the combined spinal-epidural (CSE) are neuraxial techniques that can be used to provide postoperative analgesia and, in some circumstances, anaesthesia for surgery.
They feature in many current enhanced recovery after surgery (ERAS) protocols with a bias towards epidurals for open surgery because it is thought they provide superior opiate-sparing analgesia and reduce the stress response to surgery through blunting sympathetic outflow. Both are key components of the ERAS concept.
We aim to provide an evidenced-based overview for the use of neuraxial techniques in major surgery and their use within an ERAS protocol.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-86/rc).
Methods
A PubMed search for ‘Neuraxial’, followed by ‘Epidural’ and then ‘Intrathecal’, AND ‘Enhanced recovery after surgery’ published between 2016 and 2021. Titles were reviewed and those deemed relevant, written in English, with full text available were selected. References for these articles were reviewed and again those deemed relevant selected (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 08/2021 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used (including MeSH and free text search terms and filters) | 1. Neuraxial AND Enhanced recovery after surgery |
| 2. Epidural AND Enhanced recovery after surgery | |
| 3. Intrathecal AND Enhanced recovery after surgery | |
| 4. Neuraxial AND Adjuncts | |
| Timeframe | Between 2016 and 2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | 1. English Language |
| 2. Full text available | |
| 3. Deemed relevant from title review | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Selection by one author |
| Any additional considerations, if applicable | References of selected articles were reviewed and those deemed relevant from title review, were included if full text available and in English |
Neuraxial techniques
History
In 1898 a German surgeon, August Bier, successfully performed the first operation under a spinal block using the local anaesthetic cocaine (1). He experimented on himself and his assistant by testing the block with cigarette burns to the lower limbs and hitting their shins with a hammer. Both felt no discomfort but suffered with post-dural puncture headaches (PDPHs). The spinal technique rapidly spread to the rest of Europe and within a few years had reached America (2). Epidural anaesthesia began later in 1901 using the caudal approach. It took longer to gain worldwide interest and lumbar epidurals were not described until 1921 (3).
Anatomy of neuraxial techniques
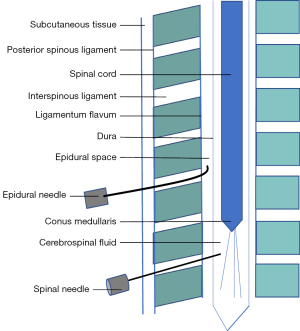
The spinal involves a needle intentionally breaching the dura and medication being placed in the cerebrospinal fluid (CSF) within the intrathecal space. This is often done as a single injection, most commonly performed between the third and fourth lumber vertebrae, so as to remain below the conus medullaris, the terminal end of the spinal cord (see Figure 1). With an epidural injection, the drug is deposited in the epidural space which is present along the entire course of the dura. Most commonly, lumbar and thoracic approaches are used and a catheter is frequently inserted. The level of the epidural procedure determines the area of anaesthesia and analgesia achieved. A CSE is an amalgamation of both techniques. Each may be performed separately, however equipment exists to perform them as a single procedure. Intrathecal medication is administered and a catheter is subsequently placed into the epidural space.
The analgesia provided by the local anaesthetic is predominantly limited to the area supplied by the nerve roots exciting the spinal canal at the site of insertion (mainly lumbar or low thoracic). Broader analgesia is augmented by the addition of neuraxial opiates.
Contraindications
There are only a few absolute contraindications to neuraxial techniques such as patient refusal, localised infection at the site of injection, allergy to the medications, raised intracranial pressure and significant, uncorrected hypovolaemia. However, many contraindications, once thought to be absolute are now relative where the risk and benefits of the procedure must be carefully balanced. These relative contraindications include coagulopathy, sepsis, demyelinating neurological disease and fixed cardiac output states such as aortic stenosis (2,4).
Complications of insertion
Insertion of a neuraxial block is not without risk (see Table 2). Failure, itch, urinary retention, shivering and hypotension are all common but minor complications. PDPH is also relatively common, quoted at rates between 1 in 200 to 1 in 500. In treating a PDPH those that do not respond to conservative management [simple analgesia, hydration and caffeine are recognised treatments (7)] may require an epidural blood patch. Nerve injury is a major, but fortunately rare, complication. It may be caused by direct trauma from the needle, a vertebral canal haematoma or due to infection such as an epidural abscess, meningitis or arachnoiditis. The 3rd National Audit project by the Royal College of Anaesthetists UK, found permanent nerve injury occurred in 1 in 24,000 patients (8). The majority were in a perioperative setting and over half occurred in patients with epidurals or CSEs. Inadvertent drug errors may also lead to major complications, with incorrect dosing causing total spinal anaesthesia, and wrong route administration causing nerve injury. Cardiovascular collapse and death are also quoted complications but are very rare.
Table 2
| Additive | Intrathecal dose | Epidural dose (bolus) | Notes |
|---|---|---|---|
| Sufentanil | 2.5–10 μg | 10–50 μg | Duration of action 1–3 hours |
| Fentanyl | 10–25 μg | 50–100 μg | Duration of action 2–4 hours |
| Pethidine | – | 25–50 mg | Duration of action 4–8 hours |
| Morphine | 50–500 μg | 2–5 mg | Delayed respiratory depression Duration of action 12–24 hours |
| Diamorphine | 300–400 μg | 2–3 mg | Duration of action 10–20 hours |
| Bicarbonate | – | 1 mL 8.4% bicarbonate for 10 mL lidocaine | Amount required differs for each local anaesthetic. Excess may result in precipitation |
| Adrenaline | – | 5 μg/mL | Less effect when used with bupivacaine as bupivacaine avidly binds to tissues and thus slower tissue absorption |
| Clonidine | 15–40 μg | 75–150 μg | 25–50 μg/hour |
| Dexmedetomidine | 5–10 μg | 70 μg | |
| Ketamine | – | 35–40 mg | Theory it reduces central sensitisation and windup (i.e., reduces chronic pain) |
Neuraxial adjuncts
Local anaesthetic given as a single dose, as intermittent boluses or as continuous infusion achieves analgesia and anaesthesia for surgery. Local anaesthetics may be hypobaric, isobaric or hyperbaric when compared to CSF. Hyperbaric solutions can be made by the addition of glucose (most commonly to bupivacaine) and tend to follow gravity once injected. Isobaric solutions travel freely from the level at which they were injected and hypobaric solutions may rise against gravity (2). Though the type of local anaesthetic and dose used determines speed of onset, duration of action and density of the block (5), other additives can also influence this (see Table 2).
Commonly used additives in central neuraxial blocks are opiates. Though often used in combination with local anaesthetics, they can also be delivered as a sole agent to provide postoperative analgesia. Sufentanil, fentanyl, diamorphine, pethidine and morphine have all been described (6). Their mechanism of action differs depending on the route of administration. Intrathecal opiates act at the dorsal horn of the spinal cord and reduce the propagation of nociceptive action potentials; the cephalad spread of the opiate modulates descending inhibitory pain pathways and it is absorbed systemically. In the epidural space, the main mechanism of action of the opiate is via systemic absorption, though some will diffuse into the intrathecal space as well. The physical properties of the drug also determine their mechanism of action. Fentanyl and sufentanil are lipophilic (5). They therefore rapidly diffuse into the intrathecal space and promptly bind at the dorsal horn thus having a faster onset of action. The duration of action, however, is short. Morphine is hydrophilic thus has a slower onset of action. As it does not rapidly bind to the dorsal horn of the spinal cord, a greater proportion is free to travel up the CSF. This explains the delayed respiratory depression seen with morphine when compared to fentanyl or even diamorphine. When diamorphine is used the respiratory depression is not delayed or as profound, as it is 280 times more lipid soluble than morphine. All central neuraxial opiates may cause pruritis (the most common side-effect), urinary retention, nausea, vomiting and respiratory depression. The incidence of all of these is higher with morphine than with other opiates, however the benefit is its much longer duration of action (5,6).
Alpha-2-agonists, such as clonidine and dexmedetomidine have been shown to increase the duration of block and improve postoperative analgesia. However, it is associated with hypotension, sedation and bradycardia (5,6).
Ketamine also acts at the dorsal horn of the spinal cord at N-methyl-D-aspartate (NMDA) receptors. It has been used individually or as an adjunct in the epidural space. It also speeds up the onset and prolongs the duration of analgesia. Sedation and headache have been reported as side effects (6).
Sodium bicarbonate speeds up the onset of action of local anaesthetics by increasing the unionised portion of the drug which is able to diffuse to its intraneural site of action. It is mostly used with lidocaine in the epidural space (6).
Vasoconstrictors such as adrenaline have been added to local anaesthetics to decrease absorption from the vascular epidural space and prolong the duration of action. It is more effective when used in conjunction with lidocaine than bupivacaine.
Midazolam, neostigmine, magnesium and tramadol have also been used as additives in central neuraxial blocks. They have a limited evidence-base, equivocal success and side effects, including potential neurotoxicity (5,6).
More recently there has been an increase in the popularity of regional anaesthetic techniques such as thoracic wall blocks (e.g., erector spinae or serratus anterior blocks) or abdominal wall blocks (e.g., transverse abdominis plane or rectus sheath block), especially when techniques are contraindicated or when appropriate regional techniques are felt to provide a suitable and effective alternative. More detail on the subject of regional anaesthesia is beyond the remit of this article.
Methods
A PubMed search for ‘Neuraxial’, followed by ‘Epidural’ and then ‘Intrathecal’, AND ‘Enhanced recovery after surgery’ over the past 5 years was made. Titles were reviewed and those deemed relevant, written in English, with full text available were selected. References for these articles were reviewed and again those deemed relevant selected.
Discussion
Analgesic benefits
A key component of the ERAS pathway is perioperative analgesia, with the aim to help dampen the physiological stress response to surgery. Neuraxial techniques provide an alternative to systemic opiates for managing pain in the perioperative period. Though opiates are a reliable and effective way to provide analgesia, they are associated with significant side-effects including nausea, respiratory depression and ileus. Poorly managed pain is associated with increased morbidity, long-term anxiety and development of chronic pain (9).
The MASTER study was a randomised trial looking at epidural analgesia and outcome after major surgery. They found a significant improvement in pain scores at rest on postoperative day 1 and on coughing on days 1 to 3 (10) when compared to a patient or physician-controlled opiate infusion. A Cochrane review also compared epidurals with opiate-based analgesic regimens. They found epidurals, with local anaesthetic alone, reduced pain on movement after abdominal surgery, however, the addition of an opiate to the epidural infusion was required for the effects to persist past 24 hours (11).
Intrathecal morphine reduces postoperative opiate requirements in major surgery (12) including open liver resection and major colorectal surgery. This, however, did not impact hospital length of stay, resolution of ileus or incidence of postoperative delirium (13,14).
Respiratory benefits
Postoperative pulmonary complications, ranging from atelectasis to respiratory failure, significantly affect patient well-being and outcome (15). They are relatively common, occurring in 2.8% of patients having non-obstetric and non-cardiac surgery (16), and account for 10–40% of complications after abdominal and vascular surgery (17).
Epidural analgesia can reduce respiratory complications with a relative risk reduction quoted to be as high as 25% (18). A meta-analysis by Pöpping et al. compared epidural with systemic analgesia in over 5,900 patients having abdominal or thoracic surgery over a 40-year period. They found the odds of pneumonia were decreased with epidural analgesia, though this effect was weaker in trials using patient-controlled systemic analgesia and in the more recent studies (19). They also found epidural analgesia was associated with reduced rates of prolonged ventilation and reintubation. This was reiterated in a more recent study of over 9,000 patients having open aortic abdominal aneurysm repair. Those that had epidural analgesia were less likely to require post-operative mechanical ventilation and those that did spent fewer days on a ventilator (20). Intrathecal morphine has also been shown to reduce postoperative complications (21).
The superior analgesia neuraxial techniques can provide may allow for improved respiratory function thus reducing postoperative pulmonary complications.
Circulatory benefits
Myocardial infarction remains a leading cause of postoperative mortality. A meta-analysis by Beattie et al. comprising 1,173 patients mostly for vascular surgery found epidural analgesia, in particular thoracic epidural, reduced the rate of myocardial infarction (but not death) when compared to systemic analgesia alone (22). More recently, similar results were found by Mohamad et al. who looked at 120 patients with a history of coronary artery disease having major abdominal cancer surgery. Thoracic patient-controlled epidural significantly reduced the incidence of not only myocardial infarction but also arrythmias, heart failure and non-fatal cardiac arrest when compared to patient-controlled systemic opiate analgesia (23). The effect may not be limited to epidurals alone and neuraxial (spinal and epidural) analgesia/anaesthesia may also reduce myocardial events (24).
Neuraxial blocks improve the circulation to the lower limbs and blunt the pro-inflammatory response to surgery thus, in theory, should reduce the risk of thrombosis. This is also seen clinically, with neuraxial techniques reducing the odds of deep vein thrombosis by as much as 44% and pulmonary embolism by 55% (24).
Ileus
Opiates are well known to reduce gut transit, causing ileus. The Cochrane review found strong evidence that epidurals with local anaesthetic alone reduced the time to first flatus when compared to systemic opiate analgesia (11). This effect can also be seen in thoracic surgery, when the bowel has not been handled, where epidural with and without opiate was superior to systemic opiates (25).
Other benefits
Attenuating the stress response to surgery is a key principle to the ERAS pathway. Neuraxial analgesia has been shown to blunt the hormonal, neuroendocrine and metabolic response to injury. Cortisol levels, blood glucose and heart rate were all lower in the spinal anaesthesia group when compared to those given a general anaesthetic (26). There are similar findings with epidural analgesia (27).
These individual benefits of neuraxial block combine and have been shown to improve mortality. Rodgers et al. looked at over 9,000 patients from 141 studies, spanning over 20 years with the earliest study published in 1977. They found a 30% reduction in all-cause mortality in patients who received neuraxial analgesia and anaesthesia when compared to those who did not (24). Pöpping et al. looked solely at epidural analgesia and similarly found a 40% decrease in the odds of death independent of level of catheter insertion, type of surgery or epidural infusion regimen (28). Again, in open abdominal aortic aneurysm repair, the use of epidural or spinal catheters lowered all-cause mortality at 90 days, when compared to general anaesthesia alone (20).
The evidence against neuraxial analgesia and anaesthesia
Much of the above evidence is specifically for epidural analgesia and more importantly from the pre-ERAS era. Meta-analyses using older studies may skew the outcome data as routine practice has changed (29). Thromboprophylaxis is now consistently prescribed for those at risk of thromboembolic events, ERAS protocols are firmly embedded into everyday practice and surgical techniques have improved. Minimally-invasive laparoscopic and robot-assisted operations cause less tissue injury therefore reducing the stress response to surgery and post-operative pain.
The MASTER’s trial prospectively compared epidural analgesia to patient or physician- controlled analgesia for major open surgery. They found no difference in mortality between these two groups. Though respiratory failure was significantly reduced and post-operative analgesia better in the epidural group, there was no difference in length of stay or any other complications including renal failure, myocardial infarction or infection (10). When looked at specifically in an ERAS pathway in patients having open abdominal surgery, epidural analgesia did again achieve better postoperative pain scores as well as a faster return of gut function, but, as before, it did not reduce complication rates or length of stay when compared to alternative analgesic regimens, including intravenous opiates and continuous wound infiltration (30). In fact, some studies show a prolonged length of stay in laparoscopic surgery (31) or increased complication rates (30,32) with epidural analgesia. As meta-analyses are reviewed in more detail the reduction in odds of death quoted in some studies may not be as high or achieve significance as was once thought and are very dependent on the studies included in analysis (28,29). A sub-study of the POISE trial compared neuraxial analgesia, with general anaesthesia alone in patients at high risk of postoperative cardiovascular comorbidity. They found an increased risk of cardiovascular death and myocardial infarction in the neuraxial group, emphasised in patients who had a thoracic epidural with general anaesthesia and reduced when lumbar epidural or spinal was used alone (32). A Cochrane review also found neuraxial anaesthesia alone reduced mortality (but not myocardial infarction) compared to general anaesthesia but not when the two were combined (33).
As described above, neuraxial techniques are not without risk. Failure rates for epidural are quoted to be as high as 30% in some studies (34). The sympathetic block from epidurals, thoracic more so than lumbar, causes hypotension (32,35) which, in the first instance is often managed with increased intravenous fluid administration (36). The fear is this will lead to ileus and anastomotic breakdown, especially in gastrointestinal surgery. Hypotension can also delay mobilisation (29). The prolonged infusion of local anaesthetic into the post-operative period, though prolonging the analgesic benefit of epidurals, may also cause a motor block of the lower limbs, again hindering the early mobilisation advocated in the ERAS pathway.
Epidural versus spinal analgesia
Epidural analgesia continues to be recommended in many ERAS protocols, but their weaknesses are discussed above. Does spinal analgesia offer a better alternative? There are obvious benefits. The failure rates with spinal analgesia are much lower (37) due to its well-defined end point. It is also less likely to cause major nerve injury due to the smaller size of the needle. Intrathecal-administered morphine also causes less haemodynamic compromise than epidural-administered (35). But does this translate to improved outcomes? Levy et al. compared epidural, spinal and patient-controlled analgesia for laparoscopic colorectal surgery. The epidural group had a longer length of stay, a longer time for return of bowel function and greater weight gain (a marker of fluid retention) when compared to the other two groups. Pain scores were significantly higher in the PCA group but only in the early postoperative period (38). Virlos et al. had similar findings in a similar patient group. Those that received intrathecal morphine were faster to mobilise, had shorter hospital stay and had better pain control and there was no difference in the rates of postoperative nausea and vomiting or ileus (39). Similar findings were also seen in open hepatectomies (40) and major open hepatic-pancreatic-biliary surgery (41).
Not all studies favour the spinal analgesia approach. In open gastrectomy surgery, intrathecal morphine was associated with greater postoperative opiate use, more nausea and vomiting, slower ambulation and a higher incidence of pulmonary complications and ileus (42). Unfortunately studies directly comparing the two techniques are limited.
Conclusions
Central neuraxial blocks, mainly epidurals, have been deemed the gold-standard for perioperative analgesia in enhanced recovery pathways based on strong evidence from the pre-ERAS era. However, with the introduction of ERAS pathways, many of the benefits the epidural offered have been mitigated by other ERAS strategies such as thromboprophylaxis, early feeding, avoidance of drains, early mobilisation and minimally invasive surgery. The evidence for superior postoperative analgesia and reduction in pulmonary complications still remains strong. However, this doesn’t always translate into reduced mortality or shorter lengths of stay. The hypotension, reduced mobility and greater intraoperative fluid administration associated with epidurals, once acceptable as the benefits were greater, may now be a cause for harm. Regional anaesthetic techniques are also growing and may offer an alternative adjunct to intrathecal opiates. In the authors’ institution, intrathecal opiate is preferred in laparoscopic major surgery and thoracic epidural in open major surgery accommodating the greater stress response and postoperative pain experienced. The type or surgery (lower vs. upper gastrointestinal, vascular or orthopaedic surgery), the surgical approach (open vs. laparoscopic/robotic), the medication used (local anaesthetic and/or opiates) and dose of any neuraxial drug will all have an impact on the success of the central neuraxial block. Neuraxial anaesthesia, in suitable patients, undoubtedly provides considerable benefits for patients undergoing surgery as part of an ERAS pathway.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Chris Jones and Leigh Kelliher) for the series “Current Issues in Analgesia for Major Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-86/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-86/coif). The series “Current Issues in Analgesia for Major Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wulf HF. The Centennial of Spinal Anaesthesia. Anesthesiology 1998;89:500-6. [Crossref] [PubMed]
- Chin A, Van Zundert A. Spinal Anaesthesia. Available online: https://www.nysora.com/techniques/neuraxial-and-perineuraxial-techniques/spinal-anesthesia/ [Accessed September 2021]
- Mandabach MG. The History of Epidural Anaesthesia: Pages, Dpgliotti, Guiterrez and Ruiz. The Anesthesiology Annual Meeting. American Society of Anesthesiologists, 2000:abstr A-1157.
- Avila-Hernandez AN, Singh P. Epidural Anaesthesia. Stat Pearls. Treasure Island (FL): StatPearls Publishing, 2021.
- Emelife PI, Eng MR, Menard BL, et al. Adjunct medications for peripheral and neuraxial anesthesia. Best Pract Res Clin Anaesthesiol 2018;32:83-99. [Crossref] [PubMed]
- van Zyl T, Klar G. An update on effective management of postdural puncture headache. Tutorial 458. Anaesthesia Tutorial of The Week, 2021.
- Cook TM, Counsell D, Wildsmith JA, et al. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009;102:179-90. [Crossref] [PubMed]
- Khangure N. Adjuvant agents in neuraxial Blockade. Anaesthesia Tutorial of The Week 230. Anaesthesia Tutorial of The Week, 2011.
- Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res 2017;10:2287-98. [Crossref] [PubMed]
- Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002;359:1276-82. [Crossref] [PubMed]
- Guay J, Nishimori M, Kopp S. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev 2016;7:CD001893. [Crossref] [PubMed]
- Meylan N, Elia N, Lysakowski C, et al. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth 2009;102:156-67. [Crossref] [PubMed]
- Tang J, Churilov L, Tan CO, et al. Intrathecal morphine is associated with reduction in postoperative opioid requirements and improvement in postoperative analgesia in patients undergoing open liver resection. BMC Anesthesiol 2020;20:207. [Crossref] [PubMed]
- Beaussier M, Weickmans H, Parc Y, et al. Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Reg Anesth Pain Med 2006;31:531-8. [Crossref] [PubMed]
- Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg 2003;96:583-9. [Crossref] [PubMed]
- LAS VEGAS investigators. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol 2017;34:492-507. [Crossref] [PubMed]
- Gupta H, Gupta PK, Fang X, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest 2011;140:1207-15. [Crossref] [PubMed]
- Odor PM, Bampoe S, Gilhooly D, et al. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ 2020;368:m540. [Crossref] [PubMed]
- Pöpping DM, Elia N, Marret E, et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg 2008;143:990-9; discussion 1000. [Crossref] [PubMed]
- Salata K, Abdallah FW, Hussain MA, et al. Short-term outcomes of combined neuraxial and general anaesthesia versus general anaesthesia alone for elective open abdominal aortic aneurysm repair: retrospective population-based cohort study†. Br J Anaesth 2020;124:544-52. [Crossref] [PubMed]
- Ellenberger C, Sologashvili T, Bhaskaran K, et al. Impact of intrathecal morphine analgesia on the incidence of pulmonary complications after cardiac surgery: a single center propensity-matched cohort study. BMC Anesthesiol 2017;17:109. [Crossref] [PubMed]
- Beattie WS, Badner NH, Choi P. Epidural analgesia reduces postoperative myocardial infarction: a meta-analysis. Anesth Analg 2001;93:853-8. [Crossref] [PubMed]
- Mohamad MF, Mohammad MA, Hetta DF, et al. Thoracic epidural analgesia reduces myocardial injury in ischemic patients undergoing major abdominal cancer surgery. J Pain Res 2017;10:887-95. [Crossref] [PubMed]
- Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000;321:1493. [Crossref] [PubMed]
- Zoumprouli A, Chatzimichali A, Papadimitriou S, et al. Gastrointestinal motility following thoracic surgery: the effect of thoracic epidural analgesia. A randomised controlled trial. BMC Anesthesiol 2017;17:139. [Crossref] [PubMed]
- Milosavljevic SB, Pavlovic AP, Trpkovic SV, et al. Influence of spinal and general anesthesia on the metabolic, hormonal, and hemodynamic response in elective surgical patients. Med Sci Monit 2014;20:1833-40. [Crossref] [PubMed]
- Iwasaki M, Edmondson M, Sakamoto A, et al. Anesthesia, surgical stress, and "long-term" outcomes. Acta Anaesthesiol Taiwan 2015;53:99-104. [Crossref] [PubMed]
- Pöpping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg 2014;259:1056-67. [Crossref] [PubMed]
- Ballantyne JC. Does epidural analgesia improve surgical outcome? Br J Anaesth 2004;92:4-6. [Crossref] [PubMed]
- Hughes MJ, Ventham NT, McNally S, et al. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg 2014;149:1224-30. [Crossref] [PubMed]
- Wagemans MF, Scholten WK, Hollmann MW, et al. Epidural anesthesia is no longer the standard of care in abdominal surgery with ERAS. What are the alternatives? Minerva Anestesiol 2020;86:1079-88. [Crossref] [PubMed]
- Leslie K, Myles P, Devereaux P, et al. Neuraxial block, death and serious cardiovascular morbidity in the POISE trial. Br J Anaesth 2013;111:382-90. [Crossref] [PubMed]
- Guay J, Choi P, Suresh S, et al. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014;CD010108. [Crossref] [PubMed]
- Hermanides J, Hollmann MW, Stevens MF, et al. Failed epidural: causes and management. Br J Anaesth 2012;109:144-54. [Crossref] [PubMed]
- Gallegos G, Morgan CJ, Scott G, et al. Effect of Neuraxial Analgesic Procedures on Intraoperative Hemodynamics During Routine Clinical Care of Gynecological and General Surgeries: A Case-Control Query of Electronic Data. J Pain Res 2020;13:1163-72. [Crossref] [PubMed]
- Low J, Johnston N, Morris C. Epidural analgesia: first do no harm. Anaesthesia 2008;63:1-3. [Crossref] [PubMed]
- Fettes PD, Jansson JR, Wildsmith JA. Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth 2009;102:739-48. [Crossref] [PubMed]
- Levy BF, Scott MJ, Fawcett W, et al. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 2011;98:1068-78. [Crossref] [PubMed]
- Virlos I, Clements D, Beynon J, et al. Short-term outcomes with intrathecal versus epidural analgesia in laparoscopic colorectal surgery. Br J Surg 2010;97:1401-6. [Crossref] [PubMed]
- Koea JB, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg 2009;2009:271986. [Crossref] [PubMed]
- Tang JZJ, Weinberg L. A Literature Review of Intrathecal Morphine Analgesia in Patients Undergoing Major Open Hepato-Pancreatic-Biliary (HPB) Surgery. Anesth Pain Med 2019;9:e94441. [PubMed]
- Lee JH, Park JH, Kil HK, et al. Efficacy of intrathecal morphine combined with intravenous analgesia versus thoracic epidural analgesia after gastrectomy. Yonsei Med J 2014;55:1106-14. [Crossref] [PubMed]
Cite this article as: Agarwala R, Morrison B. Neuraxial anaesthesia and its role in enhanced recovery after surgery: a narrative review. Dig Med Res 2022;5:20.