
Minimally-invasive approach to synchronous colorectal cancer liver metastases: outcomes from a single centre
Introduction
Globally, colorectal cancer remains the second most common cause of oncological deaths, with Asia the highest contributor: 957,896 (51.8%) of incident cases and 461,422 (52.4%) of deaths (1). At the point of diagnosis, around 15–25% of patients have synchronous liver metastases with only a minority of them having an upfront resectable liver disease (2,3).
The available modalities of regional treatment for hepatic metastases from colorectal cancer range from surgical resection, thermal ablation, regional hepatic intraarterial chemotherapy, chemoembolization, radioembolization and radiation therapy (RT). Among these, surgery remains the gold standard because it is associated with a long-term relapse-free survival plateau (4).
While hepatic resection used to be reserved for patients with a maximum of three lesions in the same lobe, if it was possible to achieve 1 cm margins, these “rules” have evolved in the modern era with advancements of both surgical techniques as well as systemic treatment. In fact, significant improvements in patient outcomes over the last decade have been attributed to the increased use of hepatic resection in appropriate patients and effective chemotherapy (5). As a result, many surgeons are now taking a more aggressive stance in the management of hepatic metastases.
Even as surgery continues to be their main chance of cure, adjuvant chemotherapy continues to play an ever-increasing role in this group of patients; improving both their response as well as survival rates (5). Amidst this backdrop, it is imperative that patients recover speedily post-surgery, allowing the prompt commencement of chemotherapy.
The ideal scenario is of course a safe, one stage, minimally invasive liver and colorectal resection followed by the swift initiation of chemotherapy. We know from practical experience however that this is not always feasible. We often have to mull over multiple factors such as the patient’s performance status, the technical complexities of hepatic and colorectal resections such as the tumour location and extent of disease, and even local symptoms which necessitate early interventions.
Many surgeons exercise yet further caution in making a decision for combined approaches in scenarios requiring a major liver resection for fear of major morbidity and mortality. Indeed, a large multicenter study by Reddy et al. accentuated this point. The study included 327 patients requiring major liver resection for synchronous colorectal liver metastases. Patients who underwent simultaneous colorectal and major hepatectomies (n=36) had significantly higher rates of severe morbidity (36% vs. 15%, P<0.05) and mortality (8% vs. 1%, P<0.05) compared to a staged approach (n=291) (6).
Thankfully, advances in anesthetic management as well as surgical training and techniques have afforded us more autonomy in recent years. Fretland et al. demonstrated in his study that laparoscopic liver resection was associated with significantly less perioperative complications and a shorter post-operative hospital stay compared to open liver resection for colorectal liver metastases (7). Martin et al. further described in his series of 240 patients that patients who underwent simultaneous resections had fewer complications and a shorter median hospital length of stay (LOS) with similarly low mortality rates compared to a traditional staged approach (8).
Such favorable results have led to a growing number of experienced surgeons proposing a minimally invasive and where possible, one stage approach to the management of colorectal liver metastases. Nevertheless, these enticing approaches remain fairly uncommon (9,10). We aim to review the outcomes of minimally invasive resection of colorectal liver metastases in our institutional series. We present the following article in accordance with the STROBE reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-20-155/rc).
Methods
Using a prospectively maintained colorectal cancer database at Koo Foundation Sun Yat-Sen Cancer Centre, patients who underwent a minimally invasive resection of a primary colorectal cancer and synchronous liver metastases between Jan 2008 to December 2019 were identified. Synchronous liver metastases were defined as those identified at the diagnosis of primary colorectal cancer. This study was approved by the Ethics Committee of Koo Foundation Sun Yat-Sen Cancer Center (IRB No. 20220118A). Informed consent was not taken from all the patients due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
All patients were discussed at a colorectal cancer multi-disciplinary meeting pre-operatively.
Preoperative workup was standardized: colonoscopy with biopsy; computed topography (CT) of chest, abdominal and pelvis and carcinoembryonic antigen (CEA). Where indicated, a magnetic resonance imaging (MRI) of the liver or rectum was organized.
The decision to perform either a staged or simultaneous resection was determined by the hepatobiliary surgeon in consultation with the colorectal surgeon. In general, our first-choice treatment for colorectal cancer with synchronous liver metastases was a minimally invasive approach, even if a low anterior resection and/or major hepatectomy was required, unless the patient was in poor health or had a symptomatic primary colorectal cancer (i.e., perforation, bleeding or obstruction). Patients with a limited liver function or potentially low future liver remnant were naturally recommended for a staged resection.
Patient baseline demographics, tumour characteristics, post-operative as well as oncological outcomes were available from the institutional database.
Surgical technique
Our series included both laparoscopic and robotic-assisted cases. For both surgical approaches, the colorectal dissection and anastomosis was performed first. This allowed us to work on healthy colorectal tissue, circumventing potential venous congestion of bowel caused by Pringle maneuverer, particularly in cases of technically challenging liver resections.
After completion of the colorectal segment, a laparoscopic ultrasound exploration of the liver was performed to precisely establish the limits of hepatic resection. Following adequate liver mobilization, ensuing hepatic resection was performed using the Harmonic Scalpel (Ethicon. Johnson & Johnson Medical). Further control of larger vessels and biliary ducts were obtained using a combination of bipolar cauterization, laparoscopic clips or laparoscopic linear staplers.
Once the tumour had been removed, the residual resection margins were further coagulated both for hemostasis as well as an adjunct attempt to obtain further oncological clearance in cases of microscopic residual disease. At the end of the procedure, fibrin glue was applied if required. Extraction of the specimens was obtained through a periumbilical incision extended from the initial 10 mm umbilical port site.
Statistical analysis
Baseline preoperative characteristics and surgical outcomes were summarized using descriptive statistics. Chi-square test or Fisher’s exact test were used for categorical variables and t-test for continuous variables to compare differences between the simultaneous or staged resection groups.
Statistical analysis was performed using SAS software (version 9.4 for SAS Institute, Inc., USA).
Results
During the study period, a total of 66 cases of liver resection for synchronous colorectal cancer liver metastasis were performed using a minimally invasive approach. 34 cases underwent simultaneous colorectal and liver resections while the remaining 32 underwent a staged approach. Three cases required conversion to open surgery. The mean age in our series was 56.4 (range, 34–81) years with a mean patient body mass index (BMI) of 24.8 (range, 18.2–35.7) kg/m2. Other patient demographics as well as pre-operative characteristics are outlined in Table 1.
Table 1
| Variables | Simultaneous (n=34) | Staged (n=32) | Combined (n=66) | P value |
|---|---|---|---|---|
| Mean age [range], yrs | 55.8 [34–81] | 57.1 [34–77] | 56.4 [34–81] | 0.5082 |
| Gender, n (%) | 0.0925 | |||
| Female | 14 (41.2) | 7 (21.9) | 21 (31.8) | |
| Male | 20 (58.8) | 25 (78.1) | 45 (68.2) | |
| Mean BMI [range], kg/m2 | 24.5 [18.2–35.7] | 25.0 [18.3–31.7] | 24.8 [18.2–35.7] | 0.5856 |
| ASA score, n (%) | 0.7276 | |||
| 1 | 0 | 0 | 0 | |
| 2 | 22 (64.7) | 22 (68.8) | 44 (66.7) | |
| 3 | 12 (35.3) | 10 (31.3) | 22 (33.3) | |
| 4 | 0 | 0 | 0 | |
| Neoadjuvant treatment, n (%) | 0.1635 | |||
| No | 20 (58.8) | 24 (75.0) | 44 (66.7) | |
| Yes | 14 (41.2) | 8 (25.0) | 22 (33.3) | |
| Colorectal tumour location, n (%) | 0.5527 | |||
| Right colon | 4 (11.8) | 2 (6.3) | 6 (9.1) | |
| Left colon | 16 (47.1) | 16 (50.0) | 32 (48.5) | |
| Rectosigmoid/upper rectum | 9 (26.5) | 8 (25.0) | 17 (25.8) | |
| Middle rectum | 2 (5.9) | 3 (9.4) | 5 (7.6) | |
| Lower rectum | 3 (8.8) | 3 (9.4) | 6 (9.1) | |
| Number of liver lesions, n (%) | 0.1744 | |||
| 1 | 17 (50.0) | 7 (21.9) | 24 (36.4) | |
| 2 | 7 (20.6) | 5 (15.6) | 12 (18.2) | |
| 3 | 3 (8.8) | 4 (12.5) | 7 (10.6) | |
| 4 | 3 (8.8) | 5 (15.6) | 8 (12.1) | |
| ≥5 | 4 (11.8) | 11 (34.3) | 15 (22.7) |
BMI, body mass index; ASA, American Society of Anesthesiologists.
The bulk of our patients (72.7%) underwent laparoscopic resections (Table 2). A hand port was utilized to facilitate hand-assisted laparoscopic surgical (HALS) liver resection in 3 of these cases. A significant number of cases (37.9%) involved rectal resections with 5 abdominoperineal resections (APRs) performed. Fourteen patients (21.2%) also underwent a major hepatic resection (involving ≥4 segments).
Table 2
| Variables | Simultaneous (n=34) | Staged (n=32) | Combined (n=66) | P value |
|---|---|---|---|---|
| Surgical approach, n (%) | 0.6691 | |||
| Laparoscopic/hand-assisted | 27 (79.4) | 21 (65.6) | 48 (72.7) | |
| Robotic-assisted | 7 (20.6) | 8 (25.0) | 15 (22.7) | |
| Conversion to open | 0 | 3 (9.4) | 3 (4.5) | |
| Primary resection, n (%) | 0.7478 | |||
| Right hemicolectomy | 4 (11.8) | 2 (6.3) | 6 (9.1) | |
| Left hemicolectomy | 3 (8.8) | 1 (3.1) | 4 (6.1) | |
| Anterior resection | 13 (38.2) | 17 (53.1) | 30 (45.5) | |
| Low anterior resection | 11 (32.3) | 9 (28.1) | 20 (30.3) | |
| APR | 2 (5.9) | 3 (9.4) | 5 (7.6) | |
| Liver resection, n (%) | 0.0122 | |||
| Ablation | 7 (20.6) | 1 (3.1) | 8 (12.1) | |
| Wedge | 7 (20.6) | 3 (9.4) | 10 (15.2) | |
| Segmental | 16 (47.1) | 18 (56.3) | 34 (51.5) | |
| Lobectomy and above (≥4 segments) | 4 (11.8) | 10 (31.3) | 14 (21.2) |
APR, abdominoperineal resection.
The median operation time was 460 (range, 225–1,053) minutes and the median estimated blood loss (EBL) was 150 (range, 25–3,600) mL (Table 3). The mean time of discharge was 10.1 (range, 3–27) days. There were no 30-day surgical mortalities, with a global morbidity of 13.6% (9 patients). Six patients (9.1%) had minor complications (Clavien-Dindo I–II) and 3 patients (4.5%) had major complications (Clavien-Dindo III–IV) (Table 3). One of the patients was re-operated for anastomotic leakage while the other 2 patients underwent drainage of intra-abdominal abscesses (one surgically and one via radiological guidance). We had a cumulative liver R0 resection rate of 89.4% (59/66 patients).
Table 3
| Variables | Simultaneous (n=34) | Staged (n=32) | Combined (n=66) |
|---|---|---|---|
| Liver resection margins, n (%) | |||
| R0 | 32 (94.1) | 27 (84.4) | 59 (89.4) |
| R1 | 2 (5.9) | 5 (15.6) | 7 (10.6) |
| Median operative time [range], min | 367 [265–1,053] | 533 [225–1,016] | 460 [225–1,053] |
| Median EBL [range], mL | 150 [25–650] | 300 [50–3,600] | 150 [25–3,600] |
| Mean LOS [range], days | 8.5 [3–19] | 11.9 [7–27] | 10.1 [3–27] |
| Complications1, n (%) | |||
| Grade 1–2 | 2 (13.3) | 4 (26.7) | 6 (9.1) |
| Grade 3 & above | 1 (6.7); intra-abdominal abscess | 2 (13.3); anastomotic leak; intra-abdominal abscess | 3 (4.5) |
| 30-day mortality | 0 | 0 | 0 |
1, Clavien-Dindo classification. EBL, estimated blood loss; LOS, length of stay.
Discussion
Our series looks predominantly at minimally invasive modalities for the resection of synchronous colorectal cancer liver metastases. The aim of a minimally invasive approach is naturally to reduce postoperative pain and wound complications facilitating a smoother post-operative recovery. This is particularly relevant for simultaneous liver and colorectal resections where the field of surgery concurrently involves the pelvis as well as upper abdomen. Coupled with a rising global incidence of obesity (11); which necessitates larger wounds for adequate surgical exposure, this can create considerable downstream issues in the post-operative recovery.
Suffice to say the use of minimally invasive surgery in the treatment of colorectal cancer is now conventional with better results in terms of transfusions, shorter recovery and discharge timings demonstrated; with corresponding surgical complications and oncological outcomes similar (12,13). Minimally invasive treatment of liver metastases has also gained traction in recent years, with evidence of improved short-term outcomes without any oncological compromise (14,15)
In our series, we achieved an overall morbidity rate of 13.6% (9/66 patients) with 4.5% (3 patients) suffering from a major complication. This is credible considering the reported rates of morbidity in the literature for isolated minimally invasive colorectal (19–45%) and liver (10–15%) resections (16,17). Furthermore, our series also had a higher proportion of major hepatic resections when compared to similar studies on hepatic resections (Table 4). The limited number of patients as well as the retrospective nature of this study however prevented further analysis.
Table 4
| Author | Year | Study type | N1 | Right sided CR | Left sided CR | Rectal resect | Minor Hep | Major Hep | Operative time2, min | EBL2, mL | LOS3, days | Cx | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Our series | 2020 | CS | 63/66 | 6 | 49 | 11 | 52 | 14 | 460 | 150 | 10.1 [3–27] | 9/66 | 0 |
| Bizzoca et al. (18) | 2019 | CS | 17/17 | 2 | 6 | 9 | 16 | 1 | 165 | 158 | 8.6 [5–36] | 8/17 | 0 |
| Chen et al. (19) | 2019 | CM | 61/122 | 15 | 6 | 40 | 61 | 0 | 206 | 200 | 6.0 [5–6] | 14/61 | 0 |
| Shim et al. (9) | 2018 | CM | 22/123 | 3 | 1 | 18 | 20 | 2 | 135 | 100 | 8.5 [5–22] | 2/22 | 0 |
| Ratti et al. (10) | 2016 | CM | 25/25 | 5 | 8 | 12 | 19 | 6 | 420 | 350 | 9.0 [4–17] | 6/25 | 0 |
| Ferretti et al. (20) | 2015 | MCS | 127/142 | 38 | 46 | 55 | 125 | 17 | 360 | 200 | 8.0 [3–84] | 44/142 | 3/142 |
| Jung et al. (21) | 2014 | CM | 24/24 | 2 | 1 | 21 | 18 | 6 | 290 | 325 | 8.0 [5–23] | 4/24 | 0 |
| Hu et al. (22) | 2012 | CM | 13/26 | 3 | 4 | 6 | 11 | 2 | 313 | 258 | 8.5 | 1/13 | 0 |
| Sasaki et al. (23) | 2009 | CS | 9/82 | 2 | – | 7 | 9 | 0 | 418 | 219 | 9.0 [3–37] | NA | 0 |
1, no. of minimally invasive cases/no. of cases in the series; 2, median values; 3, mean values [range]. CS, case series; CM, case matched; MCS, multi-centre study; CR, colon resection; Hep, hepatectomy; EBL, estimated blood loss; LOS, length of stay; Cx, complications.
Despite the listed complications, there were no 30-day mortality and our median hospital stay was 10.1 days. This was higher than the other reported series (20). This can largely be attributed to the existing national health insurance system in Taiwan which cumulates in a high level of health seeking behavior and an accompanying inclination among patients to fulfil the majority of their post-operative recovery inpatient (24). Enhanced recovery after surgery (ERAS) programs have not surprisingly failed to take off in a big way locally.
The mean age of our cohort was 56.4 (range, 34–81) years. We know that age at diagnosis is an independent prognostic factor of overall survival. A population-based study by Yang et al. of 30,000 patients revealed a mean survival time of 23 months for young patients (<50 years) with colorectal liver metastases, 17 and 6 months for the middle aged (50–69 years) and older aged (>69 years) groups (25). This is likely multi-factorial with a lower immune response and a higher level of chronic inflammation resulting in poorer survival. In addition, these elderly patients are also less likely to receive optimal treatment as a result of age-related increases in organ deterioration or comorbidities.
Other known independent prognostic factors for disease free survival include the largest initial liver metastases >5 cm and possibly a positive surgical margin (26). An R0 resection on the liver specimen was achieved in 89.4% of our cases. There are obviously no prospective randomized trials examining the impact of positive or close margins, but several large retrospective studies have suggested that positive margins do not increase the risk of death in the presence of post resection chemotherapy (27). It is the authors belief however that resection margins of >10 mm should always be targeted where anatomically feasible but surgery should still be offered when such a wide margin is not feasible.
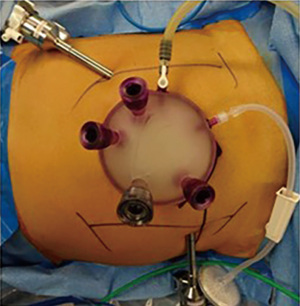
From a technical standpoint, we managed to complete all 34 simultaneous resection cases without a need for conversion. The liver surgeon however required adaptation to HALS in 3 cases via the periumbilical specimen site. For the 7 simultaneous robotic-assisted resections in our series, we further elected to utilize a single incision platform to reduce the total number of robotic ports keeping in mind the contrasting fields of surgery. This single incision platform incorporated two robotic arms (30-degree camera scope and hot shears with monopolar diathermy) as well as an assistant laparoscopic port (Figure 1) and was located at the eventual specimen extraction site. This is consistent with documented evidence that robotic simultaneous colorectal liver resection is safe, technically feasible and has an acceptable morbidity in specialized centres with well trained teams, even in cases requiring major liver resections (28).
Interestingly, we also found that 27.3% (18/66) of our patients eventually required repeat abdominal surgery. These included redo liver resections, incisional hernia repairs and excision of peritoneal recurrences. Of these patients, the majority (16/18) of them continued to receive minimally invasive surgery and only 2 patients required conversion to open for major liver resections. This highlights that an initial minimally invasive approach significantly limits the amount of adhesions, providing an enormous advantage in the patient’s eventual quality of life. Just as importantly, the option of yet another minimally invasive redo surgery persists.
The still evolving COVID-19 pandemic has also placed unprecedented stress on many healthcare systems worldwide. This sudden scale back in operative resources has led to re-triaging and prioritization of elective surgeries including such cancer cases. Delays in complex multi-disciplinary cancer surgery could risk losing a window for resection and ultimately compromise curative-intent surgeries. It is thus imperative that despite the limitations imposed by the pandemic, surgeons should still strive to provide standard of care treatment and all treatment decisions particularly those in which standard treatment is delayed or altered, should be made in a multidisciplinary team. For patients with minor liver disease requiring resection, more than ever, a minimally invasive approach is even more enticing with a lower morbidity and a shorter ICU or hospital stay (29).
We acknowledge the limitations of our study. Our study numbers are relatively small and the retrospective nature of this study with its inherent biases precludes easy generalizability. However, our surgical outcomes compare favorably to other reported case series. Ferretti et al. for example, which has one of the largest series of 142 patients, described a comparable median Op time of 360 min, a median EBL of 200 mL and a mean hospital LOS of 8.0 (range, 3–84) days (20).
Our series suggest that a combined minimally invasive approach can be performed safely under mature hands. As surgical techniques and minimally invasive modalities continue to advance unabated in tandem with emerging evidence on long term outcomes, a combined minimally invasive approach to synchronous colorectal liver metastases may well be the norm rather than exception in the not-so-distant future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Andrew A. Gumbs) for the series “Hepatic, Pancreatic and Biliary Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-20-155/rc
Data Sharing Statement: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-20-155/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-20-155/coif). The series “Hepatic, Pancreatic and Biliary Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Koo Foundation Sun Yat-Sen Cancer Center (IRB No. 20220118A). Informed consent was not taken from all the patients due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Onyoh EF, Hsu WF, Chang LC, et al. The Rise of Colorectal Cancer in Asia: Epidemiology, Screening, and Management. Curr Gastroenterol Rep 2019;21:36. [Crossref] [PubMed]
- Abu Hilal M, Underwood T, Zuccaro M, et al. Short- and medium-term results of totally laparoscopic resection for colorectal liver metastases. Br J Surg 2010;97:927-33. [Crossref] [PubMed]
- Garritano S, Selvaggi F, Spampinato MG. Simultaneous Minimally Invasive Treatment of Colorectal Neoplasm with Synchronous Liver Metastasis. Biomed Res Int 2016;2016:9328250. [Crossref] [PubMed]
- Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438-47; discussion 447-50. [Crossref] [PubMed]
- Tohme S, Goswami J, Han K, et al. Minimally Invasive Resection of Colorectal Cancer Liver Metastases Leads to an Earlier Initiation of Chemotherapy Compared to Open Surgery. J Gastrointest Surg 2015;19:2199-206. [Crossref] [PubMed]
- Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 2007;14:3481-91. [Crossref] [PubMed]
- Fretland ÅA, Kazaryan AM, Bjørnbeth BA, et al. Open versus laparoscopic liver resection for colorectal liver metastases (the Oslo-CoMet Study): study protocol for a randomized controlled trial. Trials 2015;16:73. [Crossref] [PubMed]
- Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 2003;197:233-41; discussion 241-2. [Crossref] [PubMed]
- Shim JR, Lee SD, Park HM, et al. Outcomes of liver resection in patients with colorectal liver metastases by laparoscopic or open surgery. Ann Hepatobiliary Pancreat Surg 2018;22:223-30. [Crossref] [PubMed]
- Ratti F, Catena M, Di Palo S, et al. Impact of totally laparoscopic combined management of colorectal cancer with synchronous hepatic metastases on severity of complications: a propensity-score-based analysis. Surg Endosc 2016;30:4934-45. [Crossref] [PubMed]
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. [Crossref] [PubMed]
- Tanis PJ, Buskens CJ, Bemelman WA. Laparoscopy for colorectal cancer. Best Pract Res Clin Gastroenterol 2014;28:29-39. [Crossref] [PubMed]
- Chen K, Cao G, Chen B, et al. Laparoscopic versus open surgery for rectal cancer: A meta-analysis of classic randomized controlled trials and high-quality Nonrandomized Studies in the last 5 years. Int J Surg 2017;39:1-10. [Crossref] [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Morneau M, Boulanger J, Charlebois P, et al. Laparoscopic versus open surgery for the treatment of colorectal cancer: a literature review and recommendations from the Comité de l'évolution des pratiques en oncologie. Can J Surg 2013;56:297-310. [Crossref] [PubMed]
- Tranchart H, Dagher I. Laparoscopic liver resection: a review. J Visc Surg 2014;151:107-15. [Crossref] [PubMed]
- Bizzoca C, Delvecchio A, Fedele S, et al. Simultaneous Colon and Liver Laparoscopic Resection for Colorectal Cancer with Synchronous Liver Metastases: A Single Center Experience. J Laparoendosc Adv Surg Tech A 2019;29:934-42. [Crossref] [PubMed]
- Chen YW, Huang MT, Chang TC. Long term outcomes of simultaneous laparoscopic versus open resection for colorectal cancer with synchronous liver metastases. Asian J Surg 2019;42:217-23. [Crossref] [PubMed]
- Ferretti S, Tranchart H, Buell JF, et al. Laparoscopic Simultaneous Resection of Colorectal Primary Tumor and Liver Metastases: Results of a Multicenter International Study. World J Surg 2015;39:2052-60. [Crossref] [PubMed]
- Jung KU, Kim HC, Cho YB, et al. Outcomes of simultaneous laparoscopic colorectal and hepatic resection for patients with colorectal cancers: a comparative study. J Laparoendosc Adv Surg Tech A 2014;24:229-35. [Crossref] [PubMed]
- Hu MG, Ou-yang CG, Zhao GD, et al. Outcomes of open versus laparoscopic procedure for synchronous radical resection of liver metastatic colorectal cancer: a comparative study. Surg Laparosc Endosc Percutan Tech 2012;22:364-9. [Crossref] [PubMed]
- Sasaki A, Nitta H, Otsuka K, et al. Ten-year experience of totally laparoscopic liver resection in a single institution. Br J Surg 2009;96:274-9. [Crossref] [PubMed]
- Wu TY, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. London J Prim Care (Abingdon) 2010;3:115-9. [Crossref] [PubMed]
- Yang L, Yang X, He W, et al. Comparisons of metastatic patterns of colorectal cancer among patients by age group: a population-based study. Aging (Albany NY) 2018;10:4107-19. [Crossref] [PubMed]
- Andreou A, Brouquet A, Abdalla EK, et al. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB (Oxford) 2011;13:774-82. [Crossref] [PubMed]
- Miller CL, Taylor MS, Qadan M, et al. Prognostic Significance of Surgical Margin Size After Neoadjuvant FOLFOX and/or FOLFIRI for Colorectal Liver Metastases. J Gastrointest Surg 2017;21:1831-40. [Crossref] [PubMed]
- Navarro J, Rho SY, Kang I, et al. Robotic simultaneous resection for colorectal liver metastasis: feasibility for all types of liver resection. Langenbecks Arch Surg 2019;404:895-908. [Crossref] [PubMed]
- Lassen K, Nymo LS, Olsen F, et al. Contemporary practice and short-term outcomes after liver resections in a complete national cohort. Langenbecks Arch Surg 2019;404:11-9. [Crossref] [PubMed]
Cite this article as: Ng JY, Chen CC, Tsai TJ. Minimally-invasive approach to synchronous colorectal cancer liver metastases: outcomes from a single centre. Dig Med Res 2022;5:10.


