
A narrative review on COMMD1, the promiscuous ATP7B chaperone bridging the gap between inherited canine copper toxicosis and Wilson disease
Introduction
To accommodate the various functions of the liver, such as biotransformation, lipid homeostasis, glutamine, and urea production, the different cell types in the liver are structured in an orderly way and the functions are zonally distributed. For instance, the periportal area covering the portal vein, the hepatic artery, and the bile duct is involved in gluconeogenesis, beta-oxidation, cholesterol biosynthesis, and ureagenesis, whereas the central vein area is mainly responsible for glycolysis, bile acid production, glutamine synthesis, and biotransformation. Midway the Portal-Central axis the iron homeostasis is regulated, whereas the copper concentration is highest in the periportal zone (1,2). The very first in-depth single-cell RNA sequencing results confirmed the zonation pattern and indicated that around 50% of all liver genes are zonally distributed (3). The most abundant liver cell type is the hepatocyte, accounting for 2-thirds of all liver cells. Liver sinusoidal endothelial cells (blood vessel lining) around 15–20%, slightly more than the percentage of Kupffer cells (the liver-specific macrophages), and hepatic stellate cells (HSC) responsible for vitamin A storage. Cholangiocytes, the cells of the bile duct, are the least numbered cells in the liver, 5% at max (4-7). The crucial cell and driving force in liver fibrosis is the HSC. In a healthy liver, the HSCs are the main storage cells for vitamin A stored as Retinyl-Esters in large lipid droplets (LDs). LDs are intracellular organelles consisting of a hydrophobic core containing neural lipids (triacylglycerols, cholesteryl esters, retinyl esters) surrounded by a phospholipid monolayer and a specific set of proteins such as perilipins (8-10). In case of liver damage, the HSCs rapidly lose their LDs and transactivate into a profibrogenesis phenotype that is responsible for the production of extracellular matrix (ECM) proteins. This highly dynamic phenotypical shift, or transactivation, is the hallmark of fibrosis and occurs irrespective of the type of damage trigger. It is important to realise the crucial role of fibrogenesis in the deteriorating progression of liver diseases which often follows a pattern of (I) initial hepatitis, (II) liver fibrosis that extends to (III) liver cirrhosis which is correlated with (IV) increased risk to develop liver cancer.
Hepatic diseases have various underlying causes, for instance, (I) viruses like HAV, HBV, HCV, HDV, HEV, and (II) toxins, such as the peanut mold-derived aflatoxin, and drugs like the frequently used paracetamol, but also (III) inherited liver diseases caused for instance by elevated levels of intrahepatic copper. With on the one hand greatly improved prevention and treatment options for virally-induced hepatitis as a good sign of clinical achievements in recent years, the rise in nonalcoholic fatty liver diseases (NAFLD) is very worrisome. The importance and global burden of NAFLD do not need further introduction in this special issue given the fact that the quality of life of around 1 in every 4 humans worldwide is affected by some form of NAFLD, which includes simple steatosis or more advanced nonalcoholic steatohepatitis (NASH) (11,12). Taking into account that NAFLD is associated with an increased risk to develop type 2 diabetes, cardiovascular diseases, chronic kidney failure, and hepatocellular carcinoma (HCC) the term metabolic-associated fatty liver diseases (MAFLD) is suggested as being more appropriate (13). Surprisingly, in developed and developing countries the numbers are equally rising at a disturbing pace. In sharp contrast to the enormous large part of the world population affected by hepatic diseases presenting as a form of NAFLD/MAFLD, are the rare inherited copper-related hepatic disorders. This rarity might be explained by the specific chemical properties of copper.
The trace element copper is indispensable for various biochemical processes, yet at the same time, copper is involved in deleterious reactions caused by the involvement of copper in the formation of reactive oxygen species (ROS) (14,15). The transition element copper can be present in a reduced form as Cu+ and in an oxidized state as Cu++, this makes copper ions particularly strong mediators of ROS formation in Fenton and Haber-Weiss chemistry. Because of these opposing positive and negative effects of copper in biological systems, intracellular free copper concentrations need to be kept within very narrow limits (16). The intricate intracellular copper homeostasis occurs at various levels such as the site of uptake, intracellular-binding and -distribution, and excretion. Uptake of copper is mediated via Copper Transporter 1 (CTR1), the main transmembrane copper import protein (17). CTR1 does not only transport copper; it can also transport zinc, which explains some of the protective effects of dietary zinc on copper toxicosis. Once inside the cell, copper-binding proteins maintain intracellular free copper levels low, these copper-chaperones include Cytochrome c Oxidase Copper Chaperone (COX17), Copper Chaperone for Superoxide Dismutase (CCS), and Antioxidant protein1 (ATOX1) (14,16). In addition, intracellular copper can be sequestered by glutathione and metallothionein, to minimize its disastrous radical-mediated impact on cellular components such as lipids, proteins, and DNA. To excrete copper the P-type ATPases ATP7A and ATP7B are crucial (18). Generally speaking, ATP7A determines the excretion of copper from the intestine into the bloodstream, whereas ATP7B, highly expressed in hepatocytes, dictates hepatic excretion. Once copper is excreted, ceruloplasmin mediates copper transport through the bloodstream. When the intrahepatic levels raise above 250 mg/kg dry weight liver (dwl) it may lead to the development of Wilson Disease (WD) in men (19). Clinical cases of hepatic copper accumulation are not restricted to men, but also are often observed in men’s best friend, the dog.
This chapter in the special issue “the pathogenesis of hepatic fibrosis: basic facts and clinical challenges” addresses the role of COMMD1 (COpper Metabolism Murr1 Domain-containing protein 1) which was discovered 20 years ago in a specific dog breed with inherited copper toxicosis (20), a disease, to a large extent similar to human WD. The key questions addressed here focus on how mutations in the COMMD1 gene lead to a COpper Mineral Mediated Disease in dogs and highlight how the promiscuous behavior of the COMMD1 protein explains a phenotype that is similar to WD which is caused by mutations in the ATP7B gene (21-23). The clinical challenges for WD are exemplary for the challenges most patients and researchers coping with rare diseases are confronted with. These typical challenges will be addressed and the overlap between ATP7B and COMMD1 might provide a solution for some of these hurdles such as difficulties in genetic screens and lack of proper animal models will be appreciated. The rational for this narrative is based on the promise that dogs can bridge between fundamental research findings and clinical practice. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-96/rc).
Methods
A monthly PubMed-search is done every month (latest time point for this manuscript October 2021), with keywords such as COMMD1, Wilson diseases, modifier genes, copper toxicosis and combinations hereof. Selected language was English. In addition, a search was done including specific authors such as Lutsenko, Weiss, Stremmel, and Czlonkowska, van de Sluis, Weiskirchen, to make sure to include as much as possible potentially relevant papers. Second screen was based on the abstract text of the papers in the first two searches. Lastly, whole manuscript text was analyzed of the selected group of papers that was finally included in this manuscript (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | Monthly, latest October 15th, 2021 |
| Databases and other sources searched | PubMed |
| Search terms used (including MeSH and free text search terms and filters) | COMMD1 |
| Canine copper toxicosis | |
| Wilson disease (reviews only in first round) | |
| Authors: Lutsenko, Weiss, Stremmel, Czlonkowska, van de Sluis, Weiskirchen | |
| Timeframe | For reviews last 5 years was used, for others no time limit was set so from January 1979 onwards, up to October 15th, 2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Language: English |
| Exclusion: case report | |
| Additional screen: on specific authors to make sure the topic was covered well | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Selection on WD and coper toxicosis by LCP, FGvS was responsible for the papers on genetic screens as presented in the figure legend. Consensus was reached after two rounds of bilateral discussions |
| Any additional considerations, if applicable | It is always possible that papers may have been missed |
Discussion
The unique population structure of dog breeds facilitates genetic research on rare inherited diseases
Domestication of dogs occurred thousands of years ago, but it was only in the latest century that selective inbreeding resulted in severe phenotypic features, such as excessive muscle formation, short limbs, or specific coat color in dogs (24). Several examples demonstrate how selection for phenotypic characteristics resulted in severe health issues for canines like the selection for a short nose leading to breathing problems (25). Fast-growing canine breeds may develop osteosarcoma, and breeds with long backs often get hernias. Different diseases occur in extraordinarily high prevalence within specific breeds selected for phenotypic extremes. Selective breeding has also resulted in increased prevalence of more complex genetic disorders including cancer, epilepsy, and cardiovascular diseases. The canine and human genome show high conserved synteny of over 95% similarity. The combination of this high similarity with the increased prevalence makes the dog a suitable naturally occurring genetic disease model.
Essential to note: diseases in the dog arise spontaneously. Therefore, these diseases are more comparable to human disease than artificially induced rodent models. Clinical presentation of disease and genomic structure in dogs is much more homogeneous than in humans (26), which increases power in genetic mapping studies. Mapping a complex trait is performed using a few hundred cases and controls. In humans, many thousands of patients are needed, and the number of necessary SNPs is 10-fold higher than in dogs (27). Also, the dog’s unique genomic structure allows for the detection of genetic modifiers. Especially, when due to inbreeding, fixation of a disease-causing variant occurred, the difference between being healthy and affected is determined by such a modifier, changing it into a disease-determining variant. After identification of new mutations in the current approach, canine models can be used in pre-clinical therapeutic intervention studies, for example for gene therapy (28).
Besides the benefit of being bred in a genetically isolated population, the dog has another benefit: the availability of affected tissue originating from a spontaneous disease model. Since the patients are no experimental animals, but client-owned animals, we are allowed under informed consent to isolate tissue upon euthanasia. This tissue can be used for research purposes and resembles the human affected tissue by means of pathological criteria, and size. Moreover, the size of dogs permits preclinical studies on e.g., liver stem cell transplantations (29,30).
The discovery of the causative mutation leading to inherited copper toxicosis in Bedlington terriers
As a result of selective inbreeding, hepatic copper toxicosis arose in several dog breeds. Veterinarians were aware of copper disorders in dogs for decades (31). The increased levels of hepatic copper are described in several dog breeds including Bedlington terriers, Skye terriers, West-Highland White terriers, Dobermanns, Dalmatians, and Labrador retrievers (32-37). Often this copper-mediated hepatitis is passed over to the next generation by a complex mode of inheritance, as pedigree studies revealed in most breeds, except the Bedlington terrier where a simple Mendelian mode of inheritance is observed.
The first description of copper toxicosis in US-based Bedlington terriers, a mid-sized dog breed, was reported as early as in the late seventies of the previous century with intrahepatic copper to 2,000 mg/dwl (32). The histological similarities with WD were obvious, regarding high hepatic copper levels and hepatic fibrosis. It should be noted that the intrahepatic copper levels in European Bedlington terriers have been observed as high as 12,000 mg/dwl which is way above the levels observed for human WD patients. Whereas in 1993 the causative mutation leading to WD was described (21-23), it took an additional eight years before the causative mutation for Bedlington terrier copper toxicosis was found. Tools in those days for dog genetics were lagging behind the tools available for human genetics (see legend Figure 1). One of the first successes of a microsatellite approach in canine genetics screens was the localization of the gene causing Bedlington terrier copper toxicosis with a set of as little as 213 microsatellite markers (59). A few years later, in 1999 a genetic mapping study proved that the copper toxicosis locus in Bedlington terriers was located on canine chromosome 10 region 2p26 (60). Using positional cloning, a large deletion of about 40 kB covering exon-2 of the MURR1 gene was identified as the causative mutation of Bedlington terrier copper toxicosis in 2002 (20). The precise breakpoints were described three years thereafter in 2005 (61). The current name for MURR1 is COMMD1 (COpper Metabolism Murr1 domain-containing protein 1), which is more in line with the mechanism of action of COMMD1 in hepatic copper homeostasis. A few studies unequivocally proved that COMMD1 activity reduces hepatic copper levels and mutations in the COMMD1 gene resulted in elevated intracellular copper levels (62-65). siRNA-mediated COMMD1-gene silencing in HEK293 cells (human embryonic kidney cells) and BDE-cells (canine liver cells) resulted in elevated intracellular copper levels, even in short-term cultures (62,63). Liver-specific COMMD1 knock-out mice had moderate levels of hepatic copper accumulation, although by no means as high as in the Bedlington terrier dogs (29,64,66). In liver organoids cultured from COMMD1-deficient dogs, lentiviral reconstitution of a functional COMMD1 protein resulted in a normalization of intracellular copper levels and survival of these cells under high copper culture conditions (65). These were solid proofs to verify the crucial role of COMMD1 in hepatic copper homeostasis. Ever since the 2002 discovery of COMMD1, a plethora of functions have been linked to COMMD1, for a recent review on the multi-potency of COMMD1, the readers are directed elsewhere (67-70).
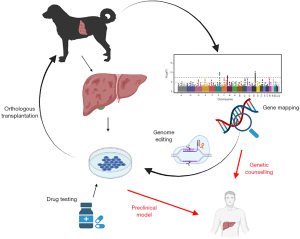
The work on copper toxicosis in Bedlington terriers resulted in the discovery of a novel copper homeostasis gene product, COMMD1 (20). This prompted geneticists to screen for COMMD1 mutations in copper storage diseases with thus far unknown genetic backgrounds such as Indian Childhood Cirrhosis, Endemic Tyrolean Infantile Cirrhosis, or Idiopathic Copper Toxicosis, very rare diseases with hepatic copper accumulation. No mutations in the COMMD1 gene were discovered in these patients (71). In WD patients COMMD1 mutations are described at very low frequencies and the COMMD1 variants seemed unrelated to the clinical presentation of the patients (72-76). Together, the involvement of COMMD1 mutations in human copper-related disorders is very limited, but this does not mean that the work on COMMD1 is of little value for WD research.
Challenges in WD diagnosis and partnering of the ATP7B and COMMD1 proteins
The timeline of milestones for WD, first described as early as 1912, is described in a historical review (77). The main drive of this disease, which presents with large clinical variations, is excessive hepatic copper accumulation. The ERN RARE-LIVER database (www.rare-liver.eu) states WD as a rare disease, with an estimated clinical prevalence of 1 per 30,000 in the general European population. A higher prevalence is described in several isolated communities (consanguinity) within Europe, such as Sardinia or the Canary Islands, with a 5–10 fold higher prevalence compared to the general European population (78). In several Asian regions, the prevalence of WD is doubled compared to the general European population (79,80). More recent population-based estimates suggest that the genetic prevalence in some areas might be 3 to 4 times the clinical presentation (81). Even larger differences in the prevalence in heath registers (±1.6/100,000) compared to mutation in the general population (±20/100,000) were reported (82). This points to the involvement of additional factors mediating genotype-phenotype differences, such as the activity of modifier genes, and or environmental factors.
The causative gene for WD was discovered in 1993, as the copper transporter ATPase2 (ATP7B) (21-23). The organ-specific expression of ATP7B, high in the liver but also other organs such as the brain, and parts of the small intestine, explains the clinical variations of WD including hepatic and neurological presentations (83-86). Around half of WD patients present with a clear hepatic phenotype, which has a gender predisposition, four times higher in females than in males in the case of the severe acute liver failure form. Liver-related features present already in childhood and young adults. Neurological symptoms occur later in life in a broad range of WD patients (20–65%), depending on the characteristics. A wide spectrum of movement disorders, including tremor, dystonia, and or parkinsonism present as early as the age 20–30 (80,86-88). As these neurological and psychiatric symptoms are separated from the hepatic presentation, the readers are directed to specific reviews on these aspects elsewhere (89-95). One very characteristic visual sign of WD is the presence of Kayser-Fleischer rings in the cornea, which is observed in most neurological WD patients, in hepatic or presymptomatic patients these ocular disturbances are less frequently observed (89). The variation in clinical presentation and the rarity of WD often results in a delayed clinical diagnosis with severe consequences (94).
The ATP7B gene spans over 80 kb on chromosome 13, and the longest liver transcript consists of all 21 exons covering around 8 kb. Smaller transcripts are present in the brain (95). The ATP7B protein consists of eight transmembrane regions forming a membrane channel, and the copper-binding domains are in the N-terminal part within the cytosol. The ATP7B protein channel function is responsible for about 95% of the excretion of hepatic copper into the bile. Taken into account well over 900 different mutations in the ATP7B gene (Human Gene Mutation Database professional 2021.3) (84,96,97), leading to variable activities of the ATP7B protein, and as a consequence, a large variation in the clinical presentation of WD, diagnosis of WD is challenging (81,98-100). The vast majority of the mutations (>60%) are missense and nonsense mutations. A clear genotype-phenotype correlation is lacking (101-106). Therefore, it is conceivable that modifier genes and/or environmental factors that affect the clinical presentation are into play here. Several potential modifier genes include ATOX1, XIAP (X-linked inhibitor of apoptosis), MTHFR (5,10-methylenetetrahydrofolate reductase), and COMMD1 (74,83,107-110). Less surprisingly, ATOX1, XIAP, and COMMD1 are directly or indirectly involved in copper binding. In addition to direct copper-binding within the COMMD1 protein, it turned out that COMMD1 interacts with ATP7A and ATP7B, to facilitate intracellular trafficking of the copper exporting ATPases from the Trans-Golgi-Network to the plasma membrane (111-113). Therefore, mutated COMMD1 directly affects ATP7Bs intracellular localization and the copper excretion function of ATP7B, explaining the similarities in the hepatic fibrotic phenotype of WD and canine copper toxicosis.
Discovery of novel copper homeostasis genes and modifier genes in copper toxicosis
Finding modifier genes in rare diseases is an immense genetic and clinical hurdle, given the low number of patients. Whereas copper toxicosis in Bedlington terriers segregates with a simple Mendelian mode of inheritance, for most dog breeds copper toxicosis is a more complex genetic disease. Due to selective inbreeding, genetic variants express at increased frequencies within specific dog breeds. As a result, rare human diseases can be highly prevalent in specific dog breeds.
Labrador retrievers is the most popular dog breed worldwide. In 2006 copper-associated hepatitis was described in this breed, presenting elevated ALT levels, and hepatic copper levels ranging between around 400–2,600 mg/dwl (37). A complex mode of inheritance was postulated (114). Several studies indicated that dietary restriction could improve the hepatic copper accumulation such as low copper diets especially so if zinc was elevated, and as for WD patients D-penicillamine was effective to lower hepatic copper levels (115-119). In view of the risk of hepatic copper and zinc deficiency, it was not recommended to use D-penicillamine lifelong (120). Gene expression pattern described the progression of copper toxicosis in Labrador retrievers, eluting the similarities between WD and copper toxicosis in this breed at the molecular levels (121). Considering the similarities with WD, researchers aimed to dissect the genetic background of Labrador copper toxicosis. To emphasize the power of canine genetics to dissect genetic causes in complex genetic diseases as little as 235 dogs were enrolled in a canine-specific genome wide association study (GWAS). This study revealed that an Arg1453Gln substitution in the ATP7B protein is causing increased hepatic copper levels, whereas a Thr327Ile variant in the ATP7A protein partially rescued the ATP7B phenotype (122). Similarly, an ATP7B mutation in Dutch and USA Dobermann pinchers increased hepatic copper levels. A mutation in ATP7A was also found in the Dutch Dobermann pinschers, however, there were too few cases to draw conclusions for the USA cohort (123). Beauty is in the eye of the beholder, but the fact that ATP7A is a modifier gene for ATP7B is astonishing given their high level of structural homology. In this breed, the high prevalence of mutations in ATP7A showed the power of canine genetics to find modifier genes. The fact that these mutations are in a gene that is causative for Menkes disease (MD), further highlights the potential of canine research for human genetics. Unlikely that this would have been discovered in men where MD is affecting 1 in 300,000 people. It turned out that COMMD1 mutations were involved in neither Labrador retrievers nor Dobermann pinchers copper toxicosis (124). Recently, RETN (coding for protein RESISTIN) was discovered as a novel modifier gene in copper toxicosis in Labrador retrievers (125). RESISTIN is involved in hepatic fat storage and mitochondrial defects, however, its low expression in the liver makes it difficult to directly associate RETN mutations with hepatic copper accumulation.
Dogs can solve pre-clinical problems associated with hepatic diseases
Longitudinal studies on COMMD1-deficient dogs revealed a progressive development of hepatitis similar to the development of chronic hepatitis, including WD, in men (126,127). This makes the COMMD1-deficient dogs a unique pre-clinical model to study the effects of cell-transplantations for inborn metabolic diseases. Five COMMD1-deficient dogs received autologous liver stem cells in which the functional COMMD1 gene was lentivirally-transduced (30). From a pre-clinical perspective, it was agreed upon with pediatricians that the portal vein was the preferred route of administration of the cells, as this was an approachable vein even in pediatric patients. To make a long story short, the transplanted cells could be recovered even up to two years post-transplantation. However, no functional recovery was observed on hepatic copper accumulation and copper excretion, which was explained by the limited number of transplanted cells in the recipient's livers. Only at a few locations, small clusters of transplanted cells were recovered accounting for less than 1% of the total number of hepatocytes. What caused this low engraftment is under investigation; either too few cells were transplanted (4×108–9×108 per dog in three consecutive daily injections), or the cells were not in the optimal differentiation status (partially hepatic, not purely proliferating stem cell phenotype), or despite being autologous the cells were rejected (cyclosporin treatment was given for 6 weeks), or other reasons. As the induction of ovulation in dogs is difficult, it will take a while before the offspring of the heterozygous parents will result in a sufficiently large study colony again.
Although the functional recovery was not achieved, this pre-clinical experiment in COMMD1-deficient dogs showed the possibility to provide autologous cells via the portal vein into the liver and showed long-term survival of the transplanted cells in the recipient. The 2 years longitudinal follow-up period provided critical information on the routing of transplanted cells, a basic question that could hardly be addressed in mice, given their small portal vein.
Future perspectives for health professionals and researchers
The key issue of this narrative is to create awareness that dogs can be instrumental in solving basic (genetics) and pre-clinical (transplantations) liver-related problems. First, the resultant of selected inbreeding is a narrow genetic basis within specific breeds working as a genetic magnifying glass, enabling the dissection of complex genetic and/or (very) rare disorders. Here it is to be realized that still some very rare copper storage diseases no causative mutations are discovered, such as for Indian Childhood Cirrhosis, Endemic Tyrolean Infantile Cirrhosis, or Idiopathic copper toxicosis (128-130). And the work on Labrador retrievers has indicated that modifiers genes can be found, actually a functional titration between ATP7B (WD) and ATP7A (MD) activities. Second, the size of dogs and the hepatic fibrosis progression, position dogs as relevant pre-clinical animal models to test dietary regimens and surgical interventions that are readily translatable to human clinical practice. Obviously, practical (costs, housing, reproduction) and ethical issues (appreciation of dog is often different than for mice) exclude large scale experiments in dogs. In view of the current debate on the utilization of experimental animals, liver organoid technology promises precision medicine without experimental animals. The combination of the gene-editing tool CRISPR/Cas9 with stem cell technology is gaining momentum (131-135). These technologies could be specifically rewarding for rare diseases, including inborn metabolic disorders (136,137).
Summary
WD and inherited canine copper toxicosis have several features in common. In some dog breeds mutations in ATPase transporters (involved in WD and MD in men) are also implicated in copper toxicosis. Genetic screens in dogs revealed a novel copper transport protein 20 years ago, COMMD1. In addition, COMMD1-deficient dogs were used as a pre-clinical animal model for stem cell transplantation. The intracellular interactions between ATP7B and COMMD1 explain partial overlap in clinical presentation between WD patients and dogs with copper toxicosis. As such dogs have had their fair share in solving some challenges related to rare forms of liver fibrosis. After all, the dog is men’s best friend. It is likely that in the years to come additional copper transporters and novel modifier genes will be discovered in the inbred dog population, realizing that around 35% of the idiopathic hepatic cases in dogs are related to copper (138). Mutual beneficial for patients and pets challenged with rare liver diseases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ralf Weiskirchen) for the series “The Pathogenesis of Hepatic Fibrosis: Basic Facts and Clinical Challenges” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-96/rc)
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-96/coif). The series “The Pathogenesis of Hepatic Fibrosis: Basic Facts and Clinical Challenges” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saviano A, Henderson NC, Baumert TF. Single-cell genomics and spatial transcriptomics: Discovery of novel cell states and cellular interactions in liver physiology and disease biology. J Hepatol 2020;73:1219-30. [Crossref] [PubMed]
- Manco R, Itzkovitz S. Liver zonation. J Hepatol 2021;74:466-8. [Crossref] [PubMed]
- Halpern KB, Shenhav R, Matcovitch-Natan O, et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 2017;542:352-6. [Crossref] [PubMed]
- Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol 1977;72:441-55. [Crossref] [PubMed]
- Banales JM, Prieto J, Medina JF. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol 2006;12:3496-511. [Crossref] [PubMed]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125-72. [Crossref] [PubMed]
- Steib CJ. Kupffer cell activation and portal hypertension. Gut 2011;60:1307-8. [Crossref] [PubMed]
- Walther TC, Farese RV. Lipid droplets and cellular metabolism. Annu Rev Biochem 2012;81:687-714. [Crossref] [PubMed]
- Kimmel AR, Sztalryd C. The Perilipins: Major Cytosolic Lipid Droplet-Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annu Rev Nutr 2016;36:471-509. [Crossref] [PubMed]
- Thiam AR, Beller M. The why, when and how of lipid droplet diversity. J Cell Sci 2017;130:315-24. [Crossref] [PubMed]
- Fazel Y, Koenig AB, Sayiner M, et al. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016;65:1017-25. [Crossref] [PubMed]
- Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 2019;70:531-44. [Crossref] [PubMed]
- Eslam M, Sanyal AJ, George J, et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020;158:1999-2014.e1. [Crossref] [PubMed]
- Inesi G. Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life 2017;69:211-7. [Crossref] [PubMed]
- Maung MT, Carlson A, Olea-Flores M, et al. The relationship with human pathologies. FASEB J 2021;35:e21810. [Crossref] [PubMed]
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 2008;4:176-85. [Crossref] [PubMed]
- Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci U S A 1997;94:7481-6. [Crossref] [PubMed]
- Lutsenko S, Barnes NL, Bartee MY, et al. Function and regulation of human copper-transporting ATPases. Physiol Rev 2007;87:1011-46. [Crossref] [PubMed]
- Stremmel W, Weiskirchen R. Therapeutic strategies in Wilson disease: pathophysiology and mode of action. Ann Transl Med 2021;9:732. [Crossref] [PubMed]
- van De Sluis B, Rothuizen J, Pearson PL, et al. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet 2002;11:165-73. [Crossref] [PubMed]
- Bull PC, Thomas GR, Rommens JM, et al. The Wilson’s disease gene is a putative copper transporting P-type ATPase similar to Menkes’ gene. Nat Genet 1993;5:327-37. [Crossref] [PubMed]
- Tanzi RE, Petrukhin K, Chernov I, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 1993;5:344-50. [Crossref] [PubMed]
- Yamaguchi Y, Heiny ME, Gitlin JD. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun 1993;197:271-7. [Crossref] [PubMed]
- van Steenbeek FG, Hytönen MK, Leegwater PA, et al. The canine era: the rise of a biomedical model. Anim Genet 2016;47:519-27. [Crossref] [PubMed]
- Packer RM, Hendricks A, Tivers MS, et al. Impact of Facial Conformation on Canine Health: Brachycephalic Obstructive Airway Syndrome. PLoS One 2015;10:e0137496. [Crossref] [PubMed]
- Karlsson EK, Lindblad-Toh K. Leader of the pack: gene mapping in dogs and other model organisms. Nat Rev Genet 2008;9:713-25. [Crossref] [PubMed]
- Hayward JJ, Castelhano MG, Oliveira KC, et al. Complex disease and phenotype mapping in the domestic dog. Nat Commun 2016;7:10460. [Crossref] [PubMed]
- Nowend KL, Starr-Moss AN, Murphy KE. The function of dog models in developing gene therapy strategies for human health. Mamm Genome 2011;22:476-85. [Crossref] [PubMed]
- Kruitwagen HS, Fieten H, Penning LC. Towards Bioengineered Liver Stem Cell Transplantation Studies in a Preclinical Dog Model for Inherited Copper Toxicosis. Bioengineering (Basel) 2019;6:88. [Crossref] [PubMed]
- Kruitwagen HS, Oosterhoff LA, van Wolferen ME, et al. Long-Term Survival of Transplanted Autologous Canine Liver Organoids in a COMMD1-Deficient Dog Model of Metabolic Liver Disease. Cells 2020;9:410. [Crossref] [PubMed]
- Fuentealba IC, Aburto EM. Animal models of copper-associated liver disease. Comp Hepatol 2003;2:5. [Crossref] [PubMed]
- Twedt DC, Sternlieb I, Gilbertson SR. Clinical, morphologic, and chemical studies on copper toxicosis of Bedlington Terriers. J Am Vet Med Assoc 1979;175:269-75. [PubMed]
- Haywood S, Rutgers HC, Christian MK. Hepatitis and copper accumulation in Skye terriers. Vet Pathol 1988;25:408-14. [Crossref] [PubMed]
- Thornburg LP, Rottinghaus G, Dennis G, et al. The relationship between hepatic copper content and morphologic changes in the liver of West Highland White Terriers. Vet Pathol 1996;33:656-61. [Crossref] [PubMed]
- Thornburg LP. Histomorphological and immunohistochemical studies of chronic active hepatitis in Doberman Pinschers. Vet Pathol 1998;35:380-5. [Crossref] [PubMed]
- Webb CB, Twedt DC, Meyer DJ. Copper-associated liver disease in Dalmatians: a review of 10 dogs (1998-2001). J Vet Intern Med 2002;16:665-8. [PubMed]
- Hoffmann G, van den Ingh TS, Bode P, et al. Copper-associated chronic hepatitis in Labrador Retrievers. J Vet Intern Med 2006;20:856-61. [Crossref] [PubMed]
- Seachrist L. Man's best friend may be companion in cancer research. J Natl Cancer Inst 1993;85:1455-6. [Crossref] [PubMed]
- Hitte C, Madeoy J, Kirkness EF, et al. Facilitating genome navigation: survey sequencing and dense radiation-hybrid gene mapping. Nat Rev Genet 2005;6:643-8. [Crossref] [PubMed]
- Parker HG, Ostrander EA. Canine genomics and genetics: running with the pack. PLoS Genet 2005;1:e58. [Crossref] [PubMed]
- Ostrander EA, Wayne RK. The canine genome. Genome Res 2005;15:1706-16. [Crossref] [PubMed]
- Clark LA, Tsai KL, Steiner JM, et al. Chromosome-specific microsatellite multiplex sets for linkage studies in the domestic dog. Genomics 2004;84:550-4. [Crossref] [PubMed]
- Ke X, Kennedy LJ, Short AD, et al. Assessment of the functionality of genome-wide canine SNP arrays and implications for canine disease association studies. Anim Genet 2011;42:181-90. [Crossref] [PubMed]
- Kyöstilä K, Cizinauskas S, Seppälä EH, et al. A SEL1L mutation links a canine progressive early-onset cerebellar ataxia to the endoplasmic reticulum-associated protein degradation (ERAD) machinery. PLoS Genet 2012;8:e1002759. [Crossref] [PubMed]
- Awano T, Johnson GS, Wade CM, et al. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 2009;106:2794-9. [Crossref] [PubMed]
- Roque JB, O'Leary CA, Kyaw-Tanner M, et al. PTPN22 polymorphisms may indicate a role for this gene in atopic dermatitis in West Highland white terriers. BMC Res Notes 2011;4:571. [Crossref] [PubMed]
- Seppälä EH, Koskinen LL, Gulløv CH, et al. Identification of a novel idiopathic epilepsy locus in Belgian Shepherd dogs. PLoS One 2012;7:e33549. [Crossref] [PubMed]
- Vaysse A, Ratnakumar A, Derrien T, et al. Identification of genomic regions associated with phenotypic variation between dog breeds using selection mapping. PLoS Genet 2011;7:e1002316. [Crossref] [PubMed]
- Jenkins CADog Biomedical Variant Database Consortium. Improving the resolution of canine genome-wide association studies using genotype imputation: A study of two breeds. Anim Genet 2021;52:703-13. [Crossref] [PubMed]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005;438:803-19. [Crossref] [PubMed]
- O'Brien SJ, Murphy WJ. Genomics. A dog's breakfast? Science 2003;301:1854-5. [Crossref] [PubMed]
- Lequarré AS, Andersson L, André C, et al. LUPA: a European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet J 2011;189:155-9. [Crossref] [PubMed]
- Vaysse A, Ratnakumar A, Derrien T, et al. Identification of genomic regions associated with phenotypic variation between dog breeds using selection mapping. PLoS Genet 2011;7:e1002316. [Crossref] [PubMed]
- Hoeppner MP, Lundquist A, Pirun M, et al. An improved canine genome and a comprehensive catalogue of coding genes and non-coding transcripts. PLoS One 2014;9:e91172. [Crossref] [PubMed]
- Penso-Dolfin L, Swofford R, Johnson J, et al. An Improved microRNA Annotation of the Canine Genome. PLoS One 2016;11:e0153453. [Crossref] [PubMed]
- Wucher V, Legeai F, Hédan B, et al. FEELnc: a tool for long non-coding RNA annotation and its application to the dog transcriptome. Nucleic Acids Res 2017;45:e57. [Crossref] [PubMed]
- Wang GD, Larson G, Kidd JM, et al. Dog10K: the International Consortium of Canine Genome Sequencing. Natl Sci Rev 2019;6:611-3. [Crossref] [PubMed]
- Ostrander EA, Wang GD, Larson G, et al. Dog10K: an international sequencing effort to advance studies of canine domestication, phenotypes and health. Natl Sci Rev 2019;6:810-24. [Crossref] [PubMed]
- Yuzbasiyan-Gurkan V, Blanton SH, Cao Y, et al. Linkage of a microsatellite marker to the copper toxicosis locus in Bedlington terriers. Am J Vet Res 1997;58:23-7. [PubMed]
- van de Sluis BJ, Breen M, Nanji M, et al. Genetic mapping of the copper toxicosis locus in Bedlington terriers to dog chromosome 10, in a region syntenic to human chromosome region 2p13-p16. Hum Mol Genet 1999;8:501-7. [Crossref] [PubMed]
- Forman OP, Boursnell ME, Dunmore BJ, et al. Characterization of the COMMD1 (MURR1) mutation causing copper toxicosis in Bedlington terriers. Anim Genet 2005;36:497-501. [Crossref] [PubMed]
- Ganesh L, Burstein E, Guha-Niyogi A, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature 2003;426:853-7. [Crossref] [PubMed]
- Spee B, Arends B, van Wees AM, et al. Functional consequences of RNA interference targeting COMMD1 in a canine hepatic cell line in relation to copper toxicosis. Anim Genet 2007;38:168-70. [Crossref] [PubMed]
- Vonk WI, Bartuzi P, de Bie P, et al. Liver-specific Commd1 knockout mice are susceptible to hepatic copper accumulation. PLoS One 2011;6:e29183. [Crossref] [PubMed]
- Nantasanti S, Spee B, Kruitwagen HS, et al. Disease Modeling and Gene Therapy of Copper Storage Disease in Canine Hepatic Organoids. Stem Cell Reports 2015;5:895-907. [Crossref] [PubMed]
- Su LC, Ravanshad S, Owen CA Jr, et al. A comparison of copper-loading disease in Bedlington terriers and Wilson's disease in humans. Am J Physiol 1982;243:G226-30. [PubMed]
- Bartuzi P, Hofker MH, van de Sluis B. Tuning NF-κB activity: a touch of COMMD proteins. Biochim Biophys Acta 2013;1832:2315-21. [Crossref] [PubMed]
- Fedoseienko A, Bartuzi P, van de Sluis B. Functional understanding of the versatile protein copper metabolism MURR1 domain 1 (COMMD1) in copper homeostasis. Ann N Y Acad Sci 2014;1314:6-14. [Crossref] [PubMed]
- Weiskirchen R, Penning LC. COMMD1, a multi-potent intracellular protein involved in copper homeostasis, protein trafficking, inflammation, and cancer. J Trace Elem Med Biol 2021;65:126712. [Crossref] [PubMed]
- Singla A, Chen Q, Suzuki K, et al. Regulation of murine copper homeostasis by members of the COMMD protein family. Dis Model Mech 2021;14:dmm045963. [Crossref] [PubMed]
- Müller T, van de Sluis B, Zhernakova A, et al. The canine copper toxicosis gene MURR1 does not cause non-Wilsonian hepatic copper toxicosis. J Hepatol 2003;38:164-8. [Crossref] [PubMed]
- Lovicu M, Dessì V, Lepori MB, et al. The canine copper toxicosis gene MURR1 is not implicated in the pathogenesis of Wilson disease. J Gastroenterol 2006;41:582-7. [Crossref] [PubMed]
- Wu ZY, Zhao GX, Chen WJ, et al. Mutation analysis of 218 Chinese patients with Wilson disease revealed no correlation between the canine copper toxicosis gene MURR1 and Wilson disease. J Mol Med (Berl) 2006;84:438-42. [Crossref] [PubMed]
- Weiss KH, Merle U, Schaefer M, et al. Copper toxicosis gene MURR1 is not changed in Wilson disease patients with normal blood ceruloplasmin levels. World J Gastroenterol 2006;12:2239-42. [Crossref] [PubMed]
- Bost M, Piguet-Lacroix G, Parant F, et al. Molecular analysis of Wilson patients: direct sequencing and MLPA analysis in the ATP7B gene and Atox1 and COMMD1 gene analysis. J Trace Elem Med Biol 2012;26:97-101. [Crossref] [PubMed]
- Zarina A, Tolmane I, Krumina Z, et al. Association of Variants in the CP, ATOX1 and COMMD1 Genes with Wilson Disease Symptoms in Latvia. Balkan J Med Genet 2019;22:37-42. [Crossref] [PubMed]
- Trocello JM, Brousolle E, Girardot-Tinant N, et al. Wilson’s disease, 100 years later. Rev Neurol (Paris) 2013;169:936-43. [Crossref] [PubMed]
- Lo C, Bandmann O. Epidemiology and introduction to the clinical presentation of Wilson disease. Handb Clin Neurol 2017;142:7-17. [Crossref] [PubMed]
- Xie JJ, Wu ZY. Wilson’s disease in China. Neurosci Bull 2017;33:323-30. [Crossref] [PubMed]
- Członkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers 2018;4:21. [Crossref] [PubMed]
- Sandahl TD, Laursen TL, Munk DE, et al. The prevalence of Wilson’s disease: an update. Hepatology 2020;71:722-32. [Crossref] [PubMed]
- Sánchez-Monteagudo A, Ripoles E, Berenguer M, et al. Wilson’s disease: facing the challenge of diagnosing a rare disease. Biomedicines 2021;9:1100. [Crossref] [PubMed]
- Weiss KH, Stremmel W. Clinical considerations for an effective medical therapy in Wilson's disease. Ann N Y Acad Sci 2014;1315:81-5. [Crossref] [PubMed]
- Medici V, Weiss KH. Genetic and environmental modifiers of Wilson disease. Handb Clin Neurol 2017;142:35-41. [Crossref] [PubMed]
- Stremmel W, Merle U, Weiskirchen R. Clinical features of Wilson disease. Ann Transl Med 2019;7:S61. [Crossref] [PubMed]
- Pfeiffer RF. Wilson’s disease. Semin Neurol 2007;27:123-32. [Crossref] [PubMed]
- Lutsenko S. Modifying factors and phenotypic diversity in Wilson’s disease. Ann N Y Acad Sci 2014;1315:56-63. [Crossref] [PubMed]
- Medici V, LaSalle JM. Genetics and epigenetic factors of Wilson disease. Ann Transl Med 2019;7:S58. [Crossref] [PubMed]
- Hermann W. Classification and differential diagnosis of Wilson’s disease. Ann Transl Med 2019;9S63.
- Litwin T, Dusek P, Szafrański T, et al. Psychiatric manifestations in Wilson’s disease: possibilities and difficulties for treatment. Ther Adv Psychopharmacol 2018;8:199-211. [Crossref] [PubMed]
- Dusek P, Litwin T, Członkowska A. Neurologic impairment in Wilson disease. Ann Transl Med 2019;7:S64. [Crossref] [PubMed]
- Litwin T, Dzieżyc K, Członkowska A. Wilson disease-treatment perspectives. Ann Transl Med 2019;7:S68. [Crossref] [PubMed]
- Lorincz MT. Wilson disease and related copper disorders. Handb Clin Neurol 2018;147:279-92. [Crossref] [PubMed]
- Leung M, Aronowitz PB, Medici V. The Present and Future Challenges of Wilson's Disease Diagnosis and Treatment. Clin Liver Dis (Hoboken) 2021;17:267-70. [Crossref] [PubMed]
- Moini M, To U, Schilsky ML. Recent advances in Wilson disease. Transl Gastroenterol Hepatol 2021;6:21. [Crossref] [PubMed]
- Ferenci P. Phenotype-genotype correlations in patients with Wilson’s disease. Ann NY Acad Sci 2014;1315:1-5. [Crossref] [PubMed]
- Chang IJ, Hahn SH. The genetics of Wilson disease. Handb Clin Neurol 2017;142:19-34. [Crossref] [PubMed]
- Hedera P. Wilson’s disease: a master of disguise. Parkinsonism Relat Disord 2019;59:140-5. [Crossref] [PubMed]
- Espinos C, Ferenci P. Are the new genetic tools for diagnosis of Wilson disease helpful I clinical practice J Hepatol Rep 2020;2:1-7.
- Poon KS, Teo ZH, Yap JH, et al. Challenges in molecular diagnosis of Wilson disease: viewpoint from the clinical laboratory. J Clin Pathol 2020;73:231-4. [Crossref] [PubMed]
- Usta J, Wehbeh A, Rida K, et al. Phenotype-genotype correlation in Wilson disease in a large Lebanese family: association of c.2299insC with hepatic and of p. Ala1003Thr with neurologic phenotype. PLoS One 2014;9:e109727. [Crossref] [PubMed]
- Guggilla SR, Senagari JR, Rao PN, et al. Spectrum of mutations in the ATP binding domain of ATP7B gene of Wilson Disease in a regional Indian cohort. Gene 2015;569:83-7. [Crossref] [PubMed]
- Cheng N, Wang H, Wu W, et al. Spectrum of ATP7B mutations and genotype-phenotype correlation in large-scale Chinese patients with Wilson Disease. Clin Genet 2017;92:69-79. [Crossref] [PubMed]
- Mordaunt CE, Kieffer DA, Shibata NM, et al. Epigenomic signatures in liver and blood of Wilson disease patients include hypermethylation of liver-specific enhancers. Epigenetics Chromatin 2019;12:10. [Crossref] [PubMed]
- Sapuppo A, Pavone P, Praticò AD, et al. Genotype-phenotype variable correlation in Wilson disease: clinical history of two sisters with the similar genotype. BMC Med Genet 2020;21:128. [Crossref] [PubMed]
- Roy S, McCann CJ, Ralle M, et al. Analysis of Wilson disease mutations revealed that interactions between different ATP7B mutants modify their properties. Sci Rep 2020;10:13487. [Crossref] [PubMed]
- Simon I, Schaefer M, Reichert J, et al. Analysis of the human Atox 1 homologue in Wilson patients. World J Gastroenterol 2008;14:2383-7. [Crossref] [PubMed]
- Weiss KH, Runz H, Noe B, et al. Genetic analysis of BIRC4/XIAP as a putative modifier gene of Wilson disease. J Inherit Metab Dis 2010;33:S233-40. [Crossref] [PubMed]
- Stuehler B, Reichert J, Stremmel W, et al. Analysis of the human homologue of the canine copper toxicosis gene MURR1 in Wilson disease patients. J Mol Med (Berl) 2004;82:629-34. [Crossref] [PubMed]
- Gromadzka G, Rudnicka M, Chabik G, et al. Genetic variability in the methylenetetrahydrofolate reductase gene (MTHFR) affects clinical expression of Wilson’s disease. J Hepatol 2011;55:913-9. [Crossref] [PubMed]
- de Bie P, van de Sluis B, Burstein E, et al. Distinct Wilson’s disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology 2007;133:1316-26. [Crossref] [PubMed]
- Weiss KH, Lozoya JC, Tuma S, et al. Copper-induced translocation of the Wilson disease protein ATP7B independent of Murr1/COMMD1 and Rab7. Am J Pathol 2008;173:1783-94. [Crossref] [PubMed]
- Vonk WI, de Bie P, Wichers CG, et al. The copper-transporting capacity of ATP7A mutants associated with Menkes disease is ameliorated by COMMD1 as a result of improved protein expression. Cell Mol Life Sci 2012;69:149-63. [Crossref] [PubMed]
- Hoffmann G, Heuven HC, Leegwater PAJ, et al. Heritabilities of coper-accumulating traits in Labrador retrievers. Anim Genet 2008;39:454. [Crossref] [PubMed]
- Hoffmann G, Jones PG, Biourge VC, et al. Dietary management of hepatic copper accumulation in abrador retrievers. J Vet Intern Med 2009;23:957-63. [Crossref] [PubMed]
- Fieten H, Hooijer-Nouwens BD, Biourge VC, et al. Association of dietary copper and zinc levels with hepatic copper and zinc concentration in Labrador Retrievers. J Vet Intern Med 2012;26:1274-80. [Crossref] [PubMed]
- Fieten H, Hugen S, van den Ingh TS, et al. Urinary excretion of copper, zinc and iron with and without D-penicillamine administration in relation to hepatic copper concentration in dogs. Vet J 2013;197:468-73. [Crossref] [PubMed]
- Fieten H, Biourge VC, Watson AL, et al. Nutritional management of inherited copper-associated hepatitis in the Labrador retriever. Vet J 2014;199:429-33. [Crossref] [PubMed]
- Fieten H, Biourge VC, Watson AL, et al. Dietary management of labrador retrievers with subclinical hepatic copper accumulation. J Vet Intern Med 2015;29:822-7. [Crossref] [PubMed]
- Fieten H, Dirksen K, van den Ingh TS, et al. D-penicillamine treatment of copper-associated hepatitis in Labrador retrievers. Vet J 2013;196:522-7. [Crossref] [PubMed]
- Dirksen K, Spee B, Penning LC, et al. Gene expression patterns in the progression of canine copper-associated chronic hepatitis. PLoS One 2017;12:e0176826. [Crossref] [PubMed]
- Fieten H, Gill Y, Martin AJ, et al. The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador retrievers: a new canine model for copper-metabolism disorders. Dis Model Mech 2016;9:25-38. [Crossref] [PubMed]
- Wu X, Mandigers PJJ, Watson AL, et al. Association of the ATP7A and ATP7B with hepatic copper accumulation in Dobermann dogs. J Vet Intern Med 2019;33:1646-52. [Crossref] [PubMed]
- Wu X, Mandigers PJJ, Fieten H, et al. Evaluation of COMMD1 in copper toxicosis in Labrador retrievers and Dobermans. Vet J 2020;265:105561. [Crossref] [PubMed]
- Wu X, den Boer ER, Vos-Loohuis M, et al. Investigation of Genetic Modifiers of Copper Toxicosis in Labrador Retrievers. Life (Basel) 2020;10:266. [Crossref] [PubMed]
- Favier RP, Spee B, Schotanus BA, et al. COMMD1-deficient dogs accumulate copper in hepatocytes and provide a good model for chronic hepatitis and fibrosis. PLoS One 2012;7:e42158. [Crossref] [PubMed]
- Favier RP, Spee B, Fieten H, et al. Aberrant expression of copper associated genes after copper accumulation in COMMD1-deficient dogs. J Trace Elem Med Biol 2015;29:347-53. [Crossref] [PubMed]
- Tanner MS. Role of copper in Indian childhood cirrhosis. Am J Clin Nutr 1998;67:1074S-81S. [Crossref] [PubMed]
- Muller T, Feichtinger H, Berger H, et al. Endemic Tyrolean infantile cirrhosis: an ecogenetic disorder. Lancet 1996;347:877-80. [Crossref] [PubMed]
- Scheinberg IH, Sternlieb I. Wilson disease and idiopathic copper toxicosis. Am J Clin Nutr 1996;63:842S-5S. [Crossref] [PubMed]
- Pierson H, Yang H, Lutsenko S. Copper Transport and Disease: What Can We Learn from Organoids? Annu Rev Nutr 2019;39:75-94. [Crossref] [PubMed]
- Kim D, Kim SB, Ryu JL, et al. Human embryonic stem cell-derived Wilson’s disease model for screening drug efficacy. Cells 2020;9:872. [Crossref] [PubMed]
- Pöhler M, Guttmann S, Nadzemova O, et al. CRISPR/Cas9-mediated correction of mutated copper transporters ATP7B. PLoS One 2020;15:e0239411. [Crossref] [PubMed]
- Schene IF, Joore IP, Oka R, et al. Prime editing for functional repair in patient-derived disease models. Nat Commun 2020;11:5352. [Crossref] [PubMed]
- Jiang W, Liu L, Chang Q, et al. production of Wilson disease model rabbits with homology-directed precision point mutations in the ATP7B gene using the CRISP/Cas9 system. Sci Rep 2018;8:132.
- Bose S, Clevers H, Shen X. Promises and Challenges of Organoid-Guided Precision Medicine. Med (N Y) 2021;2:1011-26. [Crossref] [PubMed]
- Ricci S, Cacialli P. Stem Cell Research Tools in Human Metabolic Disorders: An Overview. Cells 2021;10:2681. [Crossref] [PubMed]
- Poldervaart JH, Favier RP, Penning LC, et al. Primary hepatitis in dogs: a retrospective review (2002-2006). J Vet Intern Med 2009;23:72-80. [Crossref] [PubMed]
Cite this article as: Penning LC, van Steenbeek FG. A narrative review on COMMD1, the promiscuous ATP7B chaperone bridging the gap between inherited canine copper toxicosis and Wilson disease. Dig Med Res 2022;5:13.


