
Combined targeted therapy for BRAF mutant metastatic colorectal cancer: are we there yet?
Recently, the updated results from the BEACON study defined encorafenib plus cetuximab as a new standard of care for patients with previously treated BRAFV600E mutated metastatic colorectal cancer (mCRC) (1). This combination is thereby amongst the very few effective chemotherapy-free treatment options for a selected subgroup of patients with mCRC.
Mutations in the BRAF oncogene are present in approximately 10% to 15% of patients with mCRC, resulting in a molecularly distinct subpopulation with poor prognosis and poor response to standard treatment options (2,3). The majority of BRAF mutations (>95%) concern a T1799A transversion mutation in codon 600 of exon 15 leading to a valine-to-glutamic acid (V600E) amino acid substitution (4). These mutations mimic regulatory phosphorylation of the BRAF protein, causing a 10-fold increased BRAF activity and a hyper activated mitogen activated protein kinase (MAPK) pathway, thereby continuously stimulating tumor cell proliferation and survival (2).
Ever since BRAF was recognized as an important oncogenic driver, directly targeting the BRAF protein using tyrosine kinase inhibitors (TKIs) has become a promising treatment strategy. Although BRAF inhibitors, either as single agent or in combination with MEK inhibitors, demonstrated significant improvement in the prognosis of patients with BRAFV600E mutant metastatic melanoma, the clinical benefit of these compounds in BRAFV600E mutated mCRC was less encouraging (5,6). Already 10 years ago, preclinical studies demonstrated the presence of a negative feedback activation loop that activates the epidermal growth factor receptor (EGFR) upon inhibition of BRAF in BRAFV600E mutant CRC cells. As a result, the MAPK- and phosphoinositide 3-kinse (PI3K) signaling pathways get reactivated, explaining the intrinsic resistance of BRAFV600E mutant CRC cells against BRAF inhibitors as single agent (7,8). Based on this strong rationale to combine BRAF inhibitors with EGFR inhibitors in patients with BRAFV600E mutated colorectal cancer, multiple combinations were investigated in early-phase clinical studies. These included vemurafenib plus cetuximab, vemurafenib combined with panitumumab, dabrafenib plus panitumumab, and encorafenib plus cetuximab. Interestingly, not all combinations demonstrated equally promising efficacy results, as response rates ranged from 3.7% (vemurafenib-cetuximab) to 10% (dabrafenib-panitumumab), 13% (vemurafenib-panitumumab) and 18–22% (encorafenib-cetuximab) in pretreated patients (majority received ≥2 previous treatment regimens) (6,9-12). Of course one should not draw definitive conclusions from efficacy results demonstrated in different phase I studies. However theoretically, inhibitors with high selectivity and high potency against both BRAFV600E and CRAF may be more effective in blocking both the intrinsic tumorigenic activity of mutated BRAF and the potential MAPK pathway reactivation through CRAF upon BRAF inhibition (Figure 1). Indeed half-maximal inhibitory concentration (IC50) values of encorafenib are significantly lower than that of vemurafenib and dabrafenib for BRAFV600E and CRAF (13,14). In addition, encorafenib has an exceptionally long dissociation half-life from BRAFV600E of more than 30 hours, resulting in prolonged target inhibition and increased potency compared to vemurafenib and dabrafenib which have dissociation half-lives of only 0.5 and 2 hours from BRAFV600E, respectively (15). Furthermore, when MEK inhibitor trametinib was added to the dabrafenib-panitumumab combination for improved suppression of MAPK signaling, response rate and progression-free survival (PFS) results improved, but did not seem to be better compared to encorafenib-cetuximab, while tolerability became less favorable (9-11). Therefore, encorafenib might just be the most appropriate BRAF inhibitor currently available for application in combination with EGFR inhibition in patients with BRAFV600E mutated colorectal cancer.
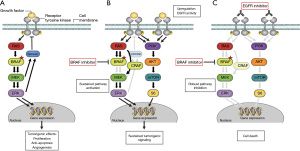
In addition to the triple BRAF-EGFR-MEK combination of dabrafenib-panitumumab-trametinib, the encorafenib-cetuximab backbone was also combined with other TKIs, such as MEK inhibitor binimetinib and PI3K inhibitor alpelisib. However, this caused a significant increase in toxicity without clinically meaningful improvement in efficacy outcomes as demonstrated in phase II and pivotal phase III studies (1,10,11).
The BEACON study was a randomized phase III trial amongst patients with BRAFV600E mutant mCRC who progressed on one or two regimens. It compared a triple combination consisting of encorafenib (300 mg once daily), cetuximab (400 mg/m2 loading dose, followed by 250 mg/m2 once a week) and binimetinib (45 mg twice daily), a dual combination of encorafenib plus cetuximab and control arm of investigators’ choice regular care consisting of either cetuximab plus irinotecan or cetuximab plus folinic acid, fluorouracil and irinotecan (FOLFIRI), although most guidelines currently recommend against the use of cetuximab in BRAFV600E mutated mCRC. The updated post hoc efficacy, safety and subgroup analysis demonstrated a significantly improved median overall survival (mOS) [hazard ratio (HR) =0.60; 95% confidence interval (CI): 0.48–0.77] for encorafenib plus cetuximab (9.3 months; 95% CI: 8.0–11.3) relative to the control arm (5.9 months; 95% CI: 5.1–7.1). As mentioned previously, addition of binimetinib to encorafenib-cetuximab did not result in further improved outcome, indicated by an identical mOS of 9.3 months (95% CI: 8.2–10.8). Compared to control, mOS with the encorafenib-cetuximab combination was favorable in all subgroups. Confirmed objective response rate (ORR) was 20% for encorafenib-cetuximab, 27% for encorafenib-cetuximab-binimetinib and 1.8% for control. In addition, the encorafenib-cetuximab combination was associated with substantial improvement in patient-reported quality of life assessments over the standard treatment arm (16). In terms of toxicity, both investigational combinations were considered tolerable but the dual combination demonstrated favorable compared to the triple combination and control arm, with grade ≥3 adverse events in 57%, 66% and 64%, respectively (1). Interestingly, dermatologic adverse events occurred substantially less frequent and were less severe with encorafenib and cetuximab combined, compared to previously reported for single-agent encorafenib or cetuximab. Palmar-plantar erythrodysesthesia syndrome of any grade was reported in only 5% of patients treated with encorafenib-cetuximab while single agent encorafenib data reported this adverse event in 67%, and papulopustular rash of any grade was seen in 82% of cetuximab treated patients compared to 45% of encorafenib-cetuximab treated patients who experienced dermatitis acneiform or any other form of skin rash (1,17,18). Furthermore, the incidence of grade ≥3 skin rash, Palmar-plantar erythrodysesthesia syndrome or dermatitis acneiform combined was only 1%, compared to >20% in patients treated with cetuximab monotherapy (1,18). This apparent protective effect that BRAF inhibitors and anti-EGFR directed antibodies have on each other’s dermatologic toxicity seems consistent with the opposing effects these drugs have in healthy, BRAF wildtype, skin tissue. Whereas anti-EGFR antibodies cause cutaneous adverse events by also inhibiting MAPK signaling in skin tissue, BRAF inhibitors counteract this effect by paradoxically activating the MAPK pathway, which may result in reduced skin toxicity when given in combination (19,10).
Taken together, these results emphasized the clinical benefit of encorafenib plus cetuximab over standard of care. Therefore, this combination received Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval, making it the new standard of care for patients with BRAFV600E mutated mCRC who progressed upon at least one prior line of systemic therapy.
Although this is a big step forward for a selected group of patients, all patients eventually develop resistance. Huijberts and colleagues investigated mutation profiles in pretreatment and post progression tumor biopsies of patients treated with cetuximab and encorafenib ± binimetinib or alpelisib. Intrinsic resistance was more frequently seen in patients with at least one genetic alteration in other genes than TP53, APC or BRAF. Secondary resistance was mostly linked to newly observed mutations in the PI3K pathway and upstream receptor tyrosine kinases, leading to (re)activation of MAPK and PI3K pathway signaling (20). Given the large extent of heterogeneity, both between patients as well as intra patient, this research emphasizes that, although difficult, an even more personalized approach may be necessary to further optimize patient outcomes.
In addition, BRAFV600E mutant CRC is not a homogeneous group and not all patients respond equally well. Previously, two distinct subtypes of BRAFV600E mutant CRC were identified based on gene expression profiles, called BM1 and BM2. BM1 was characterized by MAPK/PI3K pathway activation, epithelial-mesenchymal transition (EMT) and increased immune reactivity, whereas BM2 displays dysregulation of cell cycle-related proteins such as cyclin dependent kinase 1 (CDK1) (21). Middleton and colleagues demonstrated that upon treatment with dabrafenib-panitumumab-trametinib, confirmed ORR in patients with BM subtype 1 (n=16) was 38% compared to 7% in patients with BM2 (n=31). The same holds true for survival outcomes, with a median PFS of 7.4 vs. 3 months and median OS of 19.8 vs. 6.3 months in BM1 compared to BM2, respectively (22). Interestingly, BM1 CRC is known as an aggressive molecular phenotype with poor clinical outcomes, making the outstanding PFS and OS data with dabrafenib-panitumumab-trametinib in this subgroup even more noteworthy. As these data suggest that further subtyping may be necessary to identify the subgroup of patients who benefit most from combined BRAF and EGFR inhibition, it would be interesting to investigate the clinical relevance of these subtypes in larger studies and particularly in encorafenib-cetuximab treated patients. Especially given the multiple reports that indicated encorafenib plus cetuximab is unlikely to be cost-effective under the current pricing (23,24).
Furthermore, approximately 15–30% of BRAFV600E mutant metastatic colorectal tumors also demonstrate deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H) (25). As immune checkpoint inhibitors targeting programmed death-1 (PD-1) such as pembrolizumab and nivolumab provide durable antitumor activity and seem to outperform encorafenib-cetuximab in patients with BRAFV600E mutant MSI-H mCRC, immunotherapy should get precedence in this subgroup (25,26). Pembrolizumab monotherapy indeed represents the standard of care as first-line treatment for this patient group, based on the pivotal phase III study demonstrating superior median PFS of 16.5 months compared to 8.2 months for 5-fluorouracil-based chemotherapy (27). Upon progression, encorefanib-cetuximab may be a reasonable second-line treatment option.
Currently, the ANCHOR phase II and BREAKWATER phase III studies investigate encorafenib-cetuximab in the first-line setting. In a total of 92 included patients in ANCHOR, the investigator-assessed confirmed ORR was 48%, with median PFS and OS being 5.8 and 17.2 months, respectively (28). BREAKWATER randomizes patients with BRAFV600E mutant mCRC between encorafenib-cetuximab, encorafenib-cetuximab + mFOLFOX6 or investigator’s choice chemotherapy ± bevacizumab (29). Study results are eagerly awaited, but until then, standard chemotherapy ± bevacizumab remains the treatment of choice in the first-line setting for patients with BRAFV600E mutant microsatellite stable mCRC, upon progression followed by encorafenib-cetuximab.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-22-15/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol 2021;39:273-84. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Seligmann JF, Fisher D, Smith CG, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol 2017;28:562-8. [Crossref] [PubMed]
- De Roock W, De Vriendt V, Normanno N, et al. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 2011;12:594-603. [Crossref] [PubMed]
- Kopetz S, Desai J, Chan E, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol 2015;33:4032-8. [Crossref] [PubMed]
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726-36. Erratum in: N Engl J Med 2018;379:1585. [Crossref] [PubMed]
- Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483:100-3. [Crossref] [PubMed]
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2:227-35. [Crossref] [PubMed]
- Corcoran RB, André T, Atreya CE, et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov 2018;8:428-43. [Crossref] [PubMed]
- van Geel RMJM, Tabernero J, Elez E, et al. A Phase Ib Dose-Escalation Study of Encorafenib and Cetuximab with or without Alpelisib in Metastatic BRAF-Mutant Colorectal Cancer. Cancer Discov 2017;7:610-9. [Crossref] [PubMed]
- Tabernero J, van Geel RMJM, Guren TK, et al. Phase 2 results: Encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol 2016;34:3544. [Crossref]
- Yaeger R, Cercek A, O'Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res 2015;21:1313-20. [Crossref] [PubMed]
- Koelblinger P, Thuerigen O, Dummer R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr Opin Oncol 2018;30:125-33. [Crossref] [PubMed]
- Pickles OJ, Drozd A, Tee L, et al. Paradox breaker BRAF inhibitors have comparable potency and MAPK pathway reactivation to encorafenib in BRAF mutant colorectal cancer. Oncotarget 2020;11:3188-97. [Crossref] [PubMed]
- Delord JP, Robert C, Nyakas M, et al. Phase I Dose-Escalation and -Expansion Study of the BRAF Inhibitor Encorafenib (LGX818) in Metastatic BRAF-Mutant Melanoma. Clin Cancer Res 2017;23:5339-48. [Crossref] [PubMed]
- Kopetz S, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab with or without binimetinib for BRAF V600E-mutant metastatic colorectal cancer: Quality-of-life results from a randomized, three-arm, phase III study versus the choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC). J Clin Oncol 2020;38:8. [Crossref]
- Gomez-Roca CA, Delord J, Robert C, et al. Encorafenib (LGX818), an oral BRAF inhibitor, in patients (pts) with BRAF V600E metastatic colorectal cancer (MCRC): results of dose expansion in an open-label, Phase 1 study. Ann Oncol 2014;25:iv182. [Crossref]
- Jaka A, Gutiérrez-Rivera A, López-Pestaña A, et al. Predictors of Tumor Response to Cetuximab and Panitumumab in 116 Patients and a Review of Approaches to Managing Skin Toxicity. Actas Dermosifiliogr 2015;106:483-92. [Crossref] [PubMed]
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010;464:431-5. [Crossref] [PubMed]
- Huijberts SCFA, Boelens MC, Bernards R, et al. Mutational profiles associated with resistance in patients with BRAFV600E mutant colorectal cancer treated with cetuximab and encorafenib +/- binimetinib or alpelisib. Br J Cancer 2021;124:176-82. [Crossref] [PubMed]
- Barras D, Missiaglia E, Wirapati P, et al. BRAF V600E Mutant Colorectal Cancer Subtypes Based on Gene Expression. Clin Cancer Res 2017;23:104-15. [Crossref] [PubMed]
- Middleton G, Yang Y, Campbell CD, et al. BRAF-Mutant Transcriptional Subtypes Predict Outcome of Combined BRAF, MEK, and EGFR Blockade with Dabrafenib, Trametinib, and Panitumumab in Patients with Colorectal Cancer. Clin Cancer Res 2020;26:2466-76. [Crossref] [PubMed]
- Patel KK, Stein S, Lacy J, et al. Evaluation of the Cost-effectiveness of Doublet Therapy in Metastatic BRAF Variant Colorectal Cancer. JAMA Netw Open 2021;4:e2033441. [Crossref] [PubMed]
- Giuliani J, Mantoan B, Bonetti A. Cost-effectiveness of encorafenib plus cetuximab in BRAF V600E-mutated colorectal cancer. J Oncol Pharm Pract 2022;28:199-202. [Crossref] [PubMed]
- Morris VK, Bekaii-Saab T. Improvements in Clinical Outcomes for BRAFV600E -Mutant Metastatic Colorectal Cancer. Clin Cancer Res 2020;26:4435-41. [Crossref] [PubMed]
- Grassi E, Corbelli J, Papiani G, et al. Current Therapeutic Strategies in BRAF-Mutant Metastatic Colorectal Cancer. Front Oncol 2021;11:601722. [Crossref] [PubMed]
- André T, Shiu KK, Kim TW, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 2020;383:2207-18. [Crossref] [PubMed]
- Van Cutsem E, Tabernero J, Taieb J, et al. O-10 ANCHOR CRC: Results from a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E–mutant metastatic colorectal cancer. Ann Oncol 2021;32:S222. [Crossref]
- Kopetz S, Grothey A, Yaeger R, et al. BREAKWATER: Randomized phase 3 study of encorafenib (enco) + cetuximab (cetux) ± chemotherapy for first-line (1L) treatment (tx) of BRAF V600E-mutant (BRAFV600E) metastatic colorectal cancer (mCRC). J Clin Oncol 2021;39:TPS3619. [Crossref]
Cite this article as: van Geel RMJM, Valkenburg-van Iersel LBJ. Combined targeted therapy for BRAF mutant metastatic colorectal cancer: are we there yet? Dig Med Res 2022;5:5.


