
Endoscopic resection of large colorectal polyps: a narrative review of the literature and best practices for management
Introduction
Colorectal cancer (CRC) is the 2nd most common cancer in the United States contributing to a leading cause of morbidity and mortality. CRC will be responsible for an estimated 52,980 deaths in year 2021, despite improvement in screening modalities and early treatment (1,2). It starts as a glandular growth in the epithelial cells of the colorectal mucosa in 90% of the cases (3) forming detectable polyps that can be removed before they turn into cancer. Colonoscopy currently offers a screening and therapeutic test of choice that is less invasive than surgery and has shown to reduce population prevalence of CRC. Although different appearance-based classifications such as Paris, Kudo, NBI international colorectal endoscopic (NICE) and Japanese NBI expert team (JNET) have been developed to predict deep submucosal invasion of ≥1,000 µm, a marker of higher risk of malignancy, the size of the neoplastic lesions remains an independent and important predictor of malignancy.
In this article, we review endoscopic recognition and management of large colorectal polyps. We also review the latest literature on conventional and novel endoscopic resection methods, the risk of cancer recurrence after endoscopic resection and the role of surgery in such lesions. Finally, we propose an easy-to-follow approach to large polyps management.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-61/rc).
Methods
A literature search was conducted using electronic databases (PubMed, Embase and Cochrane) to identify English-language articles published from the database’s inception until August 1st, 2021. Keywords included “colorectal cancer”, “colon cancer”, “colon polyps”, “large polyps”, “polyp resection”, “management” and “polypectomy”. Abstracts were individually screened and selected by both authors (AGT, MD). We further narrowed the screening by examining available randomized controlled trials, multicenter studies, comparative studies, clinical trials, and observational studies, in addition to meta-analyses, systematic reviews, evidence-based practice recommendations by societies and published guidelines (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | August 15, 2021 |
| Databases and other sources searched | PubMed, Embase, Cochrane |
| Search terms used (including MeSH and free text search terms and filters) | Colorectal cancer, colon cancer, colon polyps, large polyps, polyp resection, management, polypectomy |
| Timeframe | Database inception to August 1, 2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | English-language only articles. Single case reports were excluded |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Selection was conducted by both authors (AGT, MD) |
| Any additional considerations, if applicable | Not applicable |
Discussion
Large colorectal polyps and risk of malignancy
Any colorectal lesion larger than 10 mm is considered large (4). Depending on its morphology, it could be pedunculated or sessile. Polyps larger than 10–20 mm in size and expanding more horizontally than vertically are described as laterally spreading tumors (LST). Lesions >10 mm in size are common and have gained special interest and attention due to their higher potential for harboring malignancy, up to 2.41% for lesions 10–20 mm and 19.35% for lesions >20 mm (5-7). Reinhart et al. assessed 17,771 patients with colorectal polyps and reported a risk of high-grade dysplasia (HGD) of 8.82% in polyps >10 mm, compared to 0.87% in smaller polyps ≤10 mm (8). The risk of residual or recurrent adenoma (RRA) on follow up at the site of prior initial resection also increases as the lesion size increases, as reported by a pooled analysis of 8 prospective studies conducted by Martínez et al. which found that advanced adenoma recurred in 15.9% [95% confidence interval (CI): 14.5–17.4%] of lesions 10–20 mm and 19.3% (95% CI: 16.4–22.3%) in lesions ≥20 mm size. The risk for cancer on follow-up was 1.2% (95% CI: 0.4–2.0%) in lesions ≥20 mm (9). Endoscopic resection of large lesions, instead of surgery, has become the standard of care in the last few years, though interestingly and for unclear reasons, the rate of surgical resection of benign lesions had doubled between 2000 and 2014 in the US (10) suggestive of unnecessary surgeries and additional morbidity among this group. Surgery adds morbidity related to colectomy and subsequent complications. On the other hand, endoscopic resection offers curative removal with minimal risk of recurrence if done at early stage.
Due to their size, larger polyps, especially LSTs, are at risk for RRA. Incomplete resection of lesions contributes up to 19% of interval CRC after recent colonoscopy, hence endoscopists should aim to achieve negative histologic margins at polypectomy (R0) (11). En-bloc resection is preferred whenever possible, usually for lesions <20 mm, due to lower complications and risk of RRA (7). Nonetheless, large, nonpedunculated lesions are more commonly to be resected in a piecemeal fashion, which has up to 5.5 times higher risk of RRA compared to en-bloc resection (12). As such, the European Society of Gastrointestinal Endoscopy (ESGE) recommends the least number of pieces when performing piecemeal resection (13).
Role of endoscopic resection
Endoscopic resection is currently considered the standard method of management for precancerous polyps including those with intramucosal cancer. This is also the test of diagnosis, staging and potential curative removal before determining the next step for majority of polyps. While the role of colonoscopic resection continues to expand, it is generally limited to lesions with less than 1,000 µm of submucosal invasion (13,14). The success rate of endoscopic resection can be predicted by using the self-explanatory size, morphology, site, and access score (SMSA) developed by Sidhu et al. (15). The score assigns lesions into four main categories (SMSA 1 to 4), with the lowest procedural success rate of 93% for SMSA 4 (15).
The United States Multi-Society Task Force (USMSTF) comprised of gastroenterologists from three different American societies, as well as the ESGE provide up-to-date guidelines on colorectal lesions management in the Western world. While Eastern endoscopists primarily find such guidance provided by the Japan Gastroenterological Endoscopy Society (JGES) guidelines (13,14,16).
Conventional endoscopic resection methods
Polyp morphology, size, location, and features of submucosal invasion among other characteristics would determine the preferred resection method. Conventional endoscopic resection methods for large polyps include use of snare with thermal energy (cautery) for resection with or without submucosal injection. Pedunculated lesions measuring ≥10 mm should be removed by hot snare polypectomy (HSP) through the middle to lower stalk with ligation of the stalk if the head size ≥20 mm or stalk thickness ≥5 mm (13,14). HSP has a good complete resection rate of >80% with low complications rate (17,18).
Non-pedunculated lesions of 10–19 mm in size have also been traditionally removed by HSP with submucosal injection recommended to reduce thermal injury (13). Alternatively, cold snare polypectomy (CSP) with or without submucosal injection has shown complete resection rate of 99.3% (95% CI: 98.6–100%) with residual rate of any histology of 4.1% (95% CI: 0.2–8.4%) (19). This is reflected in the USMSTF recommendations of either cold or HSP for such lesions (14). When it comes to sessile serrated polyps (SSP) of this size, conventional or CSP with endoscopic mucosal resection (EMR) appears to be effective with low RRA as shown by a meta-analysis by Thoguluva Chandrasekar et al. (20).
Historically, larger polyps including LSTs measuring ≥20 mm used to require surgical resection, until recent emergence and improvements of endoscopic resection techniques over the past few decades. These lesions can be effectively treated endoscopically in up to 90% of the cases (21). EMR is now the preferred endoscopic method as it is widely accessible, relatively safe to perform and has high success rate achieving complete resection close to 90% (21-23). It involves lifting the polyp by injecting a solution, usually saline, into the submucosal space underneath the lesion of interest to separate it from the muscular layer and to create a cushion before tissue resection to minimize the risk of perforation. Though few drawbacks have been described with EMR, that include clinically significant post-EMR bleeding (CSPEB) in up to 10% of the cases and deep mural injury (DMI) leading to perforation in 1.5% (14,21,24-26). Another drawback is the further enlargement of the lesion after the submucosal injection, necessitating more frequent piecemeal resection which in turn could increase the risk of incomplete resection. Conventional EMR carries a recurrence rate of approximately 20% when piecemeal resection was performed and 3% when en-bloc resection was performed (27-29). Hence, the USMSTF recommends surveillance colonoscopy post-EMR resection at 6 months, 18 months, and 4.5 years, and the ESGE offers similar recommendations (13,14). Table 2 reviews the recurrence and complication rates of conventional and novel methods.
Table 2
| Polypectomy method | Lesion morphology and size | Efficacy (R0 or en-bloc) | Adverse events | Recurrence |
|---|---|---|---|---|
| HSP | Non-pedunculated lesions 10–19 mm and pedunculated lesions ≥10 mm | 81% (17) | 1–3.7% overall (17,18) | 48% of SSL (30) |
| CSP | Non-pedunculated lesions ≤19 mm | 82–89% (17) | 0.2–6.6% overall (17,18) | Up to 9.7% (31) |
| Conventional EMR | Non-pedunculated lesions and LSTs ≥20 mm, SSL 10–19 mm | 80–90% (20,21,26) | 1.2–1.5% perforation (21, 26), 6.9–9.6% bleeding (21,26) | 4.3% in serrated lesions (20), 13% (21,26,27) |
| CS-EMR | Non-pedunculated lesions and LSTs ≥20 mm | – | – | 5.5% (32) |
| UEMR | Non-pedunculated lesions and LSTs ≥20 mm | 57% (33) | 3.3% overall, 2.8% bleeding (33) | 8.8% (33) |
| ESD | Non-pedunculated lesions and LSTs ≥20 mm | 93.6% in LST (26) | 6% perforation (26), 2.8% bleeding (26) | 1.1% (26) |
| Hybrid ESD | Non-pedunculated lesions and LSTs ≥20 mm | 81.6% (34) | 4.6% perforation, 4.3% bleeding (34) | 4.5% (34) |
| eFTR (FTRD) | Any lesion morphology or size not amenable for resection by other methods | 80% (35) | 12% overall (35) | 13% (35) |
HSP, hot snare polypectomy; SSL, sessile serrated lesion; CSP, cold snare polypectomy; EMR, endoscopic mucosal resection; LST, laterally spreading tumor; CS-EMR, cold snare endoscopic mucosal resection; UEMR, underwater endoscopic mucosal resection; ESD, endoscopic submucosal dissection; eFTR, endoscopic full-thickness resection; FTRD, full-thickness resection device; R0, negative histologic margins at polypectomy.
For larger polyps, endoscopic submucosal dissection (ESD) offers a valuable alternative resection method that can achieve en-bloc resection more consistently, especially in lesions with high suspicion of superficial (<1,000 µm) submucosal invasion with virtually no size limit (14,36). Compared to EMR, ESD has higher curative resection rate (93% vs. 84%) and markedly less recurrence rate (1.1% vs. 13%) (21,26). One major downside of ESD is the high perforation rate of 6%, therefore it is usually confined to experienced centers (26).
Novel endoscopic resection methods
Novel endoscopic techniques have been suggested and studied in hope to further decrease the risk of RRA and reduce complications that are attributed to conventional methods. Such novelties are simple, such as the simple thermal ablation of post-EMR margins using snare tip soft coagulation (STSC) which could potentially reduce the recurrence rate down to 5% compared with 21% in the control group who did not receive any additional treatment post-EMR as reported by Klein et al. (37). Based on this data, STSC is currently suggested to reduce post-polypectomy recurrence mainly for LSTs >20 mm in size.
Another novelty is using cold snare EMR (CS-EMR) instead of the traditional hot snare as it was recently found to be equally effective and perhaps with less CSPEB and DMI (32). Another new approach to remove large SSP, is by forego EMR and perform piecemeal cold snare polypectomy (p-CSP). In a comparative study, no complications were identified in 121 patients in the p-CSP group, compared to 45 total events in 353 patients in the EMR group (38).
Underwater EMR (UEMR) was first described by Binmoeller et al. in 2012, that aims at achieving higher success rate in en-bloc resection of large LSTs, by eliminating submucosal lifting prior to resection and immersing the lesion with water instead of gas insufflation (39). Binmoeller et al. reported complete en-bloc resection of 29 out of 50 LSTs (55%) with R0 confirmed histologically in 79% of those 29 LSTs (40). UEMR superiority to conventional EMR, especially for flat and sessile lesions ≥20 mm, was suggested by recent meta-analyses showing improved en-bloc resection rates with odds ratio (OR) up to 2, and notably lower RRA with OR 0.3 (41-43). Additionally, UEMR showed promising results when attempted on lesions at difficult locations such as the appendiceal orifice (44).
The underwater technique can be adopted in other polypectomy methods to be used for lesions of different sizes and morphologies including pedunculated polyps. This technique has shown to be more efficient by decreasing the procedural time with significantly less immediate bleeding in comparison to traditional gas insufflation polypectomy as reported by Cadoni et al. (45).
It is common in practice to encounter colorectal lesions that are not amenable to any of the endoscopic resection methods described above, such as non-lifting lesions and those arising from a difficult location like the appendiceal orifice. However, a novel device was recently developed to achieve endoscopic full-thickness resection (eFTR) [eFTR via FTR device (FTRD)] instead of invasive surgery. This technique was recently evaluated in 65 centers across Germany in a real-world clinical setting. R0 resection was achieved in 80% of 1,178 eFTR procedures, with RRA rate of 13.5% in 683 follow-ups (35). Those excellent findings were consistent in both lesions measuring <20 mm as well as those measuring ≥20 mm (35). There is also growing interest in using eFTR for T1 CRC as both a therapeutic as well as a diagnostic option by providing full-thickness specimen allowing high quality histological assessment (46). Lesions 25–30 mm with adenoma recurrence or suspected submucosal invasion are candidates but the practical applications are much broader for eFTR which are in evolution including need for R0 resection, fibrotic lesions and previously resected benign lesions. Hybrid EMR-eFTR (or ESD) are also an option for residual fibrotic lesion or suspected area of submucosal invasion where rest of the lesion (even larger than 3 cm) can be removed by conventional technique followed by eFTR/ESD of the residual area that needs to be removed en-bloc for precise assessment.
Role of surgical resection
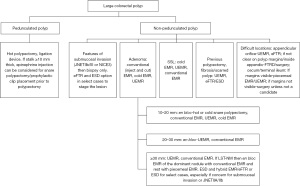
The role of surgical resection is now reserved to colorectal lesions with signs suggestive of deep submucosal invasion with probable lymphatic spread (13,14). Lesions where prior endoscopic resections have been attempted with persistent concern for submucosal invasion and lesions located in difficult anatomical locations (i.e., lesions extending inside appendicular orifice, terminal ileum) are also referred to surgery. The role of endoscopy continues to expand in management of such lesions and with advent of FTRD and ESD, now majority of benign lesions are removed by endoscopic means. Referral to advanced endoscopist or expert in managing colorectal lesions should be pursued prior to sending them for surgery. We are proposing a step-by-step approach for management of large colorectal polyps while incorporating current recommendations (Figure 1).
Summary
In conclusion, large benign colorectal lesions are amenable to endoscopic resection in majority of cases. The determination of lesion size, morphology, features of submucosal invasion and competence in resection strategy that can achieve effective complete resection with least adverse events are essential. In present time, no benign colorectal polyps should undergo surgery for removal unless absolutely needed and such cases should be reviewed with an advance endoscopist prior to surgery. The field is evolving, and more methods of endoscopic resection will be available offering personalized precision medicine for individual lesions with hope for better outcomes for all patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-61/rc
Peer Review File: Available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-61/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dmr.amegroups.com/article/view/10.21037/dmr-21-61/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250-81. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Lotfi AM, Spencer RJ, Ilstrup DM, et al. Colorectal polyps and the risk of subsequent carcinoma. Mayo Clin Proc 1986;61:337-43. [Crossref] [PubMed]
- Lieberman DA, Holub JL, Moravec MD, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA 2008;300:1417-22. [Crossref] [PubMed]
- Odom SR, Duffy SD, Barone JE, et al. The rate of adenocarcinoma in endoscopically removed colorectal polyps. Am Surg 2005;71:1024-6. [Crossref] [PubMed]
- Knabe M, Pohl J, Gerges C, et al. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol 2014;109:183-9. [Crossref] [PubMed]
- Reinhart K, Bannert C, Dunkler D, et al. Prevalence of flat lesions in a large screening population and their role in colonoscopy quality improvement. Endoscopy 2013;45:350-6. [Crossref] [PubMed]
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136:832-41. [Crossref] [PubMed]
- Peery AF, Cools KS, Strassle PD, et al. Increasing rates of surgery for patients with nonmalignant colorectal polyps in the United States. Gastroenterology 2018;154:1352-1360.e3. [Crossref] [PubMed]
- Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 2014;63:949-56. [Crossref] [PubMed]
- Mannath J, Subramanian V, Singh R, et al. Polyp recurrence after endoscopic mucosal resection of sessile and flat colonic adenomas. Dig Dis Sci 2011;56:2389-95. [Crossref] [PubMed]
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270-97. [Crossref] [PubMed]
- Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions: recommendations by the US multi-society task force on colorectal cancer. Am J Gastroenterol 2020;115:435-64. [Crossref] [PubMed]
- Sidhu M, Tate DJ, Desomer L, et al. The size, morphology, site, and access score predicts critical outcomes of endoscopic mucosal resection in the colon. Endoscopy 2018;50:684-92. [Crossref] [PubMed]
- Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2020;32:219-39. [Crossref] [PubMed]
- Gessl I, Waldmann E, Penz D, et al. Resection rates and safety profile of cold vs. hot snare polypectomy in polyps sized 5-10mm and 11-20mm. Dig Liver Dis 2019;51:536-41. [Crossref] [PubMed]
- Jegadeesan R, Aziz M, Desai M, et al. Hot snare vs. cold snare polypectomy for endoscopic removal of 4-10mm colorectal polyps during colonoscopy: a systematic review and meta-analysis of randomized controlled studies. Endosc Int Open 2019;7:E708-16. [Crossref] [PubMed]
- Thoguluva Chandrasekar V, Spadaccini M, Aziz M, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc 2019;89:929-936.e3. [Crossref] [PubMed]
- Thoguluva Chandrasekar V, Aziz M, Patel HK, et al. Efficacy and safety of endoscopic resection of sessile serrated polyps 10 mm or larger: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:2448-2455.e3. [Crossref] [PubMed]
- Hassan C, Repici A, Sharma P, et al. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut 2016;65:806-20. [Crossref] [PubMed]
- Luigiano C, Consolo P, Scaffidi MG, et al. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy 2009;41:829-35. [Crossref] [PubMed]
- Caputi Iambrenghi O, Ugenti I, Martines G, et al. Endoscopic management of large colorectal polyps. Int J Colorectal Dis 2009;24:749-53. [Crossref] [PubMed]
- Burgess NG, Bassan MS, McLeod D, et al. Deep mural injury and perforation after colonic endoscopic mucosal resection: a new classification and analysis of risk factors. Gut 2017;66:1779-89. [Crossref] [PubMed]
- Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014;12:651-61.e1-3.
- Russo P, Barbeiro S, Awadie H, et al. Management of colorectal laterally spreading tumors: a systematic review and meta-analysis. Endosc Int Open 2019;7:E239-59. [Crossref] [PubMed]
- Ortiz AM, Bhargavi P, Zuckerman MJ, et al. Endoscopic mucosal resection recurrence rate for colorectal lesions. South Med J 2014;107:615-21. [Crossref] [PubMed]
- El Rahyel A, Abdullah N, Love E, et al. Recurrence after endoscopic mucosal resection: early and late incidence, treatment outcomes, and outcomes in non-overt (histologic-only) recurrence. Gastroenterology 2021;160:949-951.e2. [Crossref] [PubMed]
- Belderbos TD, Leenders M, Moons LM, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388-402. [Crossref] [PubMed]
- Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology 2013;144:74-80.e1. [Crossref] [PubMed]
- Piraka C, Saeed A, Waljee AK, et al. Cold snare polypectomy for non-pedunculated colon polyps greater than 1cm. Endosc Int Open 2017;5:E184-9. [Crossref] [PubMed]
- Mangira D, Cameron K, Simons K, et al. Cold snare piecemeal EMR of large sessile colonic polyps ≥20 mm (with video). Gastrointest Endosc 2020;91:1343-52. [Crossref] [PubMed]
- Spadaccini M, Fuccio L, Lamonaca L, et al. Underwater EMR for colorectal lesions: a systematic review with meta-analysis (with video). Gastrointest Endosc 2019;89:1109-1116.e4. [Crossref] [PubMed]
- McCarty TR, Bazarbashi AN, Thompson CC, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy 2021;53:1048-58. [Crossref] [PubMed]
- Meier B, Stritzke B, Kuellmer A, et al. Efficacy and safety of endoscopic full-thickness resection in the colorectum: results from the german colonic FTRD registry. Am J Gastroenterol 2020;115:1998-2006. [Crossref] [PubMed]
- Rondagh EJ, Masclee AA, van der Valk ME, et al. Nonpolypoid colorectal neoplasms: gender differences in prevalence and malignant potential. Scand J Gastroenterol 2012;47:80-8. [Crossref] [PubMed]
- Klein A, Tate DJ, Jayasekeran V, et al. Thermal ablation of mucosal defect margins reduces adenoma recurrence after colonic endoscopic mucosal resection. Gastroenterology 2019;156:604-613.e3. [Crossref] [PubMed]
- van Hattem WA, Shahidi N, Vosko S, et al. Piecemeal cold snare polypectomy versus conventional endoscopic mucosal resection for large sessile serrated lesions: a retrospective comparison across two successive periods. Gut 2021;70:1691-7. [Crossref] [PubMed]
- Binmoeller KF, Weilert F, Shah J, et al. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc 2012;75:1086-91. [Crossref] [PubMed]
- Binmoeller KF, Hamerski CM, Shah JN, et al. Attempted underwater en bloc resection for large (2-4 cm) colorectal laterally spreading tumors (with video). Gastrointest Endosc 2015;81:713-8. [Crossref] [PubMed]
- Choi AY, Moosvi Z, Shah S, et al. Underwater versus conventional EMR for colorectal polyps: systematic review and meta-analysis. Gastrointest Endosc 2021;93:378-89. [Crossref] [PubMed]
- Chandan S, Khan SR, Kumar A, et al. Efficacy and histologic accuracy of underwater versus conventional endoscopic mucosal resection for large (>20 mm) colorectal polyps: a comparative review and meta-analysis. Gastrointest Endosc 2021;94:471-482.e9. [Crossref] [PubMed]
- Kamal F, Khan MA, Lee-Smith W, et al. Underwater vs conventional endoscopic mucosal resection in the management of colorectal polyps: a systematic review and meta-analysis. Endosc Int Open 2020;8:E1264-72. [Crossref] [PubMed]
- Binmoeller KF, Hamerski CM, Shah JN, et al. Underwater EMR of adenomas of the appendiceal orifice (with video). Gastrointest Endosc 2016;83:638-42. [Crossref] [PubMed]
- Cadoni S, Liggi M, Gallittu P, et al. Underwater endoscopic colorectal polyp resection: Feasibility in everyday clinical practice. United European Gastroenterol J 2018;6:454-62. [Crossref] [PubMed]
- Kuellmer A, Mueller J, Caca K, et al. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc 2019;89:1180-1189.e1. [Crossref] [PubMed]
Cite this article as: Tarakji AG, Desai M. Endoscopic resection of large colorectal polyps: a narrative review of the literature and best practices for management. Dig Med Res 2022;5:19.


