
Hypercoagulable state from COVID-19 in a patient with primary biliary cholangitis—a case report
Introduction
Manifestation of the COVID-19 can range from none to significant complications such as respiratory failure. The viral infection has emerged in a very short period as a major cause of morbidity and mortality across the globe. Since first described in 2019, there have been over 175 million infections and 3 million deaths as of June 14, 2021 (1). Patients with underlying liver disease appear to be at increased risks of severe illness (2). The viral infection can lead to a systemic illness that affects a number of organs throughout the body, including the liver.
There are a number of proposed mechanisms for how the liver is involved with Covid illness including direct hepatocyte infection, hypercoagulability leading to ischemia, drug induced liver injury from therapies used to treat COVID-19, and reflection of systemic injury (3-8). Hepatocellular involvement with elevated liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are the most common hepatic manifestations of COVID-19, and are a harbinger of outcomes (9). Increased AST and ALT is associated with greater likelihood of hospital admission, respiratory failure and death (9). A cholestatic hepatic picture is much less common. COVID-19 infection has also been associated with hepatic decompensation in patients with advanced liver disease (10). Vascular involvement of manifestations of COVID-19 have also been reported. In the liver in particular, thrombosis of the mesenteric venous thrombosis and portal vein have been described (11-16). Covid-cholangiopathy has also been described (17,18).
A subset of patients with PBC may have an inadequate response to ursodiol, the treatment of choice for PBC (19,20). The case highlights the need to consider COVID-19 infection and vascular thrombosis in the differential of patients with PBC and worsening cholestasis. In this case, we describe a patient with underlying PBC whose manifestation of COVID-19 infection was worsening cholestasis due to hypercoagulability.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-60).
Case presentation
The patient is a 48-year-old woman was evaluated for an elevated alkaline phosphatase (AP) level of 344 (international units per liter) IU/L in July 2019 (Table 1). A diagnosis of PBC was made based an elevated AP level and a detectable anti-mitochondrial antibody. She was started on ursodiol (1,000 mg/day) and within three months, her AP improved to 259 IU/L. The patient was status post cholecystectomy in 1999 and was under treatment for coccidiomycosis with fluconazole. There was no family history of hyper coagulable disease or autoimmune disorder. There was no history of alcohol disorder. She denied use of oral contraceptives. She had three live births, with the first one when she was 17 years at age.
Table 1
| Event | Date |
|---|---|
| Diagnosis of Primary Biliary Cholangitis | 7/2/2019 |
| Start of ursodiol | 7/10/2019 |
| Diagnosis of COVID | 6/21/2020 |
| Diagnosis of clot | 6/21/2020 |
| Treatment of clot | 9/10/2020 |
| Date of follow-up imaging | 7/20/2020 |
| Last clinical follow-up | 2/24/2021 |
Approximately a year later in June 2020, she contracted COVID-19 when she presented with symptoms of fever, cough and dyspnea. Her physical examination was unchanged and unremarkable. She was treated symptomatically with ibuprofen and acetaminophen and discharged from the emergency room. The results of liver associated test at the time of her COVID-19 presentation was remarkable for increased AP value of 1,050 IU/L. She claimed to be compliant with her ursodiol. Because of her worsening cholestasis, she underwent an abdominal computed tomography (CT) which demonstrated a thrombosis extending from infrarenal inferior vena cava (IVC) to the suprahepatic IVC and further extending into bilateral renal veins as well as an accessory right hepatic vein (Figures 1,2). When she was admitted for thrombectomy and anticoagulation (unfractionated heparin), her labs were notable for worsening renal function (Table 2). Her serum creatinine increased from her baseline value of 1.19 to 1.6 mg/dL. The results of 24-hour urine showed a total protein excretion of 5,291 mg per 24 hours. Urine protein electrophoresis showed no evidence of paraproteinemia. Scrum immunoglobulins: IgA 473, IgG 1,708 mg, IgM 326 mg. There was also no paraprotein seen on serum protein electrophoresis. Her Kappa Lambda light chain ratio was normal.
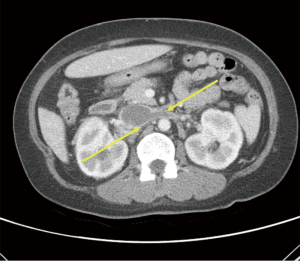
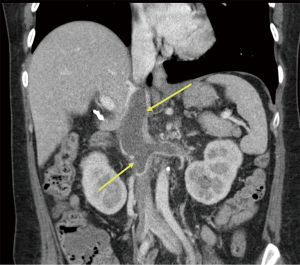
Table 2
| Date | AST (U/L) | ALT (U/L) | AP (U/L) | TB (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|---|
| 8/21/2019 | 62 | 63 | 254 | 0.7 | 0.9 |
| 10/17/2019 | 62 | 66 | 259 | 0.7 | 0.9 |
| 6/21/2020 | 90 | 32 | 1,050 | 0.3 | 1.6 |
| 7/9/2020 | 59 | 31 | 983 | 0.2 | 1.32 |
| 8/18/2020 | 62 | 30 | 742 | 0.6 | 1.3 |
| 11/25/2020 | 41 | 22 | 284 | 0.2 | 1.3 |
| 2/24/2021 | 36 | 19 | 361 | 0.2 | 1.36 |
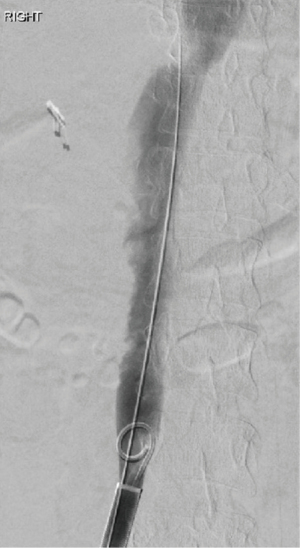
She underwent successful thrombectomy by Interventional Radiology on September 2020 (Figure 3). At the time of the procedure, the radiologist noted near complete occlusion of the infrarenal IVC by the thrombus and complete occlusive thrombosis of the left common iliac vein (CIV). Although thrombectomy of the IVC and CIV was achieved, the renal vein thrombosis was so organized and adherent that it could not be removed. Following the completion of the procedure, heparin infusion was resumed. After the procedure, renal function improved and she was discharged on apixaban. She tolerated the procedure without any complications there were no unforeseen complications.
The results of a follow-up abdominal CT in March 2021 revealed no residual IVC thrombus suggesting improved prognosis. However, her serum AP remained elevated at 361 IU/L.
This study was approved by the Institutional Review Board of University of California at Los Angeles (IRB#20-002295). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
The goal AP in patients with PBC treated with ursodiol is less than 1.67 times the upper limit of normal according to the (19). However, between 20% to 40% of patients do not respond to pharmacologic therapy. In the subset of patients that do not respond and have been compliant with ursodiol, treatment options may include obetocholic acid and/or fenofibrate (20). However, other causes of ursodiol nonresponse need to be investigated before a diagnosis of refractoriness is considered. Ursodiol treatment adherence, use of herbs/supplements, medication drug-induced liver disease should be explored. Our case report highlights that COVID-19 infection need to be added to the differential of worsening cholestasis.
The most common liver associated laboratory abnormality in COVID-19 patients are elevation of AST and ALT (21). The cause of elevation of the aminotransferases is unclear but likely multifactorial (22). Moreover, elevation of these enzymes is a harbinger of outcomes with increase related of hospitalization, intensive care unit level of care, respiratory failure and death (9). However, elevation of AP appear less common but a cholestatic presentation may be associated with higher risk of morbidity and mortality (21,23). A number of case reports have described secondary biliary cholangitis as consequence of COVID-19 infection (15,16).
A number of potential mechanisms have been proposed to explain the hypercoagulability that has been described in patients infected with COVID-19 (13,14). Some of the proposed mechanisms include cytokine storm, activation of the complement pathway, viral-induced autoimmunity, decreased ACE2 expression, endothelial damage from severe inflammation, and fibrinolysis shutdown (13). Although most authors have contemplated anticoagulation to treat thrombosis, we sought urgent thrombectomy. An important limitation in our study is the lack of coagulation data obtained. Future studies should include information on D-Dimers, which should help with a timely diagnosis. Proteinuria in our patient was mostly likely due to renal vein thrombosis.
The strength of case report is the biochemical and radiologic evaluation underscoring the need to assess for COVID-19 infection in patient that has worsening of their underlying liver disorder. However, the report is limited by the lack of liver histological data. Our case report highlights that hypercoagulability needs to be considered in the differential in worsening AP even in a patient with PBC. Both biliary and vascular disorders need to be assessed in patients who have infected with COVID-19 and worsening cholestasis. The elevation in our patient was likely a result of biliary tract ischemic damage caused by hypercoagulable state from COVID-19 infection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-60
Peer Review File: Available at https://dx.doi.org/10.21037/dmr-21-60
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-60). S Saab serves as an unpaid editorial board member of Digestive Medicine Research from April 2020 to March 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of University of California at Los Angeles (IRB#20-002295). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int. Accessed June 14, 2021.
- Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. Accessed April 20, 2020.
- Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998-1004. [Crossref] [PubMed]
- Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 2004;203:622-30. [Crossref] [PubMed]
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631-7. [Crossref] [PubMed]
- Chau TN, Lee KC, Yao H, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology 2004;39:302-10. [Crossref] [PubMed]
- Jothimani D, Vij M, Sanglodkar U, et al. Severe Jaundice in a COVID-19 Patient-Virus or Drug? J Clin Exp Hepatol 2021;11:407-8. [Crossref] [PubMed]
- Kaltschmidt B, Fitzek ADE, Schaedler J, et al. Hepatic Vasculopathy and Regenerative Responses of the Liver in Fatal Cases of COVID-19. Clin Gastroenterol Hepatol 2021;19:1726-1729.e3. [Crossref] [PubMed]
- Kullar R, Patel AP, Saab S. Hepatic Injury in Patients With COVID-19. J Clin Gastroenterol 2020;54:841-9. [Crossref] [PubMed]
- Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol 2021;74:567-77. [Crossref] [PubMed]
- Alemán W, Cevallos LC. Subacute mesenteric venous thrombosis secondary to COVID-19: A late thrombotic complication in a nonsevere patient. Radiol Case Rep 2021;16:899-902. [Crossref] [PubMed]
- Sharma N, Shukla R, Kumar K, et al. Portal Vein Thrombosis-a Rare Complication of SARS-CoV-2 Infection. SN Compr Clin Med 2021; [Crossref] [PubMed]
- Alam W. Hypercoagulability in COVID-19: A review of the potential mechanisms underlying clotting disorders. SAGE Open Med 2021;9:20503121211002996. [Crossref] [PubMed]
- Berkman SA, Tapson VF. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Semin Respir Crit Care Med 2021;42:316-26. [Crossref] [PubMed]
- Da BL, Suchman K, Roth N, et al. Cholestatic liver injury in COVID-19 is a rare and distinct entity and is associated with increased mortality. J Intern Med 2021;290:470-2. [Crossref] [PubMed]
- Rahmawati PL, Tini K, Susilawathi NM, et al. Pathomechanism and Management of Stroke in COVID-19: Review of Immunopathogenesis, Coagulopathy, Endothelial Dysfunction, and Downregulation of ACE2. J Clin Neurol 2021;17:155-63. [Crossref] [PubMed]
- Ugolotti MC, Pedrazzini M, Silini EM, et al. Vascular liver injury mimicking an intrahepatic cholangiocarcinoma in a COVID-19 patient. J Med Virol 2021;93:1940-2. [Crossref] [PubMed]
- Roth NC, Kim A, Vitkovski T, et al. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol 2021;116:1077-82. [Crossref] [PubMed]
- Lindor KD, Bowlus CL, Boyer J, et al. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69:394-419. [PubMed]
- Suraweera D, Rahal H, Jimenez M, et al. Treatment of primary biliary cholangitis ursodeoxycholic acid non-responders: A systematic review. Liver Int 2017;37:1877-86. [Crossref] [PubMed]
- Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol 2021;27:377-90. [Crossref] [PubMed]
- Marjot T, Webb GJ, Barritt AS 4th, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol 2021;18:348-64. [Crossref] [PubMed]
- Da BL, Suchman K, Roth N, et al. Cholestatic liver injury in COVID-19 is a rare and distinct entity and is associated with increased mortality. J Intern Med 2021;290:470-2. [Crossref] [PubMed]
Cite this article as: Saab S, Alper M, Sekhon S, Akhtar E, Akhtar NM, Tafti B, Tower S, Lemon R, Masood S. Hypercoagulable state from COVID-19 in a patient with primary biliary cholangitis—a case report. Dig Med Res 2021;4:75.


