
Regression of hepatocellular carcinoma in a female cirrhotic patient
Regression of hepatocellular carcinoma (HCC) is a very rare phenomenon in the absence of anti-tumor treatment strategy recommended by the current practice guidelines, such as surgery, transarterial chemoembolization, radiofrequency ablation, and molecular targeted therapy. Herein, we briefly report an elderly female patient with HCC whose liver lesion retracted without any above-mentioned standard treatments.
A 71-year-old woman with a 15-year history of hepatitis C virus related liver cirrhosis and multiple comorbidities, including rheumatic diseases, coronary heart disease, type 2 diabetes mellitus, and hypertension, had developed nearly all decompensated events, such as upper gastrointestinal bleeding, hydrothorax, ascites, encephalopathy, renal dysfunction, and infection, at our department. Alpha-fetoprotein level was within the reference range during her disease course. On July 29, 2019, dynamic magnetic resonance imaging (MRI) scan using gadoterate meglumine showed a lesion with a size of 4.6×4.1×3.7 cm in the 4th segment of the liver with hyperintensity on arterial phase, isointensity on portal phase, and hypointensity on delayed phase (Figure 1). Then, a diagnosis of HCC could be established based on MRI images. Liver biopsy was refused. After consulting with the patient and her relatives, she finally received palliative treatment with cytokine-induced killer (CIK) cells infusion five times from August 2019 to February 2020, as well as thymosin alpha1 (Talpha1) intermittently, but without any currently recommended anti-tumor treatment strategy. Subsequently, abdominal plain computed tomography (CT) scans showed that the lesion gradually retracted with a size of 2.6×2.0 cm on October 12, 2019 and 1.4×1.1 cm on January 12, 2020 (Figure 2). They suggested a dynamic decrease in the tumor size to a certain extent, despite the patient refused abdominal contrast enhanced CT scans. She died of liver failure on June 27, 2021.
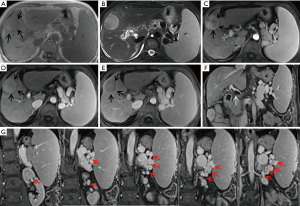
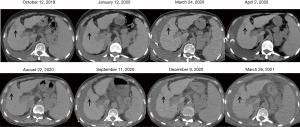
It is undeniable that the regression of HCC in this patient would be more reliable, if tumor biopsy was employed. However, based on the history of liver cirrhosis and the main imaging features of liver nodule on MRI scans, including non-rim hyperenhancement on arterial phase, non-peripheral washout appearance on portal and delayed phases, enhanced rim on delayed phase, and tumor size >20 mm, her lesion was classified as Liver Imaging Reporting and Data System-5 (LI-RADS-5) (1), and also met the diagnostic criteria of HCC according to the current practice guidelines (2,3). According to the Barcelona Clinic Liver Cancer (BCLC) staging system (2), including her tumor status (4.6×4.1×3.7 cm, single lesion, and portal invasion), liver function (Child-Pugh class B), and health status (Eastern Cooperative Oncology Group 2), she was diagnosed with advanced HCC at BCLC stage C with an expected survival time of 6–8 months (2). Surprisingly, her liver lesion regressed, which might be contributed by the effects of CIK cells (4) and Talphal (5) on treating cancer and strengthening immunity. It has been confirmed that activated CIK cells, which were used as an adjuvant immunotherapy, can increase the recurrence-free and overall survival of HCC patients. Activated CIK cells have the dual functions of T cells and NK cells, and can specifically be located in HCC cells and kill them, but with little cytotoxicity to normal cells (4). Certainly, the role of CIK cells for the management of HCC should be further validated in more well-designed prospective controlled studies. In addition, such a regression of HCC might be spontaneous, which might be related to the presence of extrahepatic portosystemic shunts and fibrous capsule around the tumor, thereby inducing tumor ischemia and hypoxia and then hindering the survival of the tumor (6).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer review: This article was a standard submission to the journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-90). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018;289:816-30. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015;148:1383-91.e6. [Crossref] [PubMed]
- Costantini C, Bellet MM, Pariano M, et al. A Reappraisal of Thymosin Alpha1 in Cancer Therapy. Front Oncol 2019;9:873. [Crossref] [PubMed]
- Randolph AC, Tharalson EM, Gilani N. Spontaneous regression of hepatocellular carcinoma is possible and might have implications for future therapies. Eur J Gastroenterol Hepatol 2008;20:804-9. [Crossref] [PubMed]
Cite this article as: Zhu M, Li H, Yu H, Yang B, Hou D, Qi X. Regression of hepatocellular carcinoma in a female cirrhotic patient. Dig Med Res 2021;4:80.


