
A narrative review of herb-induced liver injury
Background
The use of herbs as medicine or products has increased worldwide for more than a decade (1-4). Herbal products are frequently used by humans for different reasons to improve their health (1,5).
Although herbs and herbal products may have benefits for health, they also can develop adverse reactions. The rise in consuming herbs is likely due to easy access, purported harmless nature, and perceived therapeutic efficacy (2,6-8). Hepatotoxicity from herbs has been recognized for many years. The true incidence of herb-induced liver injury (HILI) is not easily determined due to the likelihood of underestimation and underreporting (9-11). There are various factors either host dependent or herb dependent that affect the clinical features and severity of HILI (10,12). HILI is diverse and classified as hepatocellular, cholestatic or mixed injury with varying clinical manifestations from asymptomatic elevation of liver tests to fulminant hepatitis, and fatal liver injury requiring liver transplantation (1,3,10,12). The true pathogenesis of each HILI is not fully understood which requires further elucidation. The diagnosis of HILI is an exclusion of other liver diseases. Roussel Uclaf Causality Assessment Method (RUCAM) has been most but not all used in causality assessment of HILI in clinical practice (9,11-14). The development of genetic testing is in progress which may predict risk for HILI and assist its early diagnosis. The exploration of definitive and valid biomarkers for HILI remains of high interests since it may help monitoring the severity, and outcome of HILI (14).
This review attempts to gather current medical knowledge of relevant and established information of HILI and advocate regulatory measures in the use of herbs and herbal products for public safety.
I present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/dmr-21-8).
Objectives
- To review current knowledge of clinical manifestations, diagnosis and management of HILI;
- To advocate the policy development in global standardization of pharmacovigilance in the use of herbs and herbal products.
Methods
Literature search was conducted through PubMed database of publications in English including retrospective, prospective studies, meta-analysis, case reports, case series, expert opinions and reviews, from different countries including but not limited to the United States (US), from year 1988 to 2020.
Discussion
Hepatotoxicity from herbs has been recognized for many years. Herbal products are categorized differently from pharmaceutical drugs and the safety profile is not regulated by standard measures. The true incidence of HILI is not easily determined due to the likelihood of underestimation and underreporting (9-11). A population-based study in Iceland at earlier time described 16% of DILI cases were due to use of herbal dietary supplements (15). HILI may have a more serious impact on different regions in the world (16). Publications between 2007 and 2013 from several Asian countries showed that prevalence of traditional medicines and dietary supplements induced liver injury ranging from 17.1% in Japan, 18.6% in China, 71% in Singapore to 72.7% in Korea respectively (17-20).
Although a retrospective study of 25,927 hospitalized DILI patients in mainland China from 2012 to 2014 showed that traditional Chinese medicines (TCM) or HDS accounted for 26.8% (21), the study was challenged by lacking rigorous entrance criteria with potential over-diagnosis, not accurately classified by causality assessment, and uncertain temporal link of medication use to liver injury given its retrospective design (22). The frequency of HDS induced liver injury in Drug Induced Liver Injury Network (DILIN) in the US remained stable around 20% (23). HDS in a retrospective cohort study in the US represented 18.8% of acute liver failure (24). A more recent systemic review of 31 studies described that HILI represented 25% of the DILI (25).
Clinical characteristics
Medicinal herbs or herbal products are originated from different parts (leaf, root, stem and flower) of the plant (2). There are various factors affecting the clinical features and severity of HILI, (I) herb associated: dose, duration, metabolism, misuse, abuse, purity, contaminant, adulterant, mislabeling and un-labeling; (II) host associated: gender, age, genetics and underlying liver disease (10,26-28).
Clinical manifestations of HILI vary from mild asymptomatic elevated liver tests, to acute hepatitis or fulminant hepatitis with liver failure requiring liver transplant, or chronic hepatitis (1,3,27,29-33). Pattern of HILI is classified as hepatocellular, cholestatic or mixed injury by R ratio based on liver biochemistry of alanine aminotransferase (ALT), and alkaline phosphatase (ALP). R = (ALT/ULN)/(ALP/ULN) (R >5 hepatocellular, 2–5 mixed pattern, <2 cholestatic) (10,12,27).
There are hundreds of medicinal herbs or products linked to different degrees of HILI per meta-analysis, reviews, case reports, case series over past 2–3 decades. Well-established HILI is listed in Table 1.
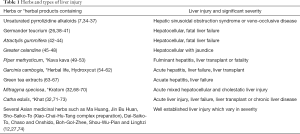
Full table
HILI presents more frequently with hepatocellular injury (75,76). It appears more prevalent in women as compared to men (75,77) but a study from China comparing HILI to DILI described that HILI from TCM affected a smaller portion of females (76).
Mechanism and pathogenesis
The true pathogenesis of HILI is not quite understood. Toxic ingredients, reactive metabolites, endoplasmic reticulum and mitochondrial stress, bile salt export inhibition have been postulated in the pathogenesis of HILI causing hepatocyte necrosis, apoptosis and lead to activation of adaptive immune response (12,27,78). Nevertheless, HILI manifests as idiosyncratic, or intrinsic liver injury. Idiosyncratic liver injury is not predictable or dose dependent, and it has a variable or long latency. Intrinsic liver injury is more predictable, experimentally reproducible, dose dependent, with a short and consistent latency period and higher incidence in humans (12,27,78).
Hepatic microsomal cytochrome P450 was shown involving in activation of pyrrolizidine alkaloids (PAs) in experiments of TCMs containing unsaturated PAs (79). Germander toxicity has been reproduced in animal experiments and transferred to human hepatotoxicity. Its toxicity is due to reactive metabolites from oxidization of neoclerodane diterpenoids through CYP3A isoform which deplete cytoskeleton associated protein thiols and hepatic glutathione leading to apoptosis of hepatocytes (80,81).
Diagnosis and causality assessment
The diagnosis of HILI remains a challenge. HILI is an exclusion of diagnosis when all alternative etiologies of liver injury such as acute viral hepatitis, autoimmune, hereditary, metabolic liver disease, ischemic hepatopathy and vascular disorder are completely ruled out (12,23,27,29,78,82).
RUCAM, a structured, quantitative, effective, and validated tool for causality assessment, is most but not all used in causality assessment of HILI in clinical practice (11-13,78,82-88). RUCAM algorithm includes 7 criteria. They are latency (time to onset), course after de-challenge, risk factors (age, alcohol/pregnancy), concomitant medications, exclusion of other etiologies, previous data on drug hepatotoxicity and response to re-challenge. The scores for each category are 0 to +2, −2 to +3, 0 to +2, −3 to 0, −3 to +2, 0 to +2, and −2 to +3, respectively. Total score ≤2: unlikely, 3–5: possible, 6–7: probable, ≥8: highly likely for liver injury (89,90).
Serum miRNAs were demonstrated as potential biomarkers with high sensitivity and specificity for diagnosis of HILI in rats (91). Biomarker research has been of great interests in improving the diagnosis of HILI. However, European Medicines Agency (EMA) and US Food and Drug Administration (FDA) officially retracted the Letter of Support affecting all biomarkers due to a significant scientific misconduct and fraud in the incriminated biomarker. This conceivably has had significant impact on biomarker research and development (92). Future exploration and validation of potential diagnostic biomarkers for HILI remain needed (22,92).
Genetic testing of human leukocyte antigen (HLA) alleles has been used in diagnosis of HILI. HLA-B*35:01 was shown to be a potential biomarker for Polygonum multiflorum (a Chinese herbal medicine) induced liver injury (93). A strong association of HLA-B*35:01 with green tea related idiosyncratic and immune mediated liver injury was recently published as well (94).
Management and outcomes
HILI is often self-limiting after offending herb or herbal product is discontinued (95-98). Majority of the patients with mild to moderate liver injury will have gradual recovery. Close monitoring of signs and symptoms of severe liver injury such as hepatic decompensation or liver failure is essential. A retrospective study of 488 patients with HILI showed that 14.1% developed chronic HILI and 4.1% had acute liver failure with death or requiring liver transplantation (99).
Liver transplantation is indicated in severe HILI with acute liver failure (100). A retrospective study of 30 patients from 2007 to 2012 also implied that Chinese medicinal herbs caused 60% mortality in acute liver failure without liver transplantation (101).
Summary
The use of herbal medicine and products continues to rise globally despite the potential of significant liver injury. The diagnosis of HILI remains challenging. It is crucial for health care providers to raise the awareness of HILI for early diagnosis and proper management. RUCAM, a structured, quantitative, effective and validated tool for causality assessment in HILI should be reinforced in clinical use.
Further research in analysis of toxic ingredients of herbs on hepatocytes and the associated injury may better elucidate the pathogenesis of HILI. The development of pathogenetic hallmarks of GIWAS, HLA alleles and non-HLA genetic variants to identify risk for HILI; along with future exploration of specific and valid new biomarkers for early diagnosis of HILI are critical. Global prospective HILI registry including HILI related issues is essential for more definitive diagnosis, better management and favorable outcomes.
Furthermore, policy development and clinical implementation of standardized regulatory surveillance and pharmacovigilance on efficacy and safety of herbs and herbal products are warranted to ensure public safety and reduce HILI associated morbidity and mortality as well.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/dmr-21-8
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/dmr-21-8). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014;4:177. [Crossref] [PubMed]
- Stournaras E, Tziomalos K. Herbal medicine-related hepatotoxicity. World J Hepatol 2015;7:2189-93. [Crossref] [PubMed]
- de Boer YS, Sherker AH. Herbal and Dietary Supplement-Induced Liver Injury. Clin Liver Dis 2017;21:135-49. [Crossref] [PubMed]
- Rashrash M, Schommer JC, Brown LM. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J Patient Exp 2017;4:108-13. [Crossref] [PubMed]
- Korth C. Drug-induced hepatotoxicity of select herbal therapies. J Pharm Pract 2014;27:567-72. [Crossref] [PubMed]
- Verma S, Thuluvath PJ. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clin Gastroenterol Hepatol 2007;5:408-16. [Crossref] [PubMed]
- Letsyo E, Jerz G, Winterhalter P, et al. Toxic pyrrolizidine alkaloids in herbal medicines commonly used in Ghana. J Ethnopharmacol 2017;202:154-61. [Crossref] [PubMed]
- Welz AN, Emberger-Klein A, Menrad K. Why people use herbal medicine: insights from a focus-group study in Germany. BMC Complement Altern Med 2018;18:92. [Crossref] [PubMed]
- Teschke R, Schwarzenboeck A, Eickhoff A, et al. Clinical and causality assessment in herbal hepatotoxicity. Expert Opin Drug Saf. 2013;12:339-66. [Crossref] [PubMed]
- Amadi CN, Orisakwe OE. Herb-induced liver injuries in developing nations: an update. Toxics 2018;6:24. [Crossref] [PubMed]
- Becker MW, Lunardelli MJM, Tovo CV, et al. Drug and herb-induced liver injury: A critical review of Brazilian cases with proposals for the improvement of causality assessment using RUCAM. Ann Hepatol 2019;18:742-50. [Crossref] [PubMed]
- Frenzel C, Teschke R. Herbal Hepatotoxicity: Clinical characteristics and listing compilation. Int J Mol Sci 2016;17:588. [Crossref] [PubMed]
- Teschke R. DILI, HILI, RUCAM algorithm, and AI, the artificial intelligence: provocative issues, progress, and proposal. Arch Gastroenterol Res 2020;1:4-11.
- Teschke R, Larrey D, Melchart D, et al. Traditional Chinese Medicine (TCM) and Herbal Hepatotoxicity: RUCAM and the Role of Novel Diagnostic Biomarkers Such as MicroRNAs. Medicines (Basel) 2016;3:18. [Crossref] [PubMed]
- Björnsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013;144:1419-25. [Crossref] [PubMed]
- Andrade RJ. Landscape of Liver Injury From Herbal and Dietary Supplements in Europe, Latin America, and Asia. Clin Liver Dis (Hoboken) 2019;14:49-50. [Crossref] [PubMed]
- Takikawa H, Murata Y, Horiike N, et al. Drug induced liver injury in Japan: An Analysis of 1676 cases between 1997 and 2006. Hepatol Res 2009;39:427-31. [Crossref] [PubMed]
- Zhou Y, Yang L, Liao Z, et al. Epidemiology of drug-induced liver injury in China: A systematic analysis of the Chinese literature including 21,789 patients. Eur J Gastroenterol Hepatol 2013;25:825-9. [Crossref] [PubMed]
- Wai CT, Tan BH, Chan CL, et al. Drug-induced liver injury at an Asian center: A prospective study. Liver Int 2007;27:465-74. [Crossref] [PubMed]
- Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol 2012;107:1380-7. [Crossref] [PubMed]
- Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology 2019;156:2230-2241.e11. [Crossref] [PubMed]
- Rosenberg JJ, Higley C, Shabazi S, et al. Selected highlights and controversies of drug -induced liver injury from the recent literature. World Journal of Gastroenterology Hepatology and Endoscopy Research 2020;1:1-16.
- Navarro V. Liver injury from herbal and dietary supplements: an introduction. Clin Liver Dis (Hoboken) 2019;14:43-4. [Crossref] [PubMed]
- Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug –induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015;148:1353-61.e3. [Crossref] [PubMed]
- Byeon JH, Kil JH, Ahn YC, et al. Systematic review of published data on herb induced liver injury. J Ethnopharmacol 2019;233:190-6. [Crossref] [PubMed]
- Nencini C, Galluzzi P, Pippi F, et al. Hepatotoxicity of Teucrium chamaedrys L. decoction: role of difference in the harvesting area and preparation method Indian J Pharmacol 2014;46:181-4. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel: Chair:; Panel members; EASL Governing Board representative:. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol 2019;70:1222-61. [Crossref]
- Jing J, Wang RL, Zhao XY, et al. Association between the concurrence of pre-existing chronic liver disease and worse prognosis in patients with an herb-polygonum multiflorum thunb. Induced liver injury: a case-control study from a specialized liver disease center in China. BMJ Open 2019;9:e023567 [Crossref] [PubMed]
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther 2013;37:3-17. [Crossref] [PubMed]
- Calitz C, du Plessis L, Gouws C, et al. Herbal hepatotoxicity: current status, examples, and challenges. Expert Opin Drug Metab Toxicol 2015;11:1551-65. [Crossref] [PubMed]
- Navarro VJ, Khan I, Björnsson E, et al. Liver injury from herbal and dietary supplements. Hepatology 2017;65:363-73. [Crossref] [PubMed]
- Pantano F, Tittarelli R, Mannocchi G, et al. Hepatotoxicity Induced by "the 3Ks": Kava, Kratom and Khat. Int J Mol Sci 2016;17:580. [Crossref] [PubMed]
- Stedman C. Herbal hepatotoxicity. Semin Liver Dis 2002;22:195-206. [Crossref] [PubMed]
- Wiedenfeld H, Edgar J. Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochem Rev 2011;10:137-51. [Crossref]
- Kakar F, Akbarian Z, Leslie T, et al. An outbreak of hepatic veno-occlusive disease in Western afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J Toxicol 2010;2010:313280 [Crossref] [PubMed]
- Neuman MG, Cohen L, Opris M, et al. Hepatotoxicity of Pyrrolizidine alkaloids. J Pharm Pharm Sci 2015;18:825-43. [Crossref] [PubMed]
- Roeder E, Wiedenfeld H, Edgar JA. Pyrrolizidine alkaloids in medicinal plants from North America. Pharmazie 2015;70:357-67. [PubMed]
- Pérez Alvarez J, Sáez-Royuela F, Gento Peña E, et al. Acute hepatitis due to ingestion of Teucrium chamaedrys infusions. Gastroenterol Hepatol 2001;24:240-3. [Crossref] [PubMed]
- Savvidou S, Goulis J, Giavazis I, et al. Herb-induced hepatitis by Teucrium polium L.: report of two cases and review of the literature. Eur J Gastroenterol Hepatol 2007;19:507-11. [Crossref] [PubMed]
- Gori L, Galluzzi P, Mascherini V, et al. Two contemporary cases of hepatitis associated with Teucrium chamaedrys L. decoction use: case reports and review of literature. Case Reports. Basic Clin Pharmacol Toxicol 2011;109:521-6. [Crossref] [PubMed]
- Dağ MS, Aydınlı M, Oztürk ZA, et al. Drug- and herb-induced liver injury: a case series from a single center. Turk J Gastroenterol 2014;25:41-5. [Crossref] [PubMed]
- Hamouda C, Hédhili A, Ben Salah N, et al. A review of acute poisoning from Atractylis gummifera L. Vet Hum Toxicol 2004;46:144-6. [PubMed]
- Georgiou M, Sianidou L, Hatzis T, et al. Hepatotoxicity due to Atractylis gummifera-L. J Toxicol Clin Toxicol 1988;26:487-93. [Crossref] [PubMed]
- Haslan H, Suhaimi FH, Das S. Herbal Supplements and Hepatotoxicity: A Short Review. Nat Prod Commun 2015;10:1779-84. [Crossref] [PubMed]
- Benninger J, Schneider HT, Schuppan D, et al. Acute hepatitis induced by greater celandine (Chelidonium majus). Gastroenterology 1999;117:1234-7. [Crossref] [PubMed]
- Teschke R, Frenzel C, Glass X, et al. Greater Celandine hepatotoxicity: A clinical review. Ann Hepatol 2012;11:838-48. [Crossref] [PubMed]
- Pantano F, Mannocchi G, Marinelli E, et al. Hepatotoxicity induced by greater celandine (Chelidonium majus L.): a review of the literature. Eur Rev Med Pharmacol Sci 2017;21:46-52. [PubMed]
- Teschke R, Glass X, Schulze J. Herbal hepatotoxicity by Greater Celandine (Chelidonium majus): causality assessment of 22 spontaneous reports. Regul Toxicol Pharmacol 2011;61:282-91. [Crossref] [PubMed]
- Bujanda L, Palacios A, Silvarino R, et al. Kava-induced acute icteric hepatitis. Gastroenterol Hepatol 2002;25:434-5. [Crossref] [PubMed]
- Brauer RB, Stangl M, Stewart JR, et al. Acute liver failure after administration of herbal tranquilizer kava-kava (Piper methysticum). J Clin Psychiatry 2003;64:216-8. [Crossref] [PubMed]
- Gow PJ, Connelly NJ, Hill RL, et al. Fatal fulminant hepatic failure induced by a natural therapy containing kava. Med J Aust 2003;178:442-3. [Crossref] [PubMed]
- Teschke R. Kava hepatotoxicity--a clinical review. Ann Hepatol 2010;9:251-65. [Crossref] [PubMed]
- Teschke R, Sarris J, Schweitzer I. Kava hepatotoxicity in traditional and modern use: the presumed Pacific kava paradox hypothesis revisited. Br J Clin Pharmacol 2012;73:170-4. [Crossref] [PubMed]
- Sharma A, Akagi E, Njie A, et al. Acute hepatitis due to Garcinia Cambogia extract, an herbal weight loss supplement. Case Rep Gastrointest Med 2018;2018:9606171 [Crossref] [PubMed]
- Kothadia JP, Kaminski M, Samant H, et al. Hepatotoxicity Associated with Use of the Weight Loss Supplement Garcinia cambogia: A Case Report and Review of the Literature. Case Reports Hepatol 2018;2018:6483605 [Crossref] [PubMed]
- Fong TL, Klontz KC, Canas-Coto A, et al. Hepatotoxicity due to hydroxycut: a case series. Am J Gastroenterol 2010;105:1561-6. [Crossref] [PubMed]
- Melendez-Rosado J, Snipelisky D, Matcha G, et al. Acute hepatitis induced by pure Garcinia cambogia. J Clin Gastroenterol 2015;49:449-50. [Crossref] [PubMed]
- Lunsford KE, Bodzin AS, Reino DC, et al. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J Gastroenterol 2016;22:10071-6. [Crossref] [PubMed]
- Corey R, Werner KT, Singer A, et al. Acute liver failure associated with Garcinia cambogia use. Ann Hepatol 2016;15:123-6. [Crossref] [PubMed]
- Crescioli G, Lombardi N, Bettiol A, et al. Acute liver injury following Garcinia cambogia weight –loss supplementation: case series and literature review. Intern Emerg Med 2018;13:857-72. [Crossref] [PubMed]
- Yousaf MN, Chaudhary FS, Hodanazari SM, et al. Hepatotoxicity associated with Garcinia cambogia: A case report. World J Hepatol 2019;11:735-742. [Crossref] [PubMed]
- Ferreira V, Mathieu A, Soucy G, et al. Acute Severe Liver Injury Related to Long-Term Garcinia cambogia Intake. ACG Case Rep J 2020;7:e00429 [Crossref] [PubMed]
- Navarro VJ, Bonkovsky HL, Hwang SI, et al. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci 2013;58:2682-90. [Crossref] [PubMed]
- Teschke R, Zhang L, Melzer L, et al. Green tea extract and the risk of drug induced liver injury. Expert Opin Drug Metab Toxicol 2014;10:1663-76. [Crossref] [PubMed]
- Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch Toxicol 2015;89:1175-91. [Crossref] [PubMed]
- Yu Z, Samavat H, Dostal AM, et al. Effect of Green Tea Supplements on Liver Enzyme Elevation: Results from a Randomized Intervention Study in the United States. Cancer Prev Res (Phila) 2017;10:571-9. [Crossref] [PubMed]
- Oketch-Rabah HA, Roe AL, Rider CV, et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep 2020;7:386-402. [Crossref] [PubMed]
- Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-Induced Cholestatic Liver Injury and Its Conservative Management. J Investig Med High Impact Case Rep 2019;7:2324709619836138 [Crossref] [PubMed]
- Quinonez J, Atwal T. Kratom Induced Hepatotoxicity: A Case Report. Int J Hepatol Gastroenterol 2020;6:001-004.
- Antony A, Lee TP. Herb-Induced Liver Injury With Cholestasis and Renal Injury Secondary to Short-Term Use of Kratom (Mitragyna speciosa). Am J Ther 2019;26:e546-7. [Crossref] [PubMed]
- Palacios Argueta P, Attar B, Sikavi C, et al. Drug induced liver injury caused by “Khat”, an herbal stimulant. ACG Case Rep J 2020;7:e00480 [Crossref] [PubMed]
- Roelandt P, Geroge C, d’Heygere F, et al. Acute liver failure secondary to Khat (Catha edulis)-induced necrotic hepatitis requiring liver transplantation: case report. Transplant Proc 2011;43:3493-5. [Crossref] [PubMed]
- Orlien SMS, Sandven I, Berhe NB, et al. Khat chewing increases the risk for developing chronic liver disease: a hospital –based case-control study. Hepatology 2018;68:248-57. [Crossref] [PubMed]
- Teschke R. Traditional Chinese medicine induced liver injury. J Clin Transl Hepatol 2014;2:80-94. [PubMed]
- Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, et al. Herbal and Dietary Supplement-Induced Liver Injuries in the Spanish DILI Registry. Clin Gastroenterol Hepatol 2018;16:1495-502. [Crossref] [PubMed]
- Jing J, Teschke R. Traditional Chinese Medicine and Herb-Induced Liver Injury: Comparison with Drug-Induced Liver Injury. J Clin Transl Hepatol 2018;6:57-68. [Crossref] [PubMed]
- Lin NH, Yang HW, Su YJ, et al. Herb induced liver injury after using herbal medicine. Medicine (Baltimore) 2019;98:e14992 [Crossref] [PubMed]
- Andrade RJ, Chalasani N, Björnsson ES, et al. Drug-induced liver injury. Nat Rev Dis Primers 2019;5:58. [Crossref] [PubMed]
- Larrey D, Faure S. Herbal medicine hepatotoxicity: A new step with development of specific biomarkers. J Hepatol 2011;54:599-601. [Crossref] [PubMed]
- Larrey D, Vial T, Pauwels A, et al. Hepatitis after germander (Teucrium chamaedrys) administration: another instance of herbal medicine hepatotoxicity. Ann Intern Med 1992;117:129-32. [Crossref] [PubMed]
- Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: Present and future. Semin Liver Dis 2014;34:123-33. [Crossref] [PubMed]
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 1990;11:272-6. [Crossref] [PubMed]
- Chen Y, Wang C, Yang H, et al. Epidemiology of Drug- and Herb-Induced Liver Injury Assessed for Causality Using the Updated RUCAM in Two Hospitals from China. Biomed Res Int 2021;2021:8894498 [PubMed]
- Danan G, Teschke R. RUCAM in drug and herb induced liver injury: The update. Int J Mol Sci 2015;17:14. [Crossref] [PubMed]
- Danan G, Teschke R. Drug-induced liver injury: why is the Roussel Uclaf Causality Assessment Method (RUCAM) still used 25 years after its launch? Drug Safety 2018;41:735-43. [Crossref] [PubMed]
- Danan G, Teschke R. Roussel Uclaf Causality Assessment Method for Drug-Induced liver injury: present and future. Front Pharmacol 2019;10:853. [Crossref] [PubMed]
- Teschke R, Zhu Y, Jing J. Herb-induced liver injury in Asia and current role of RUCAM for causality assessment in 11,160 published cases. J Clin Transl Hepatol 2020;8:200-14. [Crossref] [PubMed]
- Teschke R, Danan G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993-Mid 2020: A Comprehensive Analysis. Medicines (Basel) 2020;7:62. [Crossref] [PubMed]
- Hayashi PH. Overview of causality assessment in drug–induced liver injury. Clin Liver Dis (Hoboken) 2017;9:29-33. [Crossref] [PubMed]
- Teschke R, Danan G. Idiosyncratic Drug-Induced Liver Injury (DILI) and Herb-Induced Liver Injury (HILI): Diagnostic Algorithm Based on the Quantitative Roussel Uclaf Causality Assessment Method (RUCAM). Diagnostics (Basel) 2021;11:458. [Crossref] [PubMed]
- Su YW, Chen X, Jiang ZZ, et al. A panel of serum microRNAs as specific biomarkers for diagnosis of compound- and herb-induced liver injury in rats. PLoS One 2012;7:e37395 [Crossref] [PubMed]
- Teschke R, Eickhoff A, Brown A, et al. Diagnostic biomarkers in liver injury by drugs, herbs, and alcohol: tricky dilemma after EMA correctly and officially retracted letter of support. In J Mol Sci 2020;21:212.
- Li C, Rao T, Chen X, et al. HLA-B*35:01 allele is a potential biomarker for predicting polygonum multiflorum-induced liver injury in humans. Hepatology 2019;70:346-57. [PubMed]
- Hoofnagle JH, Bonkovsky HL, Phillips EJ, et al. HLA-B*35:01 and Green Tea–Induced Liver Injury. Hepatology 2021;73:2484-93. [Crossref] [PubMed]
- Lee WJ, Kim HW, Lee HY, et al. Systematic review on herb-induced liver injury in Korea. Food Chem Toxicol 2015;84:47-54. [Crossref] [PubMed]
- Zhu Y, Niu M, Chen J, et al. Hepatobiliary and pancreatic: comparison between Chinese herbal medicine and western medicine-induced liver injury of 1985 patients. J Gastroenterol Hepatol 2016;31:1476-82. [Crossref] [PubMed]
- Hillman L, Gottfried M, Whitsett M, et al. Clinical features and outcomes of complementary and alternative medicine Induced acute liver failure injury. Am J Gastroenterol 2016;111:958-65. [Crossref] [PubMed]
- Shahbaz O, Mahajan S, Lewis JH. Highlights of drug - and herb- induced liver injury in the literature from 2016: how best to translate new information into clinical practice? Expert Opin Drug Metab Toxicol 2017;13:935-51. [Crossref] [PubMed]
- Zhu Y, Niu M, Wang JB, et al. Predictors of poor outcomes in 488 patients with herb-induced liver injury. Turk J Gastroenterol 2019;30:47-58. [PubMed]
- Grewal P, Ahmad J. Severe liver injury due to herbal and dietary supplements and the role of liver transplantation. World J Gastroenterol 2019;25:6704-12. [Crossref] [PubMed]
- Zhao P, Wang C, Liu W, et al. Acute liver failure associated with traditional Chinese medicine: Report of 30 cases from seven tertiary hospitals in China. Crit Care Med 2014;42:e296-9. [Crossref] [PubMed]
Cite this article as: Lee TP. A narrative review of herb-induced liver injury. Dig Med Res 2021;4:28.


