
Advantages of robotic duodenopancreatectomy: a narrative review
Introduction
Thanks to the development of new technologies, modern day surgeons are faced with options for operative approach. Such options include open surgery and minimally invasive surgery (MIS). There is a vast amount of literature revealing the safety and many benefits of MIS over open surgery, including reduced hospital length of stay, reduced postoperative pain, improved cosmesis due to smaller involvement, decreased blood loss, lower risk of infection, and overall faster recovery time leading to increased patient satisfaction (1). Since its advent, robotic surgery has further revolutionized MIS by addressing the challenges posed by laparoscopy such as restriction of operative field, limitation of movement, lack of 3-dimensional vision, and lack of precision (1-7). Though its availability is still limited mostly due to cost and need for surgeon experience, it is slowly gaining popularity as more surgeons become proficient in it and more data emerges.
Pancreatic surgery in itself represents one of the most challenging fields in abdominal surgery, epitomized by the pancreaticoduodenectomy (PD). The first minimally invasive PD was performed in May 1992 (8); however, despite the long history and the multiple advances in technique and approach in pancreatic surgery, which has led to decreased morbidity and mortality, open surgery has historically been the gold standard. Though MIS has been used for pancreatic surgery, including both laparoscopic and robotic approaches, and either alone or in combination with an open approach, there is still no sufficient data to favor one over the other, though it is without question that MIS benefits continue to apply to pancreatic surgery. Gumbs et al. performed a prior review of 285 cases of laparoscopic pancreatoduodenectomy which revealed similar outcomes with respect to perioperative morbidity and mortality compared to open (8). Despite this review, there remains a lack of data to support MIS over an open approach is especially true for robotic pancreaticoduodenectomy (RPD), mainly due to the relative novelty of robotic technology as well as the need for surgeons proficient and skilled in both pancreatic and robotic surgery, limiting the procedure to only a few centers (9-11). Moreover, early research did not necessarily focus on oncologic outcomes of pancreatic MIS as much as most recent studies, further decreasing the current amount of data for overall safety especially in the setting of malignancy (8).
MIS has demonstrated superiority in many abdominal surgeries when compared to open surgery, and because robotic surgery has addressed the disadvantages of laparoscopy, the application of robotic technology to pancreatic surgery has shown to be possible while maintaining the intrinsic benefits of MIS. However, because of the morbid nature of pancreatic disease and surgery, other operative factors and perioperative complications are to be taken into consideration. Amongst the most common and feared complications of a PD are postoperative pancreatic fistulas (POPF), delayed gastric emptying, and hemorrhage. These complications as well as other surgery-specific parameters such as intraoperative time, hospital length of stay, number of lymph nodes harvested, oncologic outcomes, and number of readmissions have been recently studied in both open pancreatic surgery and MIS pancreatic surgery.
The aim of this article is to review recent literature (2016–2020) on RPD with special focus on morbidity rates and oncologic outcomes when compared to open pancreaticoduodenectomy (OPD).
We present the following article in accordance with the narrative review reporting checklist (available at http://dx.doi.org/10.21037/dmr-21-17).
Methods
Articles were collected from PUBMED since 2016–2020 with the words robotic pancreaticoduodenectomy, propensity-matched pancreaticoduodenectomy, robotic pancreaticoduodenectomy + NSQIP, robotic pancreaticoduodenectomy + NCDB. Our criteria were studies with at least 30 patients. We categorized the studies into single-institution case series (3 articles), single-institution vs NSQIP dataset (1 article), single institution matching RPD with OPD (2 articles), single institution propensity matched RPD vs. OPD (6 articles), multi-institutional (1 article) and studies that used large national datasets (8 articles) including NCDB and ACS-NSQIP.
Results
Single institution series
Guerra et al. in a retrospective analysis of a single institution series, evaluated a consecutive series of totally robotic PD with respect to surgical and oncologic outcomes (12). Their study included 59 patients who underwent RPD from March 2010 to April 2017. 96.1% of the patients received an R0 resection with average lymph node harvest of 26. Their study involved a surgical team including trainees versus one surgeon (like Valle et al.). They reported a three year overall survival of 61.9% and an estimated three year disease free survival of 37.2%. Their clinically relevant POPF rate was 11.8%, as can be seen in Table 1.
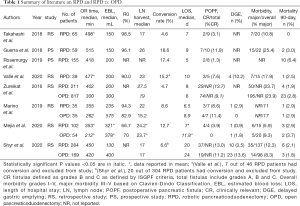
Full table
Valle et al. conducted a retrospective analysis of 39 patients who underwent RPD for pancreatic ductal and ampullary adenocarcinomas with a specific focus on long-term oncologic outcomes (13). Study end-points were postoperative morbidity and mortality, long-term overall survival and disease free survival. Thirty nine patients were included in the study. The 5-year overall survival rate for ductal and ampullary carcinoma rate was 41% (30% for adenocarcinoma and 68% for the ampullary group). Their study’s data compares favorably with most open Whipple surgeries. Cameron et al. reported a 39% 5-year overall survival for negative node and R0 resection ductal adenocarcinoma and 48% for ampullary cancers (14). Forty one percent of their study group underwent adjuvant treatment. The median time to commencement of any adjuvant treatment was 49 days, comparing favorably with other open and laparoscopic series which report 54–55 days.
Takahashi et al. published a retrospective review of a prospectively maintained database of robotic pancreatic procedures performed by a single surgeon at a single institution (15). Of the 119 procedures, 65 were RPD. For RPD, R0 resection rates were 98.5% and average number of lymph nodes removed was 17. EBL was 125 mL for all cases. Incidence of pancreatic leaks were also reported (11.8% grade A, 3.4% grade B and 1.7% grade C based on the ISGPF). Mean operative time began to decrease after 10 cases for RPD, all complication rates decreased steadily after 15 cases.
Rosemurgy et al. in a single institution prospective analysis of 155 patients undergoing RPD compared their patients’ predicted and actual outcomes with outcomes from the ACS NSQIP for pancreaticoduodenectomy from 2012 to 2017 (16). The outcomes for patients in the ACS NSQIP database (where the vast majority were OPD) and the predicted outcomes of their patients were very similar with one exception of expected discharge to nursing or rehabilitation. Actual outcomes showing improvement over predicted outcomes occurred in frequency of complications (49% of predicted), serious complications (46% of predicted), surgical site infections (28% of predicted), venous thromboembolism (100% less than predicted), return to operating room (13% of predicted), median length of stay (50% less than predicted) and discharge to rehabilitation or intermediate care facility (81% of predicted). Their in-hospital mortality rate was higher than expected (6% actual versus 2% predicted) as well as incidence of renal failure (5% actual versus 2% predicted). Deaths were due most often due to MI despite thorough cardiac clearance. The trend in their data overall showed the robotic approach yields faster recoveries and hence a shorter time delay to receive adjuvant therapy.
Single institution OPD vs. RPD
Mejia et al. published a single-center retrospective analysis focusing on clinical and financial outcomes of RPD vs. OPD from 2013–2019 (9). A total of 156 patients were included, 54 OPD and 102 RPD. Cases were performed by a single surgeon with assistance from other surgeons or residents. They found that RPD had shorter LOS, longer operative times and reduced EBL. They did not find any differences between the number of OPD and RPD that were enrolled in ERAS postoperatively. In respect to financial outcomes, operating room charges were higher with RPD than with OPD. They found lower respiratory therapy charges with RPD, and this finding combined with shorter LOS suggested that patients may experience better respiratory dynamics as expected with smaller incisions. Hospital stay charges (total charges minus OR charges) were significantly lower for RPD, indicating that RPD patients utilized significantly less hospital resources while in-house. Overall total charges were not higher for RPD. In addition, RPD patients were more likely to go home than to an extended care facility, which further drives up the cost of healthcare. They also analyzed the cases that converted from RPD to OPD and found that they had higher OR charges but lower LOS as compared to OPD. A possible explanation for this was that in some cases, significant robotic dissection had already occurred before conversion to open. They also compared the total charges for patients that were readmitted within 90 days and found no significant differences between the two groups. Their cost analysis did not include the initial cost of acquisition of the robotic system or its maintenance.
Shyr et al. recently reported on their perioperative outcomes in a large group of patients (304 RPD and 172 OPD) (17). While OR times were longer in RPD, blood loss was much lower in RPD as was delayed gastric emptying (3.5% vs. 13.6% in OPD) as was wound infection rates and hospital LOS. No significant differences were seen in lymph node yield, POPF or other perioperative outcomes. Survival outcomes at 1, 3 and 5 years were better in the RPD group. Although limited by its retrospective nature and lack of propensity score matching, RPD seemed to show comparable results for survival outcomes in pancreatic head and ampullary adenocarcinomas.
Marino et al. performed a cased-matched comparison between two groups of 35 patients with pancreatic malignant tumors who underwent RPD vs. OPD from August 2014 to April 2016 (18). While RPD was associated with longer operative times, EBL and LOS, they did not find any appreciable difference between POPF, RO resection and number of harvested lymph nodes. Overall and disease-free survival at 1 and 3 years were similar.
Single institution propensity matched RPD vs. same or other institution OPD
Over the past few years, propensity-matched studies have increasingly been published as a way to mitigate the bias inherent in a field where there are no randomized controlled trials or level 1 evidence.
The first propensity score-matched study comparing RPD to OPD was published in 2017 by McMillan and Zureikat et al. (19), aimed at assessing RPD impact on POPF as compared to OPD. Propensity score matching was used to minimize bias from nonrandom treatment assignment. All OPDs were performed at 16 institutions from January 2003 to October 2015 by 48 surgeons who had surpassed the learning curve. All RPDs were performed at one center by three surgeons who had surpassed their learning curves. The study concluded that RPD was non-inferior to OPD in terms of POPF, as can be seen in Table 2.
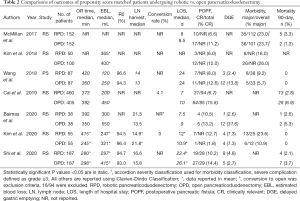
Full table
In line with this finding and with a higher powered study, Cai et al. published a propensity matched single institution study looking at the incidence of clinically-relevant post-operative pancreatic fistula (CR-POPF) in 865 patients, 405 (46.8%) who underwent OPD and 460 (53.2%) who underwent RPD over a 7-year period (20). After propensity score matching, they analyzed 229 patients in OPD arm and 414 in RPD arm. Only outcomes of surgeons beyond their learning curve for both OPD and RPD were used. POPF were graded based on the ISGS definition (biochemical leak grade A, CR-POPF grade b or c). First, they calculated a fistula risk score for every patient based on pancreatic texture, pathology, pancreatic duct diameter and intraoperative blood loss, criteria set by Callery et al. for assessing patients’ risk for developing POPF (also done by McMillan et al.) (19). This divided their patients into three fistula risk zones: low risk, moderate risk and high risk. They found that RPD was associated with overall reductions in CR-POPF across all fistula risk zones, with statistical significance in those with moderate risk for leaks. Interestingly, they found that while both OPD and RPD were associated with a similar overall POPF rate, RPD was associated with a lower incidence of CR-POPF. Multivariate analysis demonstrated that RPD was an independent predictor of lower CR-POPF (OR 0.278, P<0.001). Their study also showed that RPD was associated with shorter operative times, less EBL and lower transfusion rates as well as shorter length of stay. They conclude that while the robotic approach does not prevent fistulas, minimizing tissue trauma may serve to curtail the clinical impact of those fistulas by a decreased subsequent inflammatory response. While they make a compelling argument for the benefit of RPD over OPD, their outcomes are once again of highly trained and seasoned robotic pancreatic surgeons, making it difficult to generalize to lower volume centers and surgeons still within their learning curves.
Kim et al. (21) in an article comparing RPD vs. OPD with propensity score matching found POPF tended to be lower (6% vs. 12%, P=0.387) among RPD between a cohort of 50 RPD and 100 OPD propensity matched patients. They also found that pain scores were similar on POD1 and 3 but significantly lower in the robot group than in the open group on POD5 and 7. The number of postoperative analgesic injections were significantly lower in the RPD than in the OPD group. Patients in the RPD group started ambulation earlier and had a reduced postoperative hospital stay.
Oncologic outcomes
Wang et al. published a propensity score matched study comparing 87 OPD and 87 RPD patients (22). All surgeries were performed by the same two surgeons with a modified Blumgart pancreaticojejunostomy. After propensity score matching, significantly longer OR time, less blood loss, more lymph node harvest and less delayed gastric emptying were noted by RPD than OPD. No significant difference was detected in R0 resections or postoperative complications. Their data showed similar disease specific survival outcomes between the two groups. The study was unique in comparing three year survival outcomes between RPD and OPD with the same surgical technique by the same surgical team.
Baimas-George et al. in a propensity-matched retrospective review evaluated all RPD performed for pancreatic adenocarcinoma from 2008 to 2019 in a single institution and matched them with open cases by demographics and oncologic characteristics (23). Thirty eight RPD were matched with 38 OPD. The goal was to evaluate the long term oncologic effects of RPD versus OPD for pancreatic ductal adenocarcinoma. All surgeries were conducted by four fellowship trained hepatobiliary surgeons who perform over 120 PD cases per year. Perioperative outcomes showed no significant difference in operative time between OPD and RPD (RPD 392 min, OPD 350 minutes). EBL was similar between the two groups as was ileus, urinary retention, anastomotic leak, POPF and wound infection. The lymph node yield was significantly higher in the RPD group with 21.5 vs. 13.5 nodes in OPD. They also showed a significantly higher incidence of DGE in the OPD than RPD (32% vs. 3%, P=0.0009). Oncologic outcomes showed equivalent R1 resection rates of mid 40% in both cohorts. Open cases had two times the local recurrence rate (RPD 8% vs. OPD 16%, P=0.2870). The authors discuss that the significantly higher lymph node yield and decreased inflammatory response of robotic surgery may increase time to recurrence and improve overall survival in pancreatic ductal adenocarcinoma. In addition, the decrease in DGE in the RPD arm, while not directly related to oncologic outcomes, is of clinical significance as this results in prolonged length of stay and delays in starting adjuvant chemotherapy. The authors discuss that their results support the theory that DGE is influenced by intra-abdominal inflammation, and the overall decreased inflammation from RPD leads to less DGE events. Although the chance of a type II error exists with the small sample size, the data from their study is encouraging and in line with trends of RPD showing decreased DGE and increased lymph node harvest.
Learning curve
RPD learning curve has been extensively studied by multiple authors (not covered in detail in this review). Kim et al. (21) reported a single surgeon experience comparing surgical outcomes between early (case 1–35) and late [36–70] cases. They used propensity score matching to compare outcomes to OPD. Shorter operative times, EBL and LOS were observed in the second group of late cases. Boone et al. (24) published an article on the learning curve associated with RPD, suggesting approximately 80 cases were needed. In a large propensity matched study, Shi found two inflexion points at 100 and 250, where outcomes were better than OPD in terms of operative time, EBL and postoperative hospital stay.
Multi-institutional analysis
A comparison across more than one institution was conducted by Zureikat et al. in 2016 (25). This study involved a RPD arm from two academic surgery centers and an OPD arm from 6 centers with high volume pancreatic surgeons. All surgeons were in their post-procedure learning curve. On multivariate analysis when compared with OPD, RPD was associated with longer operative times, reduced blood loss and reductions in major complications. No statistically significant differences were found in regards to 90-day mortality or CR-POPF, wound infection, LOS or 90-day readmission. Multivariate analysis also showed that operative approach was not independently associated with positive resection margins or tumor understaging. More PDACs were resected in the OPD group than the RPD group. In addition, only two surgeons were involved in the RPD arm, further limiting generalizability. Overall, the comparison showed that robotic surgery was comparable to open in safety and short-term oncologic outcomes.
Database studies using NSQIP and NCDB
We examined several studies comparing national databases on patients who underwent OPD vs MIPD, including RPD. Both NSQIP and NCDB have been used in recent years to glean trends on a national level in comparison to single-institution studies with minimal generalizability.
Nassour et al. (26) in 2018 published a propensity-score matched study using NSQIP. Of note, minimally invasive pancreaticoduodenectomy (MIPD) included laparoscopic or robotic. In their intention to treat analysis which included MIPD with conversion to open, they found that MIPD patients were less likely to have prolonged LOS >14 days and less likely to be discharged to non-home but they were more likely to be readmitted. In their secondary analysis without conversion to open cases, there was no difference in overall complications nor in the rate of pancreas specific complications like POPF (18.5% for MIPD vs. 21.8% for OPD, P=0.5) or delayed gastric emptying (16.6% for MIPD vs. 18.7% for OPD, P=0.51). If completed in a minimally invasive approach without conversion to open assist, MIPD was associated with lower 30-day overall infectious complications and decreased perioperative transfusion requirement. Due to the study design using the NSQIP database, the surgeon experience and hospital volume were not recorded but due to the low perioperative mortality reported in both OPD and MIPD, it was assumed that the data was mostly from high-volume centers. Also, they did not separate laparoscopic and robotic outcomes due to the relatively small sample size of the MIPD group overall.
NSQIP did not report pathological or oncological outcomes, which were eventually addressed by a study from the same author in 2020 when Nassour et al. (27) published the first large national study on long term oncologic outcomes comparing OPD vs. RPD (they also compared open vs distal pancreatectomies but that is not the focus of our paper) for a national cohort of pancreatic adenocarcinoma in NCDB. Robotic pancreatic surgery began to be recorded by the NCDB in 2010 and their study is based on the release of data in 2016, allowing analysis of 5-year survival data of OPD vs. RPD. They selected patients who underwent OPD or RPD for stages I–III pancreatic adenocarcinoma. The primary outcome was 5-year overall survival. They also compared pathologic outcomes (by using number of lymph nodes examined and margin status) and perioperative outcomes (30- and 90-day mortality, 30-day readmission rate and LOS). They analyzed 17,831 patients, of which 4% [626] were robotic. They did note a six-fold increase in utilization of RPD from 2010–2016. They found a higher number of lymph nodes examined in the RPD group compared to the OPD group. Mean overall survival was similar between RPD and OPD. There was no difference in perioperative mortality among RPD and OPD. LOS was shorter in the RPD than the OPD. Interestingly, a similar earlier analysis of the NCDB from 2010–2012 showed that MIPDs were associated with higher 30-day postoperative mortality (28). That analysis, however, focused more on low volume surgeons and hospitals likely in their learning curve stage and did not involve RPD. While this study is encouraging in its findings of non-inferiority of RPD vs. OPD for 5-year overall survival, improved lymph nodes examined and decreased LOS, data on tumor involvement of vascular structures, surgeon volume and patient selection were not available in the NCDB database.
Another article by Torphy et al. (29) used NCDB to compared OPD vs. MIPD (both laparoscopic and robotic) with primary outcome of 90-day mortality and secondary outcomes of 30-day mortality, length of hospital stay, unplanned 30-day readmissions, surgical margins, number of lymph nodes harvested, and receipt of adjuvant chemotherapy. They found that patients who underwent MIPD had equivalent short term and oncologic outcomes compared to OPD. MIPD was associated with a reduction in hospital LOS. Regardless of surgical approach, patients operated on at high volume centers had reduced 90-day mortality. The interaction between hospital volume and approach was not significant, suggesting the protective effect of pancreaticoduodenectomy and MIPD institutional volume is the same regardless of whether the patient underwent an OPD vs. MIPD, with an estimated 30% reduction in 90-day mortality for institutions in the top 5th percentile. The authors conclude that the fact that institutions with high total PD volume and high MIPD volume had the lowest mortality rates for both open and minimally invasive cases suggests that system and institutional competency, such as the ability of the institution to manage the postoperative complications and avoid death from major complications, rather than technical skills is the main determinant of the differences in perioperative mortality across institutions. Interestingly, a study by Hoehn et al. using NCDB found that compared with academic centers, non-academic centers had equivalent rates of conversion to open surgery, negative margins and 90-day mortality (30). Their study also showed a trend of more RPDs being performed outside of academic centers.
Zimmerman et al. published a retrospective study of NSQIP outcomes from 2014-2015 between OPD, laparoscopic pancreaticoduodenectomy (LPD) and RPD, 3.1% of their study was RPD (31). RPD had less perioperative transfusions and more readmissions. Interestingly, LPD was independently associated with less morbidity than OPD, but whether the surgeons were past their learning curves was not extractable.
Xourafas et al. attempted to use NSQIP to define predictors associated with prolonged operative time (OpTime) and LOS across OPD, LPD and RPD (32). They found perioperative risk factors for prolonged OpTime and hospital LOS were relatively consistent across OPD, LPD and RPD. Independent predictors of a prolonged OpTime were ASA class ≥3, preoperative XRT, pancreatic duct <3 mm, T stage ≥3 and vascular resection for OPD; T stage ≥3 and vascular resection for LPD; and malignancy and conversion to laparotomy for RPD. Independent predictors of increased LOS were age ≥65 years, ASA class ≥3, hypoalbuminemia, and preoperative blood transfusion for OPD as well as an OpTime >370 min and specific postoperative complications for all surgical approaches. As with all NSQIP based analyses of RPD, the sample size of LPD and RPD was significantly smaller than OPD, which limits the statistical power and could explain why fewer independent predictors of increased OpTime and LOS were identified for LPD and RPD than with OPD.
Panni et al. used the NSQIP database to determine if national MIPD pancreatic fistula rates are decreasing with time (33). Their results were promising for groups at low risk for pancreatic fistula formation but not in those with pancreatic ducts <3 mm and soft gland texture. On multivariate analysis, increasing year of operation was independently protective against pancreatic fistula [odds ratio (OR) 0.76 per year, P<0.01] and clinically relevant pancreatic fistula (OR 0.73 per year, P<0.01). 48.8% of MIPD were RPD. This trend was thought to be due to improvement in minimally invasive anastomotic techniques.
Vining et al. recently published a propensity matched retrospective cohort study from 2014 to 2017 using the ACS-NSQIP database comparing RPD to OPD in the development of CR-POPF (34). Their study further supports the findings from Cai et al. that RPD is protective against development of POPF.
Discussion
We attempted to create a ladder of generalizability from single surgeon experience to studies that analyzed large data collection platforms. Each type of study offers both insight and is subject to its own unique bias. Overall trends in studies with increasing strengths of evidence are showing at least noninferiority to and improvement in several key parameters of RPD outcomes for patients. Important barriers to the implementation of robotic programs and robotic training are mainly the cost as well as the long and steep learning curve. Cost analysis remains a challenge especially when compared across countries and different healthcare reimbursement systems and insurances. In the US, there is some encouraging data as to the benefits of combining a robotic approach with ERAS protocols (35). Surgeon learning curves are also an issue, with data ranging from 30 for experienced robotic surgeons to 250 for optimal reproducible results showing improvement in perioperative outcomes. This curve is further accentuated if consideration is given to the fact that in order to do robotic pancreatic surgery, most surgeons are first trained in open surgery where they gain the knowledge and expertise that is then translated to robotic surgery. More resources into structured training may help to improve this drawback of RPD, particularly now that the ‘pioneers’ of the field can now teach instead of discover how to do the operation. For example, Giulianotti et al. recently published a step by step video on operative technique in RPD (36).
One of the more consistent variables in support of RPD has been decreased EBL, and when looked at in terms of research connecting blood loss to postoperative complications and overall prognosis, this is significant. As discussed in Cai et al., the only modifiable variable in the fistula risk score to predict POPF is blood loss. They go on to discuss how increased blood loss potentially serves as a surrogate for increased tissue inflammation, infectious predisposition, decreased end-organ perfusion, and decreased anastomotic perfusion. Thus, the underlying mechanism by which RPD protects against CR-POPF may be related to the precision in dissection of the robotic platform, ultimately decreasing the inflammatory response (20). Other research has shown that patients receiving perioperative blood transfusions had a significantly lower 5-year survival after curative-intent pancreatic surgery (37). While the reasons for this are multifactorial, the relevance of a reduced EBL in RPD should not be underestimated.
The protective effects on POPF that RPD is showing are also quite significant when the consequences down the road that POPF generates are taken into account. Mirrielees et al. recently published an article using a novel way of quantifying a specific complication on the outcomes of surgical populations by using a population attributable fraction (PAF) and applied it to postoperative complications of pancreaticoduodenectomies. The PAF can be used as a proportional reduction in a given adverse outcome that would result from complete prevention of the complication within a surgical cohort. They found that mitigation of POPF would result in a 19.2% reduction in the incidence of end-organ damage, a 11.3% reduction in 30-day mortality, a 14.5% decrease in the rate of prolonged postoperative hospitalization, 8.3% reduction in the need for non-home discharge, and 7.9% reduction in hospital readmission. With the exception of DGE, no other complication approaches POPF in terms of impact size or breadth (38). Such data undoubtedly supports the use of a technology that could potentially reduce both POPF and DGE.
In addition to perioperative outcomes, oncologic outcomes have also been related to POPF. One study reported POPF significantly reduced disease-free survival in pancreatic adenocarcinoma (39). Another study showed severe complications such as POPF significantly alter both overall and disease free survival and are an independent factor of recurrence (40).
More directly related to oncologic outcomes is lymph nodes harvest. Lymph node harvest has been linked to a strong prognostic role in pancreatic ductal adenocarcinomas with data showing that survival is independently predicted by total lymph node harvest (41). This plus the non-inferiority of R0 resections of most studies of RPD vs. OPD is encouraging.
Conclusions
While moving the needle on overall survival in pancreatic cancer as a direct correlation to operative approach remains nebulous, the overall results of RPD on perioperative and oncologic outcomes are encouraging. In lieu of a randomized controlled clinical trial comparing RPD vs. OPD in multiple institutions amongst many surgeons who have completed their learning curves, current evidence indicates that RPD is safe and at least non-inferior to OPD. The increase in propensity-matched studies improves generalizability but still remains vulnerable to unknown confounders. Propensity-matched multi-institutional studies could help further eliminate bias and ultimately tailor the future of pancreatic surgery to deliver the best possible patient outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Andrew A. Gumbs) for the series “Hepatic, pancreatic and biliary surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the narrative review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-21-17
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-17). The series “Hepatic, pancreatic and biliary surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Milone L, Daskalaki D, Wang X, et al. State of the art of robotic pancreatic surgery. World J Surg 2013;37:2761-70. [Crossref] [PubMed]
- Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J 2012;18:571-6. [Crossref] [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [Crossref] [PubMed]
- Winer J, Can MF, Bartlett DL, et al. The current state of robotic-assisted pancreatic surgery. Nat Rev Gastroenterol Hepatol 2012;9:468-76. [Crossref] [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [Crossref] [PubMed]
- Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas 2010;39:160-4. [Crossref] [PubMed]
- Buchs NC, Addeo P, Bianco FM, et al. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 2011;35:2739-46. [Crossref] [PubMed]
- Gumbs AA, Rodriguez Rivera AM, Milone L, et al. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol 2011;18:1335-41. [Crossref] [PubMed]
- Mejia A, Shah J, Vivian E, et al. Analysis of 102 Fully Robotic Pancreaticoduodenectomies: Clinical and Financial Outcomes. Pancreas 2020;49:668-74. [Crossref] [PubMed]
- Bao PQ, Mazirka PO, Watkins KT. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg 2014;18:682-9. [Crossref] [PubMed]
- Addeo P, Calabrese DP, Bachellier P. Robotic pancreaticoduodenectomy: fad or the future? Adv Robot Autom 2012;S1:001.
- Guerra F, Checcacci P, Vegni A, et al. Surgical and oncological outcomes of our first 59 cases of robotic pancreaticoduodenectomy. J Visc Surg 2019;156:185-90. [Crossref] [PubMed]
- Valle V, Fernandes E, Mangano A, et al. Robotic Whipple for pancreatic ductal and ampullary adenocarcinoma: 10 years experience of a US single-center. Int J Med Robot 2020;16:1-7. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Takahashi C, Shridhar R, Huston J, et al. Outcomes associated with robotic approach to pancreatic resections. J Gastrointest Oncol 2018;9:936-41. [Crossref] [PubMed]
- Rosemurgy A, Ross S, Bourdeau T, et al. Robotic Pancreaticoduodenectomy Is the Future: Here and Now. J Am Coll Surg 2019;228:613-24. [Crossref] [PubMed]
- Shyr BU, Shyr BS, Chen SC, et al. Robotic and open pancreaticoduodenectomy: results from Taipei Veterans General Hospital in Taiwan. Updates Surg 2021;73:939-46. [Crossref] [PubMed]
- Marino MV, Podda M, Gomez Ruiz M, et al. Robotic-assisted versus open pancreaticoduodenectomy: the results of a case-matched comparison. J Robot Surg 2020;14:493-502. [Crossref] [PubMed]
- McMillan MT, Zureikat AH, Hogg ME, et al. A Propensity Score-Matched Analysis of Robotic vs Open Pancreatoduodenectomy on Incidence of Pancreatic Fistula. JAMA Surg 2017;152:327-35. [Crossref] [PubMed]
- Cai J, Ramanathan R, Zenati MS, et al. Robotic Pancreaticoduodenectomy Is Associated with Decreased Clinically Relevant Pancreatic Fistulas: a Propensity-Matched Analysis. J Gastrointest Surg 2020;24:1111-8. [Crossref] [PubMed]
- Kim H, Park SY, Park Y, et al. Assessment of learning curve and oncologic feasibility of robotic pancreaticoduodenectomy: A propensity score-based comparison with open approach. J Hepatobiliary Pancreat Sci 2020; Epub ahead of print. [Crossref] [PubMed]
- Wang SE, Shyr BU, Chen SC, et al. Comparison between robotic and open pancreaticoduodenectomy with modified Blumgart pancreaticojejunostomy: A propensity score-matched study. Surgery 2018;164:1162-7. [Crossref] [PubMed]
- Baimas-George M, Watson M, Murphy KJ, et al. Robotic pancreaticoduodenectomy may offer improved oncologic outcomes over open surgery: a propensity-matched single-institution study. Surg Endosc 2020;34:3644-9. [Crossref] [PubMed]
- Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- Nassour I, Wang SC, Christie A, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy: A Propensity-matched Study From a National Cohort of Patients. Ann Surg 2018;268:151-7. [Crossref] [PubMed]
- Nassour I, Winters SB, Hoehn R, et al. Long-term oncologic outcomes of robotic and open pancreatectomy in a national cohort of pancreatic adenocarcinoma. J Surg Oncol 2020;122:234-42. [Crossref] [PubMed]
- Adam MA, Choudhury K, Dinan MA, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer: Practice Patterns and Short-term Outcomes Among 7061 Patients. Ann Surg 2015;262:372-7. [Crossref] [PubMed]
- Torphy RJ, Friedman C, Halpern A, et al. Comparing Short-term and Oncologic Outcomes of Minimally Invasive Versus Open Pancreaticoduodenectomy Across Low and High Volume Centers. Ann Surg 2019;270:1147-55. [Crossref] [PubMed]
- Hoehn RS, Nassour I, Adam MA, et al. National Trends in Robotic Pancreas Surgery. J Gastrointest Surg 2021;25:983-90. [Crossref] [PubMed]
- Zimmerman AM, Roye DG, Charpentier KP. A comparison of outcomes between open, laparoscopic and robotic pancreaticoduodenectomy. HPB (Oxford) 2018;20:364-9. [Crossref] [PubMed]
- Xourafas D, Pawlik TM, Cloyd JM. Independent Predictors of Increased Operative Time and Hospital Length of Stay Are Consistent Across Different Surgical Approaches to Pancreatoduodenectomy. J Gastrointest Surg 2018;22:1911-9. [Crossref] [PubMed]
- Panni RZ, Guerra J, Hawkins WG, et al. National Pancreatic Fistula Rates after Minimally Invasive Pancreaticoduodenectomy: A NSQIP Analysis. J Am Coll Surg 2019;229:192-199.e1. [Crossref] [PubMed]
- Vining CC, Kuchta K, Berger Y, et al. Robotic pancreaticoduodenectomy decreases the risk of clinically relevant post-operative pancreatic fistula: a propensity score matched NSQIP analysis. HPB (Oxford) 2021;23:367-78. [Crossref] [PubMed]
- Kowalsky SJ, Zenati MS, Steve J, et al. A Combination of Robotic Approach and ERAS Pathway Optimizes Outcomes and Cost for Pancreatoduodenectomy. Ann Surg 2019;269:1138-45. [Crossref] [PubMed]
- Giulianotti PC, Mangano A, Bustos RE, et al. Educational step-by-step surgical video about operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique-Lessons learned since the first worldwide RPD performed in the year 2001. Surg Endosc 2020;34:2758-62. [Crossref] [PubMed]
- Mavros MN, Xu L, Maqsood H, et al. Perioperative Blood Transfusion and the Prognosis of Pancreatic Cancer Surgery: Systematic Review and Meta-analysis. Ann Surg Oncol 2015;22:4382-91. [Crossref] [PubMed]
- Mirrielees JA, Weber SM, Abbott DE, et al. Pancreatic Fistula and Delayed Gastric Emptying Are the Highest-Impact Complications After Whipple. J Surg Res 2020;250:80-7. [Crossref] [PubMed]
- Serrano PE, Kim D, Kim PT, et al. Effect of Pancreatic Fistula on Recurrence and Long-Term Prognosis of Periampullary Adenocarcinomas after Pancreaticoduodenectomy. Am Surg 2016;82:1187-95. [Crossref] [PubMed]
- Lubrano J, Bachelier P, Paye F, et al. Severe postoperative complications decrease overall and disease free survival in pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Eur J Surg Oncol 2018;44:1078-82. [Crossref] [PubMed]
- Showalter TN, Winter KA, Berger AC, et al. The influence of total nodes examined, number of positive nodes, and lymph node ratio on survival after surgical resection and adjuvant chemoradiation for pancreatic cancer: a secondary analysis of RTOG 9704. Int J Radiat Oncol Biol Phys 2011;81:1328-35. [Crossref] [PubMed]
Cite this article as: Saad El-Dein S, Panta S, Ocasio A, Banayan E, Genato R, Milone L. Advantages of robotic duodenopancreatectomy: a narrative review. Dig Med Res 2021;4:30.


