Targeted therapy for pancreatic cancer: lessons learned and future opportunities
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the third-leading cause of cancer mortality in the United States (1) and is projected to become the second-leading cause in the next decade (2). PDAC has an extremely poor prognosis, with 1- and 5-year survival rates of only 18% and 7%, respectively (3), primarily due to late-stage diagnosis, its aggressive nature regarding early local invasion and metastasis, and high levels of resistance to conventional chemotherapies and radiotherapies. The increasing incidence and poor prognosis for PDAC patients demonstrate the unmet need for both earlier diagnosis and effective treatment strategies.
The current clinical standard of care for PDAC patients revolves around surgical resection and/or cytotoxic chemotherapy regimens (4). Surgical resection represents the only curative treatment, especially in cases where PDAC has no arterial and/or limited venous contact with the vasculature, with only 10–15% of patients meeting these criteria (5). In patients with advanced disease with metastases or in cases where PDAC is recurring, cytotoxic chemotherapy regimens are the standard treatment, with overall survival (OS) in the range of weeks to a few months (4). Single-agent gemcitabine was approved in 1997, and it remained the standard of care for PDAC for more than two decades, despite having a dismal clinical response with a median survival of approximately 6 months (6). Erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, in combination with gemcitabine, improved OS of PDAC patients by 10 days compared with gemcitabine alone and received FDA approval in 2005 (7). In 2011, a more intense chemotherapeutic regimen, FOLFIRINOX (oxaliplatin, irinotecan, and fluorouracil/leucovorin), was approved for PDAC treatment, with improved survival of approximately 11 months (8). However, as expected, this regimen has higher toxicity, so only patients with high-performance status are eligible to receive this treatment. In 2013, nab-paclitaxel (an albumin-bound formulation of paclitaxel) in combination with gemcitabine (NPT + Gem) demonstrated median survival of 8.5 months, which led to FDA approval of this combination as a first-line treatment for PDAC patients (9) (Table 1).

Full table
A challenging aspect in improving the treatment of PDAC is the lack of accurate predictive biomarkers that can be used to evaluate response to chemotherapies and targeted therapies. In addition, most of the time, promising results on preclinical animal models do not translate to clinical trials (4). Patient-derived xenograft, organoid, and genetically engineered preclinical models that enable a better understanding of the disease progression at molecular levels may enable the improved translation of therapies (4,10). Genomic testing to determine specific genetic PDAC mutations might also help in tailoring targeted treatment regimens for improved efficacy and OS (4).
Targeted therapies that directly block specific oncogenic pathways in PDAC progression have thus far played a limited role in the treatment of this disease. The consensus statement from the National Cancer Institute (NCI) indicated the need for targeted agents, predictive biomarkers, and improved preclinical models for PDAC (11). Additional molecular pathways and genetic mutations of PDAC can be utilized for targeted or precision therapies (12,13). Targeting oncogenes (such as KRAS), reactivating inactivated tumor suppressors (such as p53, CDKN4, p16, BRCA1/2 and SMAD4), and exploiting DNA repair pathways and the immune system might be potential treatment options for PDAC (5,14). Aberrant genes and signaling pathways for microRNAs (miRNAs) as biomarkers or therapeutic targets also have potential in PDAC (15). In this review, we will discuss potential oncogenic molecular pathways involved in PDAC progression and targeted therapies to block these pathways for improved clinical PDAC therapy.
Oncogenic pathways involved in PDAC progression
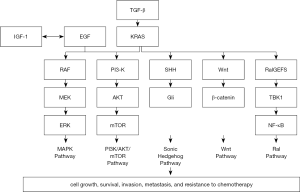
The growth and progression of PDAC involve many different, interconnected signaling pathways (Figure 1). The KRAS signaling pathway is predominant in PDAC, as oncogenic mutations of the KRAS gene facilitate many downstream pathways promoting cancer development and metastasis and impact metabolism (16). Once mutated, KRAS remains bound to GTP, leading to greater PDAC growth (16). Two well-known pathways activated by oncogenic KRAS include the RAF-MEK-ERK (MAPK) pathway and the PI3K-AKT-mTOR pathway, both promoting tumor cell proliferation, division, and survival as well as angiogenesis and invasion/migration (Figure 1). KRAS can be understood as a central mediating point regarding the network of oncogenic signaling in PDAC, as KRAS is implicated with many other downstream proteins involved in PDAC initiation, maintenance and progression, and growth of the characteristic PDAC stroma (17). Activation of hedgehog signaling pathways, initiated by oncogenic KRAS activity, causes greater cancer cell proliferation and invasive activity and promotes cancer cell survival through increased resistance to apoptotic mechanisms (18). Oncogenic KRAS signaling also regulates Wnt protein signaling, as inhibition of Wnt/Ca2+ activity is conducive towards supporting PDAC tumor growth and development (19). Another pathway affected by oncogenic KRAS signaling involves Ral guanine nucleotide exchange factors (RalGEFs) (20) (Figure 1).
Other related pathways that intersect with oncogenic KRAS signaling are as follows: EGFR signaling pathways are pertinent in PDAC progression, as EGFR proteins are overexpressed in most PDAC cases (21). Additionally, EGFR-mediated signaling can also activate the MAPK and the PI3K-AKT signaling pathways (21). Higher activity of insulin-like growth factor-1 (IGF-1) and its receptor (IGF-1R) has also been shown to play a role in PDAC progression (22) (Figure 1). EGFR pathways also intersect with IGF receptors, as such intersectionality between these signaling pathways is conducive to greater growth and development of PDAC. Accordingly, mechanisms of resistance to pathway inhibitors emerge across these signaling pathways (22). Also, vascular endothelial growth factor (VEGF) and its receptor 2 (VEGFR2) signaling are involved in activating angiogenesis and promoting vascular growth, which in turn facilitates PDAC progression (23). The TGF-β signaling in PDAC can also promote disease progression by activating Ras and consequently ERK protein signaling facilitating angiogenesis, metastasis, and suppression of immune cells as part of the pancreatic tumor microenvironment (13) (Figure 1). However, other TGF-β signaling pathways, specifically SMAD protein-dependent pathways, have tumor-suppressive functions (13). Regardless, such signaling is prevented in many cases of PDAC, as there are mutations that inactivate SMAD-dependent TGF-β signaling, which in turn also promotes further progression of this disease (13). Some oncogenic pathways are more directly implicated in the PDAC microenvironment. Met (hepatocyte growth factor receptor) signaling regulates the relationship between pancreatic stellate cells (PSCs) of the tumor stroma and PDAC epithelial cells, promoting PDAC growth and metastasis (24). Moreover, integrins and their related signaling pathways also function within the PDAC microenvironment, as they facilitate PSCs transitioning into different kinds of cancer-associated fibroblasts (CAFs) through differentiation as well as regulate paracrine signaling effects mediated within the tumor microenvironment promoting greater PDAC growth and progression (25).
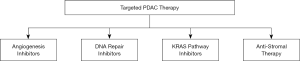
Targeted therapies investigated for PDAC
Antiangiogenic therapies
Angiogenesis plays an integral role in promoting tumor growth and metastasis in many solid tumors (26). Although many types of cancers present with hypervascularity, corresponding to angiogenic processes, PDAC, in contrast, is characterized by its hypovascular nature, having fewer blood vessels associated with its tumors (27). Nonetheless, pro-angiogenic processes and factors are still important in the growth and development of PDAC, as these tumors often have multiple areas consisting of high levels of vascular, microvessel networks (27). Therefore, targeted therapies to block angiogenesis are important to consider for potential PDAC treatment modalities (Figure 2).
Antiangiogenic therapies for PDAC have focused on targeting VEGF signaling, as it facilitates tumor angiogenesis, creating greater tumor blood vessel density (28). Two types of antiangiogenic agents studied in PDAC are (I) monoclonal antibodies, such as bevacizumab (anti-VEGF antibody) and ramucirumab (anti-VEGFR2 antibody), and (II) small-molecule tyrosine kinase inhibitors (TKIs), including sunitinib, sorafenib, imatinib, and axitinib as early-generation TKIs, as well as newer therapeutic agents, such as nintedanib, which have shown greater promise with better safety profile.
Preclinical studies involving VEGF-targeted inhibition presented promising results regarding antitumor activity (27). However, such trends did not translate in clinical studies involving gemcitabine in combination with bevacizumab or axitinib, as there was no significant OS difference observed (29,30). Therefore, it is imperative to extend the scope of antiangiogenic drugs considered beyond targeting only VEGF to inhibit angiogenesis more effectively (28).
Sunitinib, a multikinase inhibitor targeting VEGFR, platelet-derived growth factor receptors (PDGFR), colony-stimulating factor receptor (CSFR) and the stem cell factor receptor (c-KIT), demonstrated effectiveness in blocking angiogenesis in the treatment of pancreatic neuroendocrine tumors, showing improvements in OS (31). However, sunitinib’s efficacy has shown to be only temporary, as mechanisms of resistance have developed against this treatment with tumor hypoxia (32). In PDAC murine xenografts, sunitinib demonstrated antitumor activity as monotherapy and in combination with gemcitabine (33). Sorafenib, another multitarget TKI that blocks B-Raf, VEGFR, PDGFR, c-KIT and RET, demonstrated promising results in combination with gemcitabine in preclinical studies (34), but this combination was inactive in a clinical study (35). Nintedanib, a triple angiokinase inhibitor that targets VEGFR, PDGFR and FGFR, has shown significant antitumor response in preclinical models of several solid tumors (36). Treatment of PDAC tumors in xenograft models with nintedanib monotherapy or in combination with gemcitabine demonstrated significant anti-tumor activity (37). Nintedanib is currently under clinical investigation in combination with nab-paclitaxel plus gemcitabine for advanced PDAC (38).
More broadly, although antiangiogenic therapies presented some potential in preclinical contexts, there is limited evidence of their effectiveness in clinical studies of PDAC. The factors of drug resistance such as tumor hypoxia as well as alternative mechanisms of angiogenesis further complicate utilizing antiangiogenic therapies for this disease. Vascular mimicry presents an alternative mechanism of angiogenesis, as this allows for the building of compensatory vascular networks that evade antiangiogenic therapies (27,32). Antiangiogenic therapies can reduce the effectiveness of other treatment modalities, as they have been shown to compromise blood vessel structures, diminishing the efficacy of drug delivery to tumors (27,28). An important consideration for improving antiangiogenic therapies in personal treatment contexts is a better understanding of biomarkers, as these can provide information regarding which patients could have better treatment outcomes with these therapies (27,32).
DNA repair targeted therapy
Although cancer cells have inherently damaged DNA, they can block and repair DNA damage induced by therapeutic agents. Accordingly, DNA repair targeted therapy in cancerous cells is directed towards those repair mechanisms specifically involved in fixing DNA damage caused by anticancer drugs. Targeting DNA repair processes presents a promising consideration in targeted therapies for cancers, including PDAC (39) (Figure 2).
Gemcitabine is not only strongly implicated in discerning new standards of combination chemotherapy modalities in PDAC, but it is also recognized as a DNA damaging agent. Therefore, consideration of DNA damage repair inhibitors is important, as such agents could complement the effects of gemcitabine, allowing such DNA damage to remain in PDAC cells, eventually leading to apoptosis (40). Two ways of preventing the repair of such DNA damage are (I) blocking the function of cell cycle regulators to allow cancer cells with DNA damage to continue through the cell cycle process and (II) stopping the process of DNA repair directly. In PDAC, cell cycle inhibitors such as CHK1, WEE1, and ATR kinase inhibitors have shown varying levels of potential when tested in combination with gemcitabine. CHK1 inhibitors have presented as relatively ineffective (41), while WEE1 and ATR kinase inhibitors have shown some promising results in inhibiting tumor growth in xenograft models, but such research is still in its early phases (42-44). Relative to these cell cycle inhibitors, poly adenosine diphosphate-ribose polymerase (PARP) inhibitors target PARP proteins, which have DNA repair as one of their cellular functions. BRCA proteins play an important role in DNA damage repair (40). BRCA1/2 gene mutations are commonly implicated in cases of familial PDAC, which make up 5–17% of PDAC cases (45). When BRCA1/2 genes are mutated, homology-directed DNA repairs are compromised, and PARP acts as a substitute mechanism to maintain genomic integrity; consequently, the cells become very sensitive to PARP inhibitors. Olaparib (a PARP inhibitor), both alone and in combination with gemcitabine, has shown effectiveness in improving OS in PDAC patients having the BRCA mutation (46,47). Olaparib has also shown promising results in combination with bevacizumab in other cancers, whether the BRCA mutation was present or not, presenting possible relevance of this combination as a potential treatment in PDAC (48).
Another area of relevance of DNA damage repair inhibitors in developing therapies for PDAC involves its combination with radiotherapy. Utilization of radiation/chemoradiation has demonstrated potential for improving patient survival outcomes with regards to resectable as well as unresectable PDAC cases (49). Administration of chemoradiation preceding surgical resection has improved OS in patients with PDAC, although such survival benefit has been shown in limited contexts with smaller-scale studies (50-53). Given that radiotherapy results in DNA damage in cancer cells, utilizing DNA damage repair inhibitors to prevent these cells from repairing this damage can facilitate greater radiosensitization, making these cancer cells more vulnerable to radiotherapy (54,55). In PDAC xenograft models, several DNA damage repair inhibitors such as CHK1 inhibitors (AZD7762, MKK8776), ATR inhibitor (VE-822), PARP inhibitors (olaparib, veliparib) and WEE1 inhibitor (AZD1175), demonstrated sensitization effects to radiation/chemoradiation (44,56-60). The combination approach of radiotherapy with DNA damage repair inhibitors is in the early phases of PDAC clinical studies.
KRAS pathway inhibitors
Activating KRAS mutation is the most frequent mutation (>95%) in PDAC, and it is associated with the initiation, progression, and maintenance of PDAC (16). KRAS is a small GTPase that cycles between active GTP-bound and inactive GDP-bound forms. KRAS mutation occurs at three primary locations: glycine-12 (G12), glycine-13 (G13), or glutamine-61 (Q61). Activating KRAS mutation results in many oncogenic signaling pathways, including the MAPK pathway and the PI3K-AKT-mTOR pathway. PDAC is particularly addicted to KRAS mutation, further emphasizing the importance of KRAS and its related pathways as potential targets in this disease.
Therapies that directly target KRAS have been challenging to study and evaluate, one reason being the structure of the KRAS protein, which has a smooth surface, which in turn is not complimentary towards inhibitors directly binding to its surface (61). Other challenges associated with direct targeting of the KRAS protein include its similarity to a large number of other proteins involved with GDP/GTP binding, which makes specific targeting of KRAS more difficult, as well as the high affinity of KRAS for GDP/GTP and the high cellular concentrations of GDP/GTP, which diminish the efficacy of a potential direct KRAS inhibitor (62). Consequently, research involving KRAS pathway inhibitors has focused on targeting downstream effector pathways, such as MAPK and PI3K-AKT pathways (63).
Regarding the MAPK pathway, MEK inhibitors have been more widely evaluated relative to RAF and ERK inhibitors, as MEK kinases have critical functions in regulating and promoting cancer cell proliferation and tumorigenic growth (64). RAF inhibitors, such as vemurafenib and dabrafenib, proved challenging to implement in cancer treatment modalities because RAF inhibition has been demonstrated to be overcome by developed resistive mechanisms and/or paradoxical activation of downstream kinases by alternative mechanisms (65,66). Research involving ERK inhibitors has been more limited in scope, although a study reported promising antitumor effects of these inhibitors along with inhibition of autophagy in pancreatic cancer preclinical models (67). MEK inhibitors, specifically trametinib, have shown potential in PDAC. In a phase II clinical trial, trametinib combination with gemcitabine presented a 1.7-month improvement in OS compared with gemcitabine monotherapy in PDAC patients (68). In a preclinical PDAC study, trametinib demonstrated additive antitumor response in combination with nab-paclitaxel plus gemcitabine chemotherapy (69).
PI3K-AKT signaling is greatly implicated in the development and survival of cancerous cells, so this pathway presents a pertinent target for cancer treatments (70,71). PI3K inhibitors, such as BKM120 and BAY 80-6946, have been evaluated in solid tumors in clinical contexts (72,73). Additionally, MK-2206, an AKT inhibitor, has demonstrated pertinence regarding PDAC targeted therapies (74,75). AKT inhibition in combination with gemcitabine has shown promising outcomes in preclinical PDAC models (76). Recently, Awasthi et al. demonstrated that the standard chemotherapy response of PDAC can be enhanced through dual targeting of PI3K and MAPK signaling by MK-2206 and trametinib, respectively (77). Thus, PI3K-AKT pathway inhibition has the potential to be complementary towards enhancing other forms of PDAC targeted therapies (70).
An alternate approach to target the KRAS pathway uses kinases and activators upstream of the KRAS protein. The Met kinase activates KRAS and its subsequent downstream pathways, and it is often overexpressed in many cancers including PDAC (78,79). Onartuzumab, a Met inhibitor, demonstrated potential as an inhibitor of the KRAS pathway; however, further research is needed to establish Met inhibitors as being therapeutically effective in this disease (80).
Anti-stromal therapies
The PDAC microenvironment, the desmoplastic stroma, is composed of a heterogeneous variety of cell types, such as PSCs, fibroblasts, endothelial cells, immune cells, as well as non-cellular extracellular matrix (ECM) components such as collagen and growth factors (81). PDAC cells release several factors that stimulate the stroma, and stromal cells release several mitogenic/oncogenic substances that stimulate PDAC progression, invasion, and therapy resistance (82). This tumor microenvironment not only creates a hypoxic environment that is detrimental to chemotherapy delivery and radiotherapy but also releases growth factors and cytokines which further the growth of the desmoplastic stroma (81,82). Further, PDAC epithelial and stromal compartments interact to potentiate tumor aggressiveness. Thus, the therapeutic potential of targeting this dense desmoplastic stroma was evaluated in advancing PDAC therapy (81).
The Sonic hedgehog (Shh) pathway induces PSCs to become activated, which promotes greater growth of the stroma microenvironment. A preclinical study using the Shh pathway inhibitor saridegib (IPI-926) in combination with gemcitabine demonstrated promising results (83). Although phase 1 studies showed that this combination was somewhat tolerable, issues regarding toxicity arose in later clinical trials as its immense effects on depleting the stroma did not improve OS in long-term considerations (84,85). Given the complexity of the desmoplastic stroma, although it promotes PDAC growth in many ways, it also has functions that limit PDAC progression, such as restraining tumor growth. Therefore, an inordinate focus on the destruction of the desmoplastic stroma, using the Shh pathway inhibitor, can have repercussions with complete removal of the stroma, which could facilitate a more aggressive growth of this disease, thus decreasing OS. Therefore, future studies on anti-stromal therapies shifted their focus away from completely depleting the desmoplastic stroma (81).
Another pertinent consideration regarding anti-stromal targeted therapies focused on hyaluronan, a glycosaminoglycan present in the desmoplastic stroma ECM. Hyaluronan levels are much higher in PDAC tissues relative to healthy pancreatic tissues, and it has been correlated with aggressive tumor growth and therefore reduced OS (86). Depletion of hyaluronan with pegylated hyaluronidase (PEGPH20) showed some promise in PDAC, specifically in patients with higher levels of hyaluronan (87). Unfortunately, a recent randomized phase III trial of PEGPH20 plus NPT + Gem did not show any improvement in OS or progression-free survival (PFS) of PDAC patients (88).
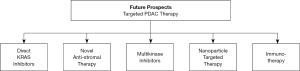
Future prospects of targeted therapies for pancreatic cancer
Direct KRAS inhibitors
Based on the critical importance of the KRAS pathway in PDAC as well as difficulties associated with its direct and downstream targeting, there are ongoing efforts to have a direct inhibitor of this pathway (Figure 3). There are different variations of the oncogenic KRAS mutation, which differ in prevalence depending on the cancer type considered. For instance, G12D, G12V, and G12R KRAS mutation isoforms are more common in PDAC, whereas the G12C mutation isoform is more common in NSCLC but extremely rare in PDAC (16). Consequently, the drugs AMG-510 and MRTX-849 that are focused towards targeting the G12C KRAS mutation isoform are less applicable as direct KRAS inhibitors in PDAC (89,90). Recently, pan-KRAS inhibitors, such as BI-1701963 and BAY-293, which address all KRAS mutation isoforms more broadly, present greater applicability in PDAC. BI-1701963 and BAY-293 are inhibitors of the protein son of sevenless homolog 1 (SOS1), which activates KRAS, inclusive of its multiple oncogenic mutation isoforms. These direct KRAS inhibitors are emerging and receiving greater attention for clinical PDAC therapy.
Another approach utilizing direct KRAS inhibitors involves disruption of the localization of KRAS proteins to plasma membranes. One example of this approach is farnesyltransferase inhibitors (FTIs), as farnesyltransferase causes post-translational modification of KRAS that assists with its association with the plasma membrane and interactions with other activating proteins (91). In a phase III study, tipifarnib, an FTI, when evaluated in combination with gemcitabine, did not demonstrate any significant benefit (92). One possible reason for this ineffectiveness could be the presence of additional compensating lipid modification mechanisms, allowing for KRAS localization on plasma membranes regardless of FTIs (93). However, FTIs could present alternative treatment benefits with regards to inhibiting cytokine secretion which promotes inflammation and supports the tumor microenvironment (63). Other inhibitors that have been evaluated regarding the association of KRAS to the plasma membrane are deltarasin and salirasib. The protein phosphodiesterase 6 delta (PDEδ) helps the KRAS protein localize at the plasma membrane, so deltarasin, an inhibitor that blocks this interaction, was evaluated and showed promise in preclinical studies (94,95). Additionally, studies involving gemcitabine in combination with salirasib, which displaces Ras proteins from plasma membranes, have shown promising results, both in preclinical and clinical contexts (96). Therefore, additional research with the combination of gemcitabine with salirasib is needed to better assess the potential efficacy of salirasib as an inhibitor of KRAS.
Novel anti-stromal therapies
Based on the detrimental effects of initial anti-stromal therapies in PDAC, novel approaches have shifted their focus towards modulating the PDAC stroma in a more conservative, balanced manner (81). Additionally, exploring novel anti-stromal therapies is also imperative due to toxicities associated with initial anti-stromal therapies, including nausea and vomiting, muscle spasms, fatigue, and impaired or altered sense of taste (84,97).
Activated PSCs play an integral role in the PDAC stroma construction, as they are implicated in the deposition of ECM proteins such as collagen, laminin, fibronectin, and elastins, all of which bolster and contribute to the fibrotic stroma (81,98). Thus, therapeutic inhibition of PSCs activation has the potential to improve PDAC therapy. A novel approach involves analogs of fat-soluble vitamins, vitamins A and D. One characteristic feature of activated PSCs is their lack of cytoplasmic retinol containing lipid droplets. Loss of these lipid droplets enhances the activity of PSCs, which is further amplified as limited, impeded functionality of the pancreas and its secretions also diminishes levels of vitamins A and D. Vitamin A analogs have demonstrated reduced activity of PSCs, causing decreased PDAC cell proliferation (99). Vitamin D analogs have demonstrated high binding affinities to PSCs, which have many vitamin D receptors. Recent studies have shown the antitumor benefits of calcipotriol, a safe vitamin D analog, in combination with gemcitabine, with modulation of the PDAC stroma to be less reactive and more passive. Such reprogramming of the PDAC stroma has shown improved OS in mice with improvement in the delivery of chemotherapeutic drugs (100).
Recently, miRNAs are receiving more attention as novel anti-stromal therapies. A miRNA with potential relevance for PDAC is miR-21, which promotes fibrosis. As elevated amounts of miR-21 are implicated in PDAC tumors, they present potential diagnostic and therapeutic opportunities in this disease (101). Another miRNA, miR-29, has been implicated in PDAC. The loss of miR-29 has been reported in activated PSCs and fibroblasts causing increased ECM deposition and further stromal growth. Therefore, overexpression of miR-29 in activated PSCs has the potential to reduce stromal density and improve PDAC therapy (98). As miRNAs have shown promise in regulating fibrotic proteins and stroma to diminish tumor progression with little to no toxicity, miRNA-based targeted therapies have great potential for utilization in PDAC therapy.
Novel small-molecule inhibitors
Since many different signaling pathways are implicated in the progression of PDAC, consideration of agents that can inhibit multiple oncogenic pathways simultaneously represents a valuable approach to future targeted therapies. Evaluation of multikinase inhibitors for potential use in such therapies has demonstrated varying levels of efficacy.
MK2461, a multikinase inhibitor, has demonstrated efficacy in preclinical studies for its interference with interactions between PSCs and PDAC cells (102). MK2461 targets MET and PDGFRβ, both of which are present in PSCs and facilitate their interactions with other PDAC cells. Higher levels of MET and PDGFRβ in PSCs are implicated in increased PDAC progression (102). Since excessively high levels of MET expression indicate greater invasiveness and aggressiveness in PDAC, utilization of MK2461 has immense potential in future clinical studies, as this drug has shown attenuation of pancreatic tumor progression without significant toxicity effects (102). Evaluation of niraparib, a PARP inhibitor, has potential in PDAC patients where the disease possesses cellular difficulties regarding responsiveness in repairing DNA damage (103). CPI-613, a metabolic inhibitor, focuses on alterations of enzymatic activity involved in mitochondrial functions in tumor cells, presenting potential as a therapeutic agent because of its specific targeting of mitochondrial activity in tumor cells. CPI-613 is being evaluated in combination with FOLFIRINOX in PDAC patients as well, with important consideration placed towards the evaluation of a tolerable dose of CPI-613 (104). BEY-1107, a cyclin-dependent kinase (CDK) inhibitor, is currently under clinical investigation regarding its safety and efficacy in PDAC patients, both as a single agent as well as in combination with gemcitabine (105,106). Galunisertib is a therapeutic agent presenting strong selectivity and efficacy towards inhibition of TGFβ receptor 1 (TGFβR1). TGFβ is involved in tumorigenic growth and metastatic progression through multiple mechanisms such as cellular proliferation, angiogenesis, and stromal management, so TGFβ pathways present promising therapeutic targets (107). Galunisertib is currently being evaluated as monotherapy and in combination with gemcitabine in PDAC patients, presenting positive potential with only slight additional toxicity effects when administered concurrently with gemcitabine (108). Apatinib, a novel agent that inhibits VEGFR2, PDGFRβ and c-kit, is very promising in PDAC, as VEGFR2 plays a significant role in angiogenesis and metastasis in this disease (109-111). In addition to reducing tumor angiogenesis, apatinib also decreases tumor cell proliferation and induces apoptosis in PDAC cells (112). Also, apatinib is particularly appealing relative to previously considered antiangiogenic approaches, as it presents a greater capability towards improving OS in cancer patients (110). As a potential targeted agent for use in PDAC treatment modalities, further research and clarification are needed with regards to apatinib’s applicability to PDAC patients.
Nanoparticle formulated targeted therapies
As the desmoplastic PDAC stroma presents a barrier to therapeutic agents, utilization of nanoparticles can lead to enhanced permeability, retention, and accumulation of anti-cancer drugs within tumors, conducive to increasing their efficacy (113). Nanoparticles have been used for improved delivery of chemotherapy drugs, combination treatments with multiple drugs, and small interfering RNA (siRNA)-derived therapeutics. siRNAs present potential for targeted treatment, as they safely facilitate suppression of gene expression, such as the KRAS gene, implicated in PDAC progression while not directly interfering with DNA. Nanoparticles can enable the delivery of siRNAs as therapeutic agents to PDAC cells and tumors.
Nanoparticle formulated targeted drugs have been shown to improve the efficacy of chemotherapeutic drugs, such as gemcitabine (114). EGFR-targeted nanoparticles in combination with gemcitabine improved treatment specificity and efficacy by delivering drugs near tumors, thereby improving cytotoxicity effects of gemcitabine at lower concentrations (115). Overall, nanoparticle-formulated targeted therapies present a direct mechanism that can improve not only the delivery of chemotherapeutic drugs to tumors but also their effectiveness in reducing tumor cell proliferation and tumor growth. Prabhuraj et al. demonstrated that administration of curcumin with gemcitabine via mesoporous silica nanoparticles (MSN) has additive antitumor effects in xenograft models and greater cytotoxicity in facilitating greater cell death among PDAC cells. As the application of curcumin presents enhanced effects of gemcitabine on PDAC, this also shows how the use of nanoparticles can complement existing chemotherapeutic agents (116).
Another combination therapy using nanoparticles, a Shh inhibitor, cyclopamine (CPA), and a chemotherapy drug paclitaxel (PTX) with a polymeric micelle formulation (M-CPA/PTX), has demonstrated improved antitumor response in PDAC preclinical studies by simultaneous remodeling of stroma by CPA and cytotoxic effects of PTX on tumor cells (117). These combination therapeutic strategies involving nanoparticles have a high potential for future PDAC therapy.
Immunotherapies
Immunotherapy approaches have faced significant challenges as potential targeted therapies for PDAC. Prominent stroma in the PDAC microenvironment presents a significant barrier to immunotherapies in multiple ways (118). The heterogeneous composition of the stroma with multiple cell types such as immunosuppressive myeloid cells and CAFs, prevents the development and activation of T-cells, creating a disadvantageous tumor microenvironment for immune responses to take effect (118). Beyond the stroma, T-regulatory cells (T-regs) tend to cause inhibitory effects localized at lymph nodes implicated with PDAC, limiting the efficacy of cytotoxic T-cells. Additionally, tumor-associated macrophages (TAMs) have been shown to facilitate tumor growth while also preventing cytotoxic T-cells from localizing into areas of tumors. The presence of CD4+ and CD8+ T-cells (which has shown the correlation with improvements in overall patient survival) into the PDAC microenvironment and subsequently the tumor itself are significantly limited by such inhibitory factors. Such immunosuppressive mechanisms in the PDAC microenvironment present immense challenges to potential targeted therapies involving immunotherapeutic considerations (118). Some approaches that have been utilized with immunotherapies include monoclonal antibody therapies, targeting immunosuppressive cells, adoptive cell therapy/transfer (ACT), and vaccines.
Targeting immune-checkpoint proteins, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) with ipilimumab and tremelimumab, or programmed cell death protein-1 (PD-1) with nivolumab has shown disappointing results as monotherapies in PDAC patients (119-121). Regardless, there is some potential for the combination of immune checkpoint inhibitors with other treatments such as chemotherapy and radiotherapy. Ipilimumab combination with gemcitabine has facilitated promising survival outcomes in PDAC patients (122,123), while tremelimumab, similarly, with gemcitabine, has demonstrated potential efficacy with manageable toxicity (124). Additionally, pembrolizumab, a PD-1 inhibitor, has shown considerable efficacy in combination with NPT + Gem (125). Another approach with immune checkpoint inhibitors in PDAC involves their administration with cancer vaccines, which has demonstrated some promise with improvements in greater T cell response (126).
Immunotherapy considerations present particular pertinence in tumors in which there are higher levels of microsatellite instability (MSI-H) facilitated by a defective DNA mismatch repair (dMMR) system, as such tumors are more vulnerable to and affected by immune system targeting. This is due to the increased prevalence of mutations in these cases, which in turn facilitates greater amounts of antigen production, inflammatory response, and stimulation of T cells (127). In PDAC, however, the prevalence of MSI-H/dMMR is very low (<2%), indicating its limited applicability (128). Despite the rare occurrence of the MSI-H/dMMR phenotype in PDAC, it is recommended to test MSI-H and/or dMMR for advanced PDAC and treat patients who test positive with pembrolizumab as second-line therapy (129,130). Based on the limited data available about MSI-H/dMMR frequency in PDAC and the potential of immune-checkpoint inhibitors in this subgroup, future studies to improve MSI-H/dMMR detection methods might benefit select PDAC patients from immunotherapy.
Monoclonal antibody therapeutics aim to target something predominately expressed in PDAC which will, in turn, cause not only greater levels of cytotoxicity towards cancer cells but also block immunosuppressive signaling to enhance the activity of cells implicated in anti-tumor efficacy (131). One such target, mesothelin (MSLN), is overexpressed in almost all PDAC cells and implicated in adverse patient outcomes (132). Targeting MSLN using the antibody amatuximab showed demonstrable safety but it did not show any significant improvement in patient outcomes (133,134). However, other monoclonal antibody therapy targets, such as KRAS mutations, could be promising in future studies (131).
Direct targeting of immunosuppressive cells, such as T-regs, by chemotherapeutic drugs, such as cyclophosphamide and gemcitabine, has been attempted in PDAC (135). Although diminishing the presence of immunosuppressive cells does not necessarily impede PDAC progression directly, such therapeutic agents can enhance the anti-tumor functioning and efficacy of other modalities, such as CD40 agonists (136).
Utilizing ACT as an immunotherapy modality also shows promise in PDAC, as genetically engineered T-cells through ACT methods enhance anti-tumor activity by supporting the functioning of CD4+ and CD8+ T-cells (131). However, some difficulties in ACT-based immunotherapy approaches are the high degree of patient-centered personalization with genetic engineering, requiring immense time and effort, as well as the emergence of unanticipated resistances despite initial effective results (63).
Cancer vaccines GVAX and CRS 207 are currently under investigation for PDAC. The GVAX cancer vaccine utilizes genetically modified PDAC cells, while CRS 207 uses a recombinant bacterial basis using the Listeria bacterial strain (63,131). These vaccines have demonstrated safety in their use and improved survival when used in combination with each other (137). However, such survival benefit was of a lesser degree relative to standard chemotherapeutic regimens, so further research is needed to determine if such efficacy implicated in cancer vaccines can demonstrate more promising survival improvements in PDAC.
Conclusions
The prognosis and survival rate for patients diagnosed with PDAC remain particularly dire. The American Cancer Society reports that while the 5-year survival rate for patients diagnosed with all PDAC stages is dismal at 9%, it reduces to only 2.9% for patients with a stage 4 diagnosis. Unfortunately, PDAC patients are typically diagnosed at a late-stage, and approximately 50% of these patients receive a stage 4 diagnosis. Improving survival rates and remission for these patients is essential. Cytotoxic chemotherapy regimens, the current clinical standard of care for PDAC, have led to moderate improvement in OS; however, PDAC remission or cure is still elusive. Targeted therapies for PDAC are therefore potentially promising avenues that can lead to improved efficacy with reduced toxicity. Targeting specific molecular targets or oncogenes involved in PDAC progression may also enable better assessment of treatment response. Along with KRAS pathway inhibitors, other targeted therapies that have been investigated for PDAC treatment include antiangiogenic, DNA repair, and traditional anti-stromal therapies. Future considerations regarding PDAC targeted therapy are direct KRAS inhibitors, novel anti-stromal therapies, small molecule multikinase inhibitors, nanoparticle-based therapies, and immunotherapies. Overall, more research is essential for the development of novel targeted therapies with reduced toxicities that can lead to improved survival rates in PDAC patients and possible remission of this intractable disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Targeted Therapy in Gastrointestinal Cancers”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-21. The series “Targeted Therapy in Gastrointestinal Cancers” was commissioned by the editorial office without any funding or sponsorship. NA served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Digestive Medicine Research from Dec 2019 to Dec 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Hidalgo M, Cascinu S, Kleeff J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 2015;15:8-18. [Crossref] [PubMed]
- Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol 2020;17:108-23. [Crossref] [PubMed]
- Mas L, Schwarz L, Bachet JB. Adjuvant chemotherapy in pancreatic cancer: state of the art and future perspectives. Curr Opin Oncol 2020;32:356-63. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-703. [Crossref] [PubMed]
- Krempley BD, Yu KH. Preclinical models of pancreatic ductal adenocarcinoma. Chin Clin Oncol 2017;6:25. [Crossref] [PubMed]
- Philip PA, Mooney M, Jaffe D, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol 2009;27:5660-9. [Crossref] [PubMed]
- Qian Y, Gong Y, Fan Z, et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol Oncol 2020;13:130. [Crossref] [PubMed]
- Ahmed S, Bradshaw AD, Gera S, et al. The TGF-β/Smad4 signaling pathway in pancreatic carcinogenesis and its clinical significance. J Clin Med 2017;6:5. [Crossref] [PubMed]
- Perkhofer L, Gout J, Roger E, et al. DNA damage repair as a target in pancreatic cancer: state-of-the-art and future perspectives. Gut 2021;70:606-17. [Crossref] [PubMed]
- Yonemori K, Kurahara H, Maemura K, et al. MicroRNA in pancreatic cancer. J Hum Genet 2017;62:33-40. [Crossref] [PubMed]
- Bryant KL, Mancias JD, Kimmelman AC, et al. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci 2014;39:91-100. [Crossref] [PubMed]
- Cowan RW, Maitra A. Genetic progression of pancreatic cancer. Cancer J 2014;20:80-4. [Crossref] [PubMed]
- Gu D, Schlotman KE, Xie J. Deciphering the role of hedgehog signaling in pancreatic cancer. J Biomed Res 2016;30:353-60. [PubMed]
- Wang MT, Holderfield M, Galeas J, et al. K-Ras promotes tumorigenicity through suppression of non-canonical Wnt signaling. Cell 2015;163:1237-51. [Crossref] [PubMed]
- Mann KM, Ying H, Juan J, et al. KRAS-related proteins in pancreatic cancer. Pharmacol Ther 2016;168:29-42. [Crossref] [PubMed]
- Oliveira-Cunha M, Newman WG, Siriwardena AK. Epidermal growth factor receptor in pancreatic cancer. Cancers (Basel) 2011;3:1513-26. [Crossref] [PubMed]
- Trajkovic-Arsic M, Kalideris E, Siveke JT. The role of insulin and IGF system in pancreatic cancer. J Mol Endocrinol 2013;50:R67-74. [Crossref] [PubMed]
- Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer 2003;2:8. [Crossref] [PubMed]
- Pothula SP, Xu Z, Goldstein D, et al. Targeting the HGF/c-MET pathway: stromal remodelling in pancreatic cancer. Oncotarget 2017;8:76722-39. [Crossref] [PubMed]
- Schnittert J, Bansal R, Mardhian DF, et al. Integrin α11 in pancreatic stellate cells regulates tumor stroma interaction in pancreatic cancer. FASEB J 2019;33:6609-21. [Crossref] [PubMed]
- Nishida N, Yano H, Nishida T, et al. Angiogenesis in cancer. Vasc Health Risk Manag 2006;2:213-9. [Crossref] [PubMed]
- Annese T, Tamma R, Ruggieri S, et al. Angiogenesis in pancreatic cancer: pre-clinical and clinical studies. Cancers (Basel) 2019;11:381. [Crossref] [PubMed]
- Craven KE, Gore J, Korc M. Overview of pre-clinical and clinical studies targeting angiogenesis in pancreatic ductal adenocarcinoma. Cancer Lett 2016;381:201-10. [Crossref] [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [Crossref] [PubMed]
- Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 2011;12:256-62. [Crossref] [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [Crossref] [PubMed]
- Pozas J, San Román M, Alonso-Gordoa T, et al. Targeting angiogenesis in pancreatic neuroendocrine tumors: resistance mechanisms. Int J Mol Sci 2019;20:4949. [Crossref] [PubMed]
- Awasthi N, Schwarz MA, Schwarz RE. Antitumour activity of sunitinib in combination with gemcitabine in experimental pancreatic cancer. HPB (Oxford) 2011;13:597-604. [Crossref] [PubMed]
- Awasthi N, Zhang C, Hinz S, et al. Enhancing sorafenib-mediated sensitization to gemcitabine in experimental pancreatic cancer through EMAP II. J Exp Clin Cancer Res 2013;32:12. [Crossref] [PubMed]
- Kindler HL, Wroblewski K, Wallace JA, et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs. 2012;30:382-6. [Crossref] [PubMed]
- Awasthi N, Schwarz RE. Profile of nintedanib in the treatment of solid tumors: the evidence to date. Onco Targets Ther 2015;8:3691-701. [Crossref] [PubMed]
- Awasthi N, Hinz S, Brekken RA, et al. Nintedanib, a triple angiokinase inhibitor, enhances cytotoxic therapy response in pancreatic cancer. Cancer Lett 2015;358:59-66. [Crossref] [PubMed]
- Beg M, Pilarski S. A phase 1b and pharmacodynamic study of nintedanib monotherapy for advanced pancreatic cancer. In: Center UoTSM, editor. ClinicalTrials.Gov Identifier: NCT02902484. 2020.
- Gavande NS, VanderVere-Carozza PS, Hinshaw HD, et al. DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol Ther 2016;160:65-83. [Crossref] [PubMed]
- Miller AL, Garcia PL, Yoon KJ. Developing effective combination therapy for pancreatic cancer: an overview. Pharmacol Res 2020;155:104740 [Crossref] [PubMed]
- Laquente B, Lopez-Martin J, Richards D, et al. A phase II study to evaluate LY2603618 in combination with gemcitabine in pancreatic cancer patients. BMC Cancer 2017;17:137. [Crossref] [PubMed]
- Rajeshkumar NV, De Oliveira E, Ottenhof N, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res 2011;17:2799-806. [Crossref] [PubMed]
- Wallez Y, Dunlop CR, Johnson TI, et al. The ATR inhibitor AZD6738 synergizes with gemcitabine in vitro and in vivo to induce pancreatic ductal adenocarcinoma regression. Mol Cancer Ther 2018;17:1670-82. [Crossref] [PubMed]
- Fokas E, Prevo R, Pollard JR, et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis 2012;3:e441 [Crossref] [PubMed]
- Wong W, Raufi AG, Safyan RA, et al. BRCA mutations in pancreas cancer: spectrum, current management, challenges and future prospects. Cancer Manag Res 2020;12:2731-42. [Crossref] [PubMed]
- Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317-27. [Crossref] [PubMed]
- Bendell J, O'Reilly EM, Middleton MR, et al. Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Ann Oncol 2015;26:804-11. [Crossref] [PubMed]
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 2019;381:2416-28. [Crossref] [PubMed]
- Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J Gastrointest Oncol 2011;2:157-67. [PubMed]
- White RR, Tyler DS. Neoadjuvant therapy for pancreatic cancer: the Duke experience. Surg Oncol Clin N Am 2004;13:675-84. ix-x. [Crossref] [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [Crossref] [PubMed]
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. [Crossref] [PubMed]
- Snady H, Bruckner H, Cooperman A, et al. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer 2000;89:314-27. [Crossref] [PubMed]
- Yang SH, Kuo TC, Wu H, et al. Perspectives on the combination of radiotherapy and targeted therapy with DNA repair inhibitors in the treatment of pancreatic cancer. World J Gastroenterol 2016;22:7275-88. [Crossref] [PubMed]
- Waissi W, Paix A, Nicol A, et al. Targeting DNA repair in combination with radiotherapy in pancreatic cancer: a systematic review of preclinical studies. Crit Rev Oncol Hematol 2020;153:103060 [Crossref] [PubMed]
- Karnak D, Engelke CG, Parsels LA, et al. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin Cancer Res 2014;20:5085-96. [Crossref] [PubMed]
- Tuli R, Surmak AJ, Reyes J, et al. Radiosensitization of pancreatic cancer cells in vitro and in vivo through poly (ADP-ribose) polymerase inhibition with ABT-888. Transl Oncol 2014;7:439-45. [Crossref] [PubMed]
- Morgan MA, Parsels LA, Zhao L, et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res 2010;70:4972-81. [Crossref] [PubMed]
- Engelke CG, Parsels LA, Qian Y, et al. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor MK8776. Clin Cancer Res 2013;19:4412-21. [Crossref] [PubMed]
- Kausar T, Schreiber JS, Karnak D, et al. Sensitization of pancreatic cancers to gemcitabine chemoradiation by WEE1 kinase inhibition depends on homologous recombination repair. Neoplasia 2015;17:757-66. [Crossref] [PubMed]
- Aslan M, Shahbazi R, Ulubayram K, et al. Targeted therapies for pancreatic cancer and hurdles ahead. Anticancer Res 2018;38:6591-606. [Crossref] [PubMed]
- McCormick F. K-Ras protein as a drug target. J Mol Med (Berl) 2016;94:253-8. [Crossref] [PubMed]
- Amanam I, Chung V. Targeted Therapies for Pancreatic Cancer. Cancers (Basel) 2018;10:36. [Crossref] [PubMed]
- Zhao Y, Adjei AA. The clinical development of MEK inhibitors. Nat Rev Clin Oncol 2014;11:385-400. [Crossref] [PubMed]
- Chan XY, Singh A, Osman N, et al. Role played by signalling pathways in overcoming BRAF inhibitor resistance in melanoma. Int J Mol Sci 2017;18:1527. [Crossref] [PubMed]
- Zhao X, Wang X, Fang L, et al. A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer. Cancer Lett 2017;402:61-70. [Crossref] [PubMed]
- Bryant KL, Stalnecker CA, Zeitouni D, et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med 2019;25:628-40. [Crossref] [PubMed]
- Infante JR, Somer BG, Park JO, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer 2014;50:2072-81. [Crossref] [PubMed]
- Awasthi N, Monahan S, Stefaniak A, et al. Inhibition of the MEK/ERK pathway augments nab-paclitaxel-based chemotherapy effects in preclinical models of pancreatic cancer. Oncotarget 2017;9:5274-86. [Crossref] [PubMed]
- Khan KH, Yap TA, Yan L, et al. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer 2013;32:253-65. [Crossref] [PubMed]
- Dienstmann R, Rodon J, Serra V, et al. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther 2014;13:1021-31. [Crossref] [PubMed]
- Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 2012;30:282-90. [Crossref] [PubMed]
- Patnaik A, Appleman LJ, Mountz JM, et al. A first-in-human phase I study of intravenous PI3K inhibitor BAY 80-6946 in patients with advanced solid tumors: Results of dose-escalation phase. J Clin Oncol 2011;29:3035. [Crossref]
- Wang Z, Luo G, Qiu Z. Akt inhibitor MK-2206 reduces pancreatic cancer cell viability and increases the efficacy of gemcitabine. Oncol Lett 2020;19:1999-2004. [Crossref] [PubMed]
- Hu C, Dadon T, Chenna V, et al. Combined inhibition of cyclin-dependent kinases (dinaciclib) and AKT (MK-2206) blocks pancreatic tumor growth and metastases in patient-derived xenograft models. Mol Cancer Ther 2015;14:1532-9. [Crossref] [PubMed]
- Massihnia D, Avan A, Funel N, et al. Phospho-Akt overexpression is prognostic and can be used to tailor the synergistic interaction of Akt inhibitors with gemcitabine in pancreatic cancer. J Hematol Oncol 2017;10:9. [Crossref] [PubMed]
- Awasthi N, Kronenberger D, Stefaniak A, et al. Dual inhibition of the PI3K and MAPK pathways enhances nab-paclitaxel/gemcitabine chemotherapy response in preclinical models of pancreatic cancer. Cancer Lett 2019;459:41-9. [Crossref] [PubMed]
- Furukawa T, Duguid WP, Kobari M, et al. Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol 1995;147:889-95. [PubMed]
- Ide T, Kitajima Y, Miyoshi A, et al. The hypoxic environment in tumor-stromal cells accelerates pancreatic cancer progression via the activation of paracrine hepatocyte growth factor/c-Met signaling. Ann Surg Oncol 2007;14:2600-7. [Crossref] [PubMed]
- Escorcia FE, Houghton JL, Abdel-Atti D, et al. ImmunoPET predicts response to Met-targeted radioligand therapy in models of pancreatic cancer resistant to Met kinase inhibitors. Theranostics 2020;10:151-65. [Crossref] [PubMed]
- Kota J, Hancock J, Kwon J, et al. Pancreatic cancer: stroma and its current and emerging targeted therapies. Cancer Lett 2017;391:38-49. [Crossref] [PubMed]
- Kanat O, Ertas H. Shattering the castle walls: Anti-stromal therapy for pancreatic cancer. World J Gastrointest Oncol 2018;10:202-10. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Jimeno A, Weiss GJ, Miller WH Jr, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res 2013;19:2766-74. [Crossref] [PubMed]
- Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med 2013;19:1410-22. [Crossref] [PubMed]
- Sato N, Kohi S, Hirata K, et al. Role of hyaluronan in pancreatic cancer biology and therapy: Once again in the spotlight. Cancer Sci 2016;107:569-75. [Crossref] [PubMed]
- Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol 2018;36:359-66. [Crossref] [PubMed]
- Van Cutsem E, Tempero MA, Sigal D, et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol 2020;38:3185-94. [Crossref] [PubMed]
- Lanman BA, Allen JR, Allen JG, et al. Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J Med Chem 2020;63:52-65. [Crossref] [PubMed]
- Hallin J, Engstrom LD, Hargis L, et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov 2020;10:54-71. [Crossref] [PubMed]
- Brunner TB, Hahn SM, Gupta AK, et al. Farnesyltransferase inhibitors: an overview of the results of preclinical and clinical investigations. Cancer Res 2003;63:5656-68. [PubMed]
- Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004;22:1430-8. [Crossref] [PubMed]
- Van Sciver RE, Lee MP, Lee CD, et al. A new strategy to control and eradicate "undruggable" oncogenic K-RAS-driven pancreatic cancer: molecular insights and core principles learned from developmental and evolutionary biology. Cancers (Basel) 2018;10:142. [Crossref] [PubMed]
- Zimmermann G, Papke B, Ismail S, et al. Small molecule inhibition of the KRAS-PDEδ interaction impairs oncogenic KRAS signalling. Nature 2013;497:638-42. [Crossref] [PubMed]
- Mottini C, Cardone L. Beyond the genomic mutation: rethinking the molecular biomarkers of K-RAS dependency in pancreatic cancers. Int J Mol Sci 2020;21:5023. [Crossref] [PubMed]
- Laheru D, Shah P, Rajeshkumar NV, et al. Integrated preclinical and clinical development of S-trans, trans-Farnesylthiosalicylic Acid (FTS, Salirasib) in pancreatic cancer. Invest New Drugs 2012;30:2391-9. [Crossref] [PubMed]
- Ko AH, LoConte N, Tempero MA, et al. A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 2016;45:370-5. [Crossref] [PubMed]
- Kwon JJ, Nabinger SC, Vega Z, et al. Pathophysiological role of microRNA-29 in pancreatic cancer stroma. Sci Rep 2015;5:11450. [Crossref] [PubMed]
- Froeling FE, Feig C, Chelala C, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 2011;141:1486-97, 97.e1-14.
- Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80-93. [Crossref] [PubMed]
- Mardin WA, Mees ST. MicroRNAs: novel diagnostic and therapeutic tools for pancreatic ductal adenocarcinoma? Ann Surg Oncol 2009;16:3183-9. [Crossref] [PubMed]
- Inoue K, Ohtsuka H, Tachikawa M, et al. MK2461, a multitargeted kinase inhibitor, suppresses the progression of pancreatic cancer by disrupting the interaction between pancreatic cancer cells and stellate cells. Pancreas 2017;46:557-66. [Crossref] [PubMed]
- Kasi A, Chalise P, Williamson SK, et al. Niraparib in metastatic pancreatic cancer after previous chemotherapy (NIRA-PANC): a phase 2 trial. J Clin Oncol 2019;37:TPS4168 [Crossref]
- Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol 2017;18:770-8. [Crossref] [PubMed]
- Sánchez-Martínez C, Lallena MJ, Sanfeliciano SG, et al. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: Recent advances (2015-2019). Bioorg Med Chem Lett 2019;29:126637 [Crossref] [PubMed]
- BeyondBio I. A single center, open-label, non-comparative, phase I/II clinical trial to assess the MTD, safety and efficacy of BEY1107 in monotherapy and in combination with gemcitabine in patient with locally advanced or metastatic pancreatic cancer. Interventional (Clinical Trial). ClinicalTrials.gov Identifier: NCT03579836. 2019.
- Yingling JM, McMillen WT, Yan L, et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-β receptor type I inhibitor. Oncotarget 2017;9:6659-77. [Crossref] [PubMed]
- Melisi D, Garcia-Carbonero R, Macarulla T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 2018;119:1208-14. [Crossref] [PubMed]
- Li CM, Liu ZC, Bao YT, et al. Extraordinary response of metastatic pancreatic cancer to apatinib after failed chemotherapy: a case report and literature review. World J Gastroenterol 2017;23:7478-88. [Crossref] [PubMed]
- Fathi Maroufi N, Rashidi MR, Vahedian V, et al. Therapeutic potentials of Apatinib in cancer treatment: Possible mechanisms and clinical relevance. Life Sci 2020;241:117106 [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- He K, Wu L, Ding Q, et al. Apatinib promotes apoptosis of pancreatic cancer cells through downregulation of hypoxia-inducible factor-1α and increased levels of reactive oxygen species. Oxid Med Cell Longev 2019;2019:5152072 [Crossref] [PubMed]
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 2008;7:771-82. [Crossref] [PubMed]
- Réjiba S, Reddy LH, Bigand C, et al. Squalenoyl gemcitabine nanomedicine overcomes the low efficacy of gemcitabine therapy in pancreatic cancer. Nanomedicine 2011;7:841-9. [Crossref] [PubMed]
- Singh A, Xu J, Mattheolabakis G, et al. EGFR-targeted gelatin nanoparticles for systemic administration of gemcitabine in an orthotopic pancreatic cancer model. Nanomedicine 2016;12:589-600. [Crossref] [PubMed]
- Prabhuraj RS, Mal A, Valvi SK, et al. Noninvasive preclinical evaluation of targeted nanoparticles for the delivery of curcumin in treating pancreatic cancer. ACS Applied Bio Materials 2020;3:4643-54. [Crossref]
- Zhao J, Wang H, Hsiao CH, et al. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials 2018;159:215-28. [Crossref] [PubMed]
- Ware MB, El-Rayes BF, Lesinski GB. Mirage or long-awaited oasis: reinvigorating T-cell responses in pancreatic cancer. J Immunother Cancer 2020;8:e001100 [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- AstraZeneca. A phase II, multi-center, open-label study of tremelimumab monotherapy in patients with advanced solid tumors. ClinicalTrials.gov Identifier: NCT02527434. 2020.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Mohindra NA, Kircher SM, Nimeiri HS, et al. Results of the phase Ib study of ipilimumab and gemcitabine for advanced pancreas cancer. J Clin Oncol 2015;33:e15281 [Crossref]
- Kalyan A, Kircher SM, Mohindra NA, et al. Ipilimumab and gemcitabine for advanced pancreas cancer: a phase Ib study. J Clin Oncol 2016;34:e15747 [Crossref]
- Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 2014;25:1750-5. [Crossref] [PubMed]
- Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs 2018;36:96-102. [Crossref] [PubMed]
- Schizas D, Charalampakis N, Kole C, et al. Immunotherapy for pancreatic cancer: a 2020 update. Cancer Treat Rev 2020;86:102016 [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Luchini C, Brosens LAA, Wood LD, et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications. Gut 2021;70:148-56. [Crossref] [PubMed]
- Sohal DPS, Kennedy EB, Khorana A, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:2545-56. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic adenocarcinoma, version 1.2019. J Natl Compr Canc Netw 2019;17:202-10. [Crossref] [PubMed]
- Fan JQ, Wang MF, Chen HL, et al. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol Cancer 2020;19:32. [Crossref] [PubMed]
- Montemagno C, Cassim S, Trichanh D, et al. 99mTc-A1 as a novel imaging agent targeting mesothelin-expressing pancreatic ductal adenocarcinoma. Cancers (Basel) 2019;11:1531. [Crossref] [PubMed]
- Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010;16:6132-8. [Crossref] [PubMed]
- Fujisaka Y, Kurata T, Tanaka K, et al. Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors. Invest New Drugs 2015;33:380-8. [Crossref] [PubMed]
- Tongu M, Harashima N, Monma H, et al. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother 2013;62:383-91. [Crossref] [PubMed]
- Winograd R, Byrne KT, Evans RA, et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res 2015;3:399-411. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
Cite this article as: Thomas AG, Awasthi N. Targeted therapy for pancreatic cancer: lessons learned and future opportunities. Dig Med Res 2021;4:32.