Biliary acids as promoters of colon carcinogenesis: a narrative review
Introduction
Biliary acids (BAs) are detergent-like amphipathic molecules synthesized in the liver from cholesterol, stored in the gallbladder, and released after food intake into the intestine. Nowadays the interest in BAs is increasing for gastroenterologists because of their critical role in several conditions such as liver and intestinal diseases (1). BAs are known to have multiple and significant functions: acting as a digestive surfactant that promotes the absorption of lipids, including fat-soluble vitamins, eliminating cholesterol and certain catabolites, participating in intestinal motility for fine regulation of bacterial flora. Almost fifty years ago, Berg noted that colorectal cancer (CRC) risk raised in the descendants who moved from rural to developed countries where the diet was characterized by meals enriched with saturated fatty acids and red meat. In fact, the close correlation with prolonged and increased consumption of saturated fatty acids and red meat has a crucial role in colorectal carcinogenesis (2). Recently, another cohort study investigating 55,487 Danish middle-aged men and women, suggested that CRC risk could arise without full adherence to healthy lifestyle recommendations (3).
Bayerdorffer’s control study confirms that deoxycholic acid (DCA) levels were significantly increased in colorectal adenomas patients (4) finding a positive and strong association between colorectal adenomas and serum DCA, this occurs when DCA derived directly to the unconjugated fraction who is formed in the colon (5), in addition, the bile discharging is stimulated by a high-fat diet that leads to increased concentrations of BAs over the upper limit physiological range (6).
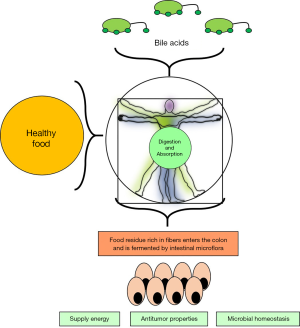
Various epidemiological nutrition studies show that subjects with high lipid and beef intake have increased levels of secondary bile acids like DCA and lithocholic acid (LCA) in feces, a similar pattern was seen in patients with CRC diagnosis (7,8). A high level of dietary fats stimulates increased primary bile acid production which forms secondary bile acids and these could be directly inductors of some gastrointestinal tumors or promoters that act on the growth of intestinal epithelium (9) (Figure 1).
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/dmr-21-23). Our review aims to analyze and summarize the possible effects of BAs as promoters of colon carcinogenesis.
Materials and methods
The research strategy is based on analyzing and reviewing principal recent research and articles regarding BAs and their influences on CRC. Another critical point has been to comprehend the physical, chemical, biological and clinical characteristics of secondary bile acids in the human. All downloaded papers were collected from PubMed Central, Medline, Scopus, CINAHL Complete, Google Scholar, Web of Science, and various open access journals.
The scientific core of this narrative review has been regrouped the principal articles of BAs physiology related to CRC disease, despite the limitation of narrative review because we didn’t analyze according to metanalysis criteria which have an incredible statistical impact.
The quality of our work has been that of to summary the principal role of BAs in physiology and pathophysiology because the scientific web sites are full of information that is sometimes different from each other, with this work we have tried to revise the main works that have the most scientific impact and bring them together in a review.
According to us it could be useful other prospective studies that aim to analyze properly BAs in the development of colon cancer, better if the choice of patients is made with exclusion criteria of all genetic impairment related to colon cancer such Lynch Syndrome, familial adenomatous polyposis.
Because it is very important to understand the real impact of BAs at the net of this genetic disease.
Discussing
Biological properties and functions of BAs
BAs were originally known only for their digestive role in the absorption of all types of dietary lipids and vitamins and in the emulsion process of the other biochemical molecules (10). More recently, they are considered to have a pleiotropic biological function: (I) into bile canaliculi, bile acids synthesis forms an osmotic pressure which is called as BA dependent-bile flow; (II) biliary lipids secretion is stimulated by bile acids (11) and their physical-chemical properties lead to the formation of mixed micelles along with phospholipids of bile and this leads to solubilization of some lipophilic compounds, cholesterol and to the emulsion of liposoluble vitamins leads to A-D-E-K vitamins absorption; (III) even free calcium is absorbed in intestine by BAs and this mechanism plays a crucial role in many biochemical reactions where calcium needed like muscle contraction, heart rate control and neuromuscular transmission. (12); (IV) releasing of cholecystokinin and the modulation of pancreatic enzyme secretion is under the control of BAs; (V) also the prevention of bacteria overgrowth in the small bowel is carried out by BAs which have a potent antimicrobial property (13,14).
The BAs’ role as endocrine and paracrine signaler has been validated by the discovery of farnesoid X receptor (FXR) (15-17) and TGR5, specific BA membrane receptors (18,19). In addition to other functions such as the synthesis regulation of their own hepatic enzymes and intestinal and hepatic transport, BAs are extremely important for the adaptive responses to liver injury and cholestasis (20-23).
Nevertheless, a relevant and vastly unexplored function of BAs could be represented by their role in the control of energy metabolism (24).
Synthesis of BAs
Cholesterol is the molecular precursor of BAs, whose synthesis involves two major pathways, named classical and alternative, in addition to several other minor pathways not equally characterized (25). The classical or neutral pathway runs only in the liver and his intermediate metabolites are neutral sterols which are involved in the synthesis of two primary BAs, cholic acid (CA) and chenodeoxycholic acid (CDCA). Cytosol organelles such as mitochondria, peroxisomes, and microsomes are involved at different levels in the CA and CDCA biosynthetic reactions (26,27).
Physical and chemical characteristics of BAs
BA molecules contain the non-equivalent hydroxyl groups but their physical and chemical characteristics are mainly conferred by the carboxylic acid group distributed across the main chain structure. The special chemical structure of this organic chain has inspired its employment in several areas of research, including novel antibiotics (28-30), chiral templates (31), new soft material (32,33), anion (34-36), artificial ion channels and cation receptors (37), drug targeting vehicles (38), dendrons (39), molecular baskets (40), scaffolds for combinatorial chemistry (41), new surfactants (42), and others (43,44).
“The solubilization of an insoluble compound” is the unique capacity of BAs that leads to lipid transport and cholesterol excretion into the intestinal tract with poor absorption of the preformed micelles (small lipidic aggregates of less than 10 monomers). The amphipathic properties are related to the presence of both a hydrophilic and a hydrophobic side: the former is composed of the carboxylic side chain and the hydroxyl group, both oriented towards the α-side, while the β-side orientation is influenced by the methyl group (45). This double-α and -β conformation confers a great surface activity where micelles are formed in aqueous solutions. In this way, the aggregates concentration is higher than the critical concentration value of a single monomer, these are known as critical micellar concentration (46). Freezing point measurements demonstrated that BAs act as 1:1 strong electrolytes below the critical micellar concentration (47).
The hydrophilic and hydrophobic properties differ within the different molecular types of bile salts, leading to different bile salts reactions with other substances depending on the balance between the various types of bile salts involved (48,49). Hydrophobic bile acids are potent inflammatory agents that cause injury to vital organs such as liver, intestine, and other tissues. Whereas hydrophilic bile acids are anti-inflammatory in nature. By inhibiting NF-κB nuclear translocation and antagonizing NF-κB-dependent induction of proinflammatory cytokines, bile acid activates FXR and TGR5 signaling pathways which suppress inflammation in macrophages, intestine, and hepatocytes (50-53).
Epidemiology of CRC in relation to BAs
According to the GLOBOCAN 2020 statistical data, more than 1.9 million new CRC (including anus) cases and 935,000 deaths were estimated to occur in 2020, representing about one in 10 cancer cases and deaths. Overall, CRC ranks third in terms of incidence, but second in terms of mortality. Incidence rates are approximately 4‐fold higher in transitioned countries compared with transitioning countries, but there is less variation in the mortality rates because of higher fatality in transitioning countries (54).
The appearance of CRC changes happens suddenly on populations migrating from low to high incidence countries: for example, the peak of CRC has already reached in people migrating from Japan to Hawaii (55). The “Japan-Hawaii migration” shows that the increase in CRC followed the moving population from “eastern diet-based fish” Country to “western diet-based meat” Country (56). BAs high level in feces indicates a major risk of CRC incidence, in these population (57-59) other data shows that association between epithelium colon exposition and increased levels of BAs lead to CRC, transforming BAs from physiological acids to potential tumor-promoting agents (60).
BAs as potential inducers of CSCs of the colon
The CSCs model proposes that a small fraction of CSCs proliferates within the tumor. These cells are capable of sustaining tumor growth and initiating carcinogenesis, as well as regenerating themselves (61).
Specific surface epitopes used to identify CSCs include CD133, CD166, EpCAM, CD44, CD24, and ALDHA1 (62-64). Some of those surface molecules are involved in tumor cell transformation, growth, and proliferation; moreover, CD44 and CD166 are associated with cross-mutation of primary CRC and increased risk of lymph node involvement and liver and lung metastatic progression (65).
Secondary BAs and CRC related to high lipids diet
The combination of low-fiber and high-fat levels in the diet is associated with increased levels of secondary BAs in feces and high incidence of CRC (66-68); moreover, increased fecal LCA/DCA ratio is associated with the presence of CRC (5,67-69). Over-consumption of a Western-style diet can represent a step linking BAs to CRC. This high-fat alimentary regimen brings excess calories, is enriched with highly saturated fats and processed carbohydrates but lacks mono-polyunsaturated fatty acids and plant-derived proteins and fiber. Following a high-fat diet, therefore, abnormally high levels of secondary BAs might unbalance the intestinal epithelial cells renewal.
The pathogenic mechanism of colon carcinogenesis induced by BAs involves the genotoxic effect exerted by CDCA and DCA in normal human colonic epithelial cells (HCoEpiCs): oxidative stress in CRC cells has been associated with genotoxic alteration of the DNA helix, which transforms normal cells into cancer cells (70). Damages caused by a prolonged exposition to BAs have been observed in the liver as well, with disruption of the normal apoptosis of hepatocytes (71-73).
DCA or LCA shows that the formation of CSCs is induced by secondary BAs and several markers of CSC’s expression, spheroids formations, and pluripotency markers such as KLF4, OCT4, Nanog, SOX2, and EMT marker’s levels such as Vimentin, Twist, Slug and Zeb2 are augmented in HCoEpiCs (74).
LCA and DCA regulate the expression of multidrug resistance (MDR) genes like ABCG2 and ABCB1 and can induce the transformation from normal HCoEpiCs to CSCs, suggesting additional roles in the pathogenesis of CRCs (75).
Muscarinic cholinergic receptors coupled to G proteins influence the CRC carcinogenesis mediated by BAs. These receptors are divided into five subtypes (M1R-M5R) and M3R is activated by LCA: this activation plays an important role in CRC progression (76,77). The simultaneous expression of mRNA of MMP1, MMP10, MMP3 in HCoEpiCs has an important role in the invasion of cancer that is accentuated by the concomitant increase of M3R expression in response to BAs. The worst biological and clinical outcomes in cancer patients are correlated to the over-expression of MMP2, MMP7, MMP1, MMP3, MMP13, and MMP9 (78-82).
Oncogenic effects of secondary BAs
The CRC lethality increases when invasion and metastasis occur and the tumor invasiveness depends on the histological phenotype of the metastatic cells. CRC growth and progression are promoted by secondary BAs, especially DCA. Another oncogenic effect is exerted by β-catenin: a protein related to the cadherin complex and involved in tumoral anoikis, a form of programmed cellular death related to the cellular detachment from the matrix.
The modulation of β-catenin can be regulated by DCA as well as the promotion of CRC growth and invasiveness: β-catenin, cyclin D1, and targeted genes of the urokinase-type plasminogen activator receptor (uPAR) are expressed in CRC and correlated to cancer invasion, growth, and metastasis. Significant increase of tyrosine phosphorylation of β-catenin, induction of uPAR and urokinase-type plasminogen activator, and expression of cyclin D1 have been demonstrated when DCA’s lower concentration enhances CRC proliferation and invasiveness. The DCA could induce uPAR and cyclin D1 expressions are markedly reduced by inhibition of β-catenin. The neutralizing antibodies are used to block uPAR that markedly suppress the DCA-induced CRC, cell proliferation, and invasiveness (9).
Protective diet against CRC
The CRC incidence is inversely proportional with the increased intake of vegetables and fruits because this food contains fibers that bind LCA and facilitate its excretion in the stool (83). Other protective factors include calcium supplementation and cholecalciferol, which can inhibit colorectal carcinogenesis caused by increased secondary bile acids and LCA detoxification following a feed-forward catabolic pathway named cholecalciferol receptor activated by LCA (84,85). The final step of the LCA detoxification is the saponification between insoluble calcium and free bile acids in the intestine lumen, these cytotoxic BAs are eliminated with stool and the colon mucosa is protected from CRC (86).
Summary and conclusions
BAs can promote tumor proliferation in human CRC. Their relationship with diet makes them an interesting target to modulate the risk for CRC associated with the consumption of high-risk food, common in the Western dietary regimen. The primary take-home message emerging from this review is that BAs, in particular DCA and LCA, are potent inducers of colon carcinogenesis and ultimately they play an important role in the progression of CRC. Moreover, BAs' co-carcinogenic activities may induce the proliferation of CSCs in the epithelium of the colon. Among the many epidemiological factors involved in CRC progression, diet is the principal inducer of BAs synthesis and therefore represents an ideal target for non-invasive preventive or treatment protocols.
Our secondary take-home message is elaborated on the consequences of uncontrolled diet and lifestyle. The association of increased intake of high-lipid and red meat, low fiber intake, and sedentary lifestyle could lead to CRC manifestation in the future of the worldwide population and to increase overall mortality for gastrointestinal cancers.
Further research should focus on understanding the pathogenic pathways involving BAs, diet, and the molecular events promoting the onset and the progression of CRC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Current Status and Future Expectations in the Management of Gastrointestinal Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-21-23
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-23). The series “Current Status and Future Expectations in the Management of Gastrointestinal Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. NMS served as the unpaid Guest Editor of the series. Dr. LA serves as an unpaid editorial board member of Digestive Medicine Research from Apr 2020 to Mar 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 1999;159:2647-58. [Crossref] [PubMed]
- Berg A. Nutrition, development, and population growth. Popul Bull 1973;29:3-37. [PubMed]
- Kirkegaard H, Johnsen NF, Christensen J. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ 2010;341:c5504. [Crossref] [PubMed]
- Bayerdörffer E, Mannes GA, Richter WO, et al. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology 1993;104:145-51. [Crossref] [PubMed]
- Bayerdörffer E, Mannes GA, Ochsenkühn T, et al. Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut 1995;36:268-73. [Crossref] [PubMed]
- Behar J. Physiology and Pathophysiology of the Biliary Tract: The Gallbladder and Sphincter of Oddi – A review. International Scholarly Research Notices 2013; [Crossref]
- Nagengast FM, Grubben MJ, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer 1995;31A:1067-70. [Crossref] [PubMed]
- Hofmann AF, Cravetto C, Molino G, et al. Simulation of the metabolism and enterohepatic circulation of endogenous deoxycholic acid in humans using a physiologic pharmacokinetic model for bile acid metabolism. Gastroenterology 1987;93:693-709. [Crossref] [PubMed]
- Pai R, Tarnawski AS, Tran T. Deoxycholic Acid Activates -Catenin Signaling Pathway and Increases Colon Cell Cancer Growth and Invasiveness. Mol Biol Cell 2004;15:2156-63. [Crossref] [PubMed]
- Erlinger S, Dhumeaux D, Berthelot P, et al. Effect of inhibitors of sodium transport on bile formation in the rabbit. Am J Physiol 1970;219:416-22. [Crossref] [PubMed]
- Coleman R. Bile salts and biliary lipids. Biochem Soc Trans 1987;15:68S-80S. [PubMed]
- Sanyal AJ, Hirsch JI, Moore EW. Premicellar taurocholate enhances calcium uptake from all regions of rat small intestine. Gastroenterology 1994;106:866-74. [Crossref] [PubMed]
- Koop I, Schindler M, Bosshammer A, et al. Physiological control of cholecystokinin release and pancreatic enzyme secretion by intraduodenal bile acids. Gut 1996;39:661-7. [Crossref] [PubMed]
- Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev 2005;29:625-51. [Crossref] [PubMed]
- Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science 1999;284:1362-65. [Crossref] [PubMed]
- Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999;284:1365-8. [Crossref] [PubMed]
- Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999;3:543-53. [Crossref] [PubMed]
- Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 2002;298:714-9. [Crossref] [PubMed]
- Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem 2003;278:9435-40. [Crossref] [PubMed]
- Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J 2006;25:1419-25. [Crossref] [PubMed]
- Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev 2002;23:443-63. [Crossref] [PubMed]
- Eloranta JJ, Meier PJ, Kullak-Ublick GA. Coordinate transcriptional regulation of transport and metabolism. Methods Enzymol 2005;400:511-30. [Crossref] [PubMed]
- Geier A, Wagner M, Dietrich CG, et al. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta 2007;1773:283-308. [Crossref] [PubMed]
- Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 2006;116:1102-9. [Crossref] [PubMed]
- Axelson M, Ellis E, Mörk B, et al. Bile acid synthesis in cultured human hepatocytes: support for an alternative biosynthetic pathway to cholic acid. Hepatology 2000;31:1305-12. [Crossref] [PubMed]
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 2003;72:137-74. [Crossref] [PubMed]
- Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 2004;40:539-51. [Crossref] [PubMed]
- Savage PB, Li C, Taotafa U, et al. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol Lett 2002;217:1-7. [Crossref] [PubMed]
- Savage PB. Cationic Steroid Antibiotics. Curr Med Chem 2002;1:293-304. [PubMed]
- Savage PB. Design, synthesis and characterization of cationic peptide and steroid antibiotics. Eur J Org Chem 2002;1:759-68. [Crossref]
- Bandyopadhyaya AK, Sangeetha NM, Maitra U. Highly diastereoselective synthesis of the 1,1'-binaphthol unit on a bile acid template. J Org Chem 2000;65:8239-44. [Crossref] [PubMed]
- Soto Tellini VH, Jover A, Galantini L, et al. New lamellar structure formed by an adamantyl derivative of cholic acid. J Phys Chem B 2006;110:13679-81. [Crossref] [PubMed]
- Soto Tellini VH, Jover A, Meijide F, et al. Supramolecular structures generated by a p-tert-butylphenyl-amide derivative of cholic acid. From vesicles to molecular tubes. Adv Mater 2007;19:1752-6. [Crossref]
- Nath S, Maitra U. A simple and general strategy for the design of fluorescent cation sensor beads. Org Lett 2006;8:3239-42. [Crossref] [PubMed]
- Davis AP, Joos JB. Steroids as organising elements in anion receptors. Coord Chem Rev 2003;240:143-56. [Crossref]
- Ghosh S, Choudhury AR, Guru Row TN, et al. Selective and unusual fluoride ion complexation by a steroidal receptor using OH...F- and CH...F- interactions: a new motif for anion coordination? Org Lett 2005;7:1441-4. [Crossref] [PubMed]
- Yoshii M, Yamamura M, Satake A, et al. Supramolecular ion channels from a transmembrane bischolic acid derivative showing two discrete conductances. Org Biomol Chem 2004;2:2619-23. [Crossref] [PubMed]
- Enhsen A, Kramer W, Wess G. Bile acids in drug discovery. Drug Discov Today 1998;3:409-18. [Crossref]
- Ropponen J, Tamminen J, Lahtinen M, et al. Synthesis, characterization, and thermal behavior of steroidal dendrons. Eur J Org Chem 2005;3:73-84. [Crossref]
- Zhao Y. Facial amphiphiles in molecular recognition: From unusual aggregates to solvophobically driven foldamers. Curr Opin Colloid Interface Sci 2007;12:92-7. [Crossref]
- del Amo V, Siracusa L, Markidis T, et al. Differentially-protected steroidal triamines; scaffolds with potential for medicinal, supramolecular, and combinatorial chemistry. Org Biomol Chem 2004;2:3320-8. [Crossref] [PubMed]
- Alvarez Alcalde M, Jover A, Meijide F, et al. Synthesis and characterization of a new gemini surfactant derived from 3alpha,12alpha-dihydroxy-5beta-cholan-24-amine (steroid residue) and ethylenediamintetraacetic acid (spacer). Langmuir 2008;24:6060-6. [Crossref] [PubMed]
- Nonappa Maitra U. Unlocking the potential of bile acids in synthesis, supramolecular/materials chemistry and nanoscience. Org Biomol Chem 2008;6:657-69. [Crossref] [PubMed]
- Davis AP. Bile acid scaffolds in supramolecular chemistry: the interplay of design and synthesis. Molecules 2007;12:2106-22. [Crossref] [PubMed]
- Hofmann AF. Bile Acids: The Good, the Bad, and the Ugly. News Physiol Sci 1999;14:24-9. [Crossref] [PubMed]
- Coello A, Meijide F, Rodríguez Nuñez E, et al. Aggregation behavior of sodium cholate in aqueous solution. J Phys Chem 1993;97:10186-91. [Crossref]
- Armstrong MJ, Carey MC. The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J Lipid Res 1982;23:70-80. [Crossref] [PubMed]
- Jover A, Meijide F, Rodríguez Núñez E, et al. Aggregation behavior of bile salts. Recent Res Dev Phys Chem 1999;3:323-35.
- Reis S, Moutinho CG, Matos C, et al. Noninvasive methods to determine the critical micelle concentration of some bile acid salts. Anal Biochem 2004;334:117-26. [Crossref] [PubMed]
- Cipriani S, Mencarelli A, Chini MG, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One 2011;6:e25637 [Crossref] [PubMed]
- Fiorucci S, Cipriani S, Mencarelli A, et al. Counter-regulatory role of bile acid activated receptors in immunity and inflammation. Curr Mol Med 2010;10:579-95. [PubMed]
- Pols TW, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab 2011;14:747-57. [Crossref] [PubMed]
- Vavassori P, Mencarelli A, Renga B, et al. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 2009;183:6251-61. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis 2004;14:431-9. [PubMed]
- Kono S, Toyomura K, Yin G, et al. A case-control study of colorectal cancer in relation to lifestyle factors and genetic polymorphisms: design and conduct of the Fukuoka colorectal cancer study. Asian Pac J Cancer Prev 2004;5:393-400. [PubMed]
- Hill MJ. Bile flow and colon cancer. Mutat Res 1990;238:313-20. [Crossref] [PubMed]
- Crowther JS, Drasar BS, Hill MJ, et al. Faecal steroids and bacteria and large bowel cancer in Hong Kong by socio-economic groups. Br J Cancer 1976;34:191-8. [Crossref] [PubMed]
- Reddy BS, Wynder EL. Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J Natl Cancer Inst 1973;50:1437-42. [Crossref] [PubMed]
- Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev 2004;36:703-22. [Crossref] [PubMed]
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011;17:313-19. [Crossref] [PubMed]
- Choi D, Lee HW, Hur KY, et al. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J Gastroenterol 2009;15:2258-64. [Crossref] [PubMed]
- Horst D, Scheel SK, Liebmann S, et al. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol 2009;219:427-34. [Crossref] [PubMed]
- Langan RC, Mullinax JE, Ray S, et al. A pilot study assessing the potential role of non-CD133 colorectal cancer stem cells as biomarkers. J Cancer 2012;3:231-40. [Crossref] [PubMed]
- Ribeiro KB, da Silva ZJ, Ribeiro-Silva A, et al. KRAS mutation associated with CD44/CD166 immunoexpression as predictors of worse outcome in metastatic colon cancer. Cancer Biomark 2016;16:513-21. [Crossref] [PubMed]
- Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer 1975;15:617-31. [Crossref] [PubMed]
- Kundu S, Kumar S, Bajaj A. Cross-talk between bile acids and gastrointestinal tract for progression and development of cancer and its therapeutic implications. IUBMB Life 2015;67:514-23. [Crossref] [PubMed]
- Rogers AE, Zeisel SH, Groopman J. Diet and carcinogenesis. Carcinogenesis 1993;14:2205-17. [Crossref] [PubMed]
- Armstrong B, Doll R. Bladder cancer mortality in diabetics in relation to saccharin consumption and smoking habits. Br J Prev Soc Med 1975;29:73-81. [Crossref] [PubMed]
- Rosignoli P, Fabiani R, De Bartolomeo A, et al. Genotoxic effect of bile acids on human normal and tumour colon cells and protection by dietary antioxidants and butyrate. Eur J Nutr 2008;47:301-9. [Crossref] [PubMed]
- Delzenne NM, Calderon PB, Taper HS, et al. Comparative hepatotoxicity of cholic acid, deoxycholic acid and lithocholic acid in the rat: in vivo and in vitro studies. Toxicol Lett 1992;61:291-304. [Crossref] [PubMed]
- Song P, Zhang Y, Klaassen CD. Dose-response of five bile acids on serum and liver bile Acid concentrations and hepatotoxicty in mice. Toxicol Sci 2011;123:359-67. [Crossref] [PubMed]
- Sousa T, Castro RE, Pinto SN, et al. Deoxycholic acid modulates cell death signaling through changes in mitochondrial membrane properties. J Lipid Res 2015;56:2158-71. [Crossref] [PubMed]
- Woolbright BL, McGill MR, Yan H, Jaeschke H. Bile Acid-Induced Toxicity in HepaRG Cells Recapitulates the Response in Primary Human Hepatocytes. Basic Clin Pharmacol Toxicol 2016;118:160-7. [Crossref] [PubMed]
- Andersen V, Svenningsen K, Knudsen LA, et al. Novel understanding of ABC transporters ABCB1/MDR/P-glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology. World J Gastroenterol 2015;21:11862-76. [Crossref] [PubMed]
- Cheng K, Chen Y, Zimniak P, et al. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta 2002;1588:48-55. [Crossref] [PubMed]
- Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol 2005;70:1035-47. [Crossref] [PubMed]
- Xie G, Cheng K, Shant J, et al. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol 2009;296:G755-63. [Crossref] [PubMed]
- Belo A, Cheng K, Chahdi A, et al. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol 2011;300:G749-60. [Crossref] [PubMed]
- Raufman JP, Cheng K, Saxena N, et al. Muscarinic receptor agonists stimulate matrix metalloproteinase 1-dependent invasion of human colon cancer cells. Biochem Biophys Res Commun 2011;415:319-24. [Crossref] [PubMed]
- Said AH, Raufman JP, Xie G. The role of matrix metalloproteinases in colorectal cancer. Cancers (Basel) 2014;6:366-75. [Crossref] [PubMed]
- Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 2004;23:101-17. [Crossref] [PubMed]
- Jenkins DJ, Wolever TM, Rao AV, et al. Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol. N Engl J Med 1993;329:21-6. [Crossref] [PubMed]
- Newmark HL, Yang K, Kurihara N, et al. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 2009;30:88-92. [Crossref] [PubMed]
- Abenavoli L, Boccuto L, Federico A, et al. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int J Environ Res Public Health 2019;16:3011. [Crossref] [PubMed]
- Lorenzen JK, Nielsen S, Holst JJ, et al. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Am J Clin Nutr 2007;85:678-87. [Crossref] [PubMed]
Cite this article as: Kulanthaivel S, Boccuto L, Zanza C, Longhitano Y, Balasundaram K, Méndez-Sánchez N, Abenavoli L. Biliary acids as promoters of colon carcinogenesis: a narrative review. Dig Med Res 2021;4:33.