
Robot-assisted revisional bariatric surgery
Introduction
Weight loss surgery is considered one of the best options for long-term management of obesity and its associated comorbidities (1-3). Bariatric surgical volume has increased exponentially in the past years and has reached over 252,000 cases annually in 2018 (4). The most commonly performed bariatric procedure is sleeve gastrectomy (SG), with 61.4% of all bariatric interventions. Other procedures, as the laparoscopic adjustable gastric banding (LAGB), are not frequently performed nowadays (5,6). Along with the increasing numbers of bariatric procedures comes a larger pool of potential candidates for revisional surgery, making it the fastest-growing field in bariatric surgery (7). Revision surgery went up from 6% of all estimated bariatric surgeries in 2011 to 15.4% in 2018 (4).
The most common causes for revisional bariatric surgery include inadequate weight loss, significant weight regain, and development or return of previously resolved or well-controlled obesity-related comorbidities such as type 2 diabetes (8). These indications account for 52.2% of revisional operations (9), while other reasons for revisional or conversional bariatric surgery vary and are related to the persistence of comorbid conditions (10). In 2018, Angrisani et al. published a global survey from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO), gathering data from 58 national societies (6). They reported that the most revised index procedures were LAGB, with a failure/revision rate of 40–50%; SG, 25–36%; and Roux-en-Y gastric bypass (RYGB), with a failure rate of 20% in morbidly obese patients and up to 35% in the super-obese population (6). As far as the most commonly performed revisional procedures, it is worth noting the single-stage conversion from LAGB to RYGB, SG to biliopancreatic derivation with duodenal switch (BPD-DS), and SG to single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) (11). These procedures have been traditionally performed laparoscopically (12-14) since it became the standard for minimally invasive bariatric surgery after being first described by Wittgrove et al. for RYGB in 1994 (15).
Robot-assisted bariatric surgery has gained popularity, and its numbers are increasing as more surgeons overcome the learning curve (16-18), and the current trend pointing towards it becoming more widespread in bariatric surgery (18,19).
The use of robot-assisted surgery has been promoted for revisional weight loss surgery in the past few years. Proponents of this technique postulate it to be the minimal access approach that resembles open surgery the most as a result of improved visualization, enhanced manipulation of tissue by the wrist-like movement adapted to almost all the instruments, and less dependence on the surgical assistants for exposure and manipulation of the camera (18,20).
This chapter describes the current role of robot-assisted surgery in the revisional bariatric surgery field, its indications with their respective outcomes, technical tips, and potential pitfalls.
Revision surgery
Causes
Although the main indication for revisional surgery seems to be weight regain or inadequate weight loss following the index procedure (9), several other chronic complications may need revision for improving outcomes. These vary depending on the primary surgery (10,12). The most commonly performed weight loss procedure, SG, has been associated with the development of gastroesophageal reflux disease (GERD), sleeve stricture or dilation, and anastomotic leaks (8). Complications related to RYGB include gastro gastric fistula, gastric pouch stricture or dilation, gastro-jejunal (G-J) stricture or dilation, marginal ulcers, severe malnutrition, vitamin deficiency, refractory hypoglycemia or hypocalcemia, and even excessive weight loss (8,21,22). It is also important to consider that weight loss surgery failure could not only be due to technical or anatomical issues but also patient-related factors (23). These include psychological and behavioral aspects that should be considered as part of the initial evaluation of a patient who is regaining weight and need to be managed promptly with a multidisciplinary team to ensure success (6,21,23).
Although some of these complications can be addressed endoscopically (24,25), this chapter will focus on the surgical management of bariatric surgery failure.
Challenges of revisional surgery
Reoperative bariatric surgery appears to be more technically challenging than the primary procedure and has been associated with higher rates of 30-day adverse events (13), leaks, and ICU stays (26). Chaar et al. (27) described a significant increase in the incidence of complications requiring 30-day reintervention, readmission, reoperation, ICU admission, and continued presence of surgical drain following revisional RYGB and SG when compared to primary operations.
Morbidity and mortality are usually higher in revisional surgery than after the primary procedure (28,29). Although patients may have lost a considerable amount of weight by the time they undergo revisional procedures, the presence of severe adhesions, obliterated surgical planes, and alteration of anatomy following the index surgery could hinder the surgeon’s ability to access the abdominal cavity safely. The surgeon might benefit from a careful examination of the operative notes from the previous procedure to develop a thorough understanding of the anatomic changes brought about by the previous procedure (28,30). This kind of understanding of the previous surgery could sometimes be unavailable, so surgeons may have to rely on imaging techniques, endoscopy, or both, pre- and intra-operatively (30). Díaz et al. propose esophagogastroduodenoscopy (EGD) as the best way to evaluate postoperative anatomy in bariatric patients (31). Lee Bion et al. recommend performing a morphological evaluation before the revision procedure, including at least a CT scan with gastric volumetry and an EGD (32). Decreased vascularization as a consequence of the primary procedure needs to be considered as well (5). Possible tissue avascularity could negatively impact the healing process of the anastomoses in the revision. Robotic platforms offer the additional advantage of allowing for near-infrared fluorescence with indocyanine green (ICG) intra-operatively. The ongoing discussion for the various uses for ICG has suggested it could be helpful to assess tissue perfusion (33,34). At our center, we favor using ICG fluorescence to check for leaks after finalizing the anastomoses.
However, most studies analyzing perioperative outcomes in revisional bariatric surgery agree that this should not preclude patients from getting a revision since the procedures are becoming safer with time and experience (35). In the hands of skilled surgeons, the risk of complications becomes justifiable when compared to the potential benefits (8). Although challenging, Sudan et al. reported that revisional bariatric surgery was deemed safe, and its higher morbidity was acceptable from a clinical perspective when considering weight loss achieved, comorbidity resolution, and overall long-term benefits (36).
In our center, we favor a robot-assisted approach for bariatric revisional surgeries. Operations such as conversion from sleeve to RYGB secondary to refractory GERD are common, as are band removals with conversion to RYGB, SG, or biliopancreatic diversion with duodenal switch (BPD-DS). Additional conversions for weight regain from sleeve to BPD-DS are excellent cases to utilize robot-assisted technology.
Although controversial, robot-assisted stricturoplasty for SG strictures has been performed with acceptable results (37-39), further studies are guaranteed in order to recommend this approach.
Our approach consists of thorough anatomy delineation using contrast-enhanced studies and endoscopy. Direct communication with the surgeon who performed the index operation is highly desired. A multidisciplinary approach with early and aggressive involvement of the nutrition and psychology departments is paramount.
Preoperative preparation follows standard antibiotic and venous thromboembolism (VTE) prophylaxis. Appropriate padding to protect pressure areas and nerves is critical, as the cases are lengthy.
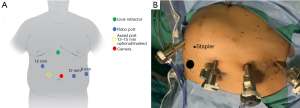
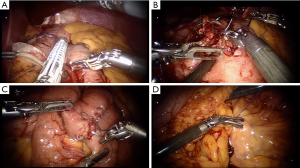
We prefer to perform laparoscopy to assess the anatomy prior to placement of robot ports. Four ports are utilized for the robotic arms and camera, with an additional assistant port, which needs to be available for additional retraction or suctioning (Figure 1). Hand-sewing methods are our preferred method of anastomoses in almost all cases (Figure 2).
Robot-assisted bariatric surgery
Cadiere et al. reported the first robotic bariatric procedure in 1999, a successful, purely robot-assisted, adjustable gastric banding (40). In 2000, after the Food and Drug Administration (FDA) cleared the Da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) for use in general surgical procedures, both robot-assisted BPD-DS (RABPD-DS) and RYGB (RARYGB) were successfully performed by Sudan et al. and Horgan et al. respectively (17,41). Since then, the applications for robotics in bariatric surgery have evolved. From initial hybrid approaches (42-44) that combined traditional laparoscopy with a robotic approach for specific tasks such as hand-sewn G-J or duodeno-ileal (D-I) anastomoses during RYGB or BPD-DS, to fully robotic primary weight loss procedures with similar benefits as the laparoscopic approach (45). The main limitations of robotic surgery have usually been the higher costs and longer operative times (OTs) with no significant differences in the outcomes (46,47). Conversely, Beckmann et al. (48) proved robotic primary RYGB (n=114) to have a shorter OT and a lower complication rate than laparoscopic primary RYGB (n=108). Lainas et al. (49) also reported a shorter OT with a robot-assisted vs. laparoscopic approach for RYGB with similar post-op complications, length of stay (LOS), and weight loss outcomes. Meanwhile, Acevedo et al. (47) found a robotic approach was associated with lower morbidity and mortality in primary RYGB, unlike in SG, where it was considered not cost-effective. A systematic review with meta-analysis published by Economopoulos et al. (50) analyzing robotic vs. laparoscopic RYGB pointed towards comparable clinical outcomes. It underlined the need for higher-quality studies in the future.
Robot-assisted surgery has proved to be safe, feasible, and effective for bariatric operations (51,52), but the challenge to determine its value remains. One area to look at for a higher robotic platform value is the revisional field in weight loss surgery. Iranmanesh et al. (53) found overall comparable outcomes between 806 patients undergoing primary robotically assisted laparoscopic (RAL) RYGB and 266 revisional RAL RYGB. They reported that this similarity in post-op complication profiles suggests the higher morbidity traditionally associated with laparoscopic revisional bariatric surgery (LRBS) could be decreased to the level of primary procedures with the robotic platform’s help.
Technical advantages
Early experience with robotic systems seems to outline that the robot’s role is not to replace the surgeon but to help them perform complicated tasks more accurately and repetitive tasks more precisely than standard laparoscopy (54).
Robot-assisted surgery provides the surgeon with tools such as improved visualization through stereoscopic 3D vision (54,55). There is increased precision by stabilizing and downscaling the amplitude of the surgeon’s motions by a factor of five or three to one (56) and eliminating or filtering physiological tremor (18). Manipulation of tissue is also enhanced by the increased range of motion provided by the wrist-like quality of the novel instruments resembling an open technique (56). Robot-assisted surgery also overcomes torque on thick abdominal walls, a characteristic limitation of the standard laparoscopic approach (55). Torque on laparoscopic instruments leads to imprecise technical movements and surgeon fatigue.
Bariatric surgery may require intervention in various quadrants of the abdomen, so hybrid techniques using a combination of laparoscopy and robot have been developed. However, these have been mitigated by the advances of the new Da Vinci Xi System by Intuitive Surgical (Sunnyvale, CA, USA). Sudan et al. reported a successful conversion from laparoscopic adjustable gastric band to totally robotic BPD-DS, using a single dock, multi quadrant strategy (57). This new approach seems helpful for the process of measuring limbs after demonstration of easy access for splenic flexure mobilization with concomitant pelvic surgery by Protyniak et al. (58).
Benefits from a robotic surgical approach may extend even to the surgeon. Laparoscopic surgery has been associated with ergonomic issues due to prolonged operation time in unnatural and sometimes awkward postures (18,56,59). This has led to more surgeons suffering from excessive fatigue and career-shortening occupational musculoskeletal injuries (55,60,61). The robotic platform offers a more neutral position with comfortable seating, avoids forced movements, and decreases the strain on the surgeon’s shoulders, neck, and back muscles by relying on the robotic arms to overcome torque forces (16,18). This ergonomic approach, improved posture, and reduced operator fatigue could help the surgeon prolong their career and perform hand-sewn anastomoses and difficult dissection with fewer challenges and more freedom of movement (55).
Challenges of robotic surgery
Costs
Adair et al. described a cohort of 753 robotic SG (RSG) matched to 1,506 laparoscopic SG (LSG) patients from data extracted from the National Inpatient Sample (NIS) database between 2011 and 2013 (62). Although they found no statistically significant differences in postoperative complications or in-hospital mortality, the LOS and total hospital charge (THC) were significantly higher in the robotic cohort when compared to laparoscopic operation in multivariable analysis adjusted for type of hospital, region, and a comorbidity index (62).
Although robot-assisted surgery is considerably more expensive regarding equipment, it can be made cost-effective by reducing the cost of complications, such as G-J or jejuno-jejunal (J-J) anastomoses leaks (63). Hagen et al. compared 524 laparotomies with 323 laparoscopic and 143 robotic cases of RYGB between 1997 and 2010 (63). After taking the steep learning curve for robotic surgery into account, they found RARYGB cost-effective compared to laparoscopic RYGB when the anastomotic leak rate during the laparoscopic procedure was at 2% or more. However, if the leak rate fell under 2% for laparoscopy, the robotic procedures were not as effective. Sensitivity analysis performed in this study also showed that an increase in the monthly robotic caseload also helped lower the overall cost per procedure, making it cheaper than laparoscopic RYGB. The significance threshold was ten monthly cases if the leak rate was 2% or seven cases if the leak rate was above 2%. The savings found by Hagen et al. were related to the prevention of leaks with hand-sewn anastomoses in robotic surgery compared to stapled anastomoses during laparoscopy. Leaks usually generated higher costs, and preventing the complications altogether made the net cost difference more favorable. Hagen et al. concluded that the robotic approach could be better suited for very complex procedures where the laparoscopic skills are at their limit, such as revision bariatric procedures. It is also essential to consider that costs may peak during the initial part of the learning curve (63). Combining relative inexperience with more expensive technology may cause the costs to increase until operating room (OR) time can be lowered to an acceptable point. This is also true when starting a full-on new robotic program. Initial costs of the robotic equipment will seem steep at first but will pay off later on when the increase in surgical volume and better allocation of material resources can amortize the total cost.
El Chaar et al. used data from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database between 2017 and 2018 to compare the costs of RSG vs. LSG, including OR time, LOS, and supplies, on patients undergoing the same preoperative workup and postoperative protocol (64). The analysis showed no statistically significant overall cost differences between 39 RSG and 59 LSG patients. Median cost for RSG was $5,308.99 vs. $4,918.88 for LSG (P>0.05). However, the median cost associated with OR time was significantly higher in the RSG cohort, $1,341 vs. $1,112 (P<0.0001), despite median OR duration being similar in the two groups, 2'08" for RSG vs. 1'43" for LSG (64). The use of robotic staplers versus laparoscopic staplers may account for this difference. A trend towards lower LOS in RSG and potentially reduced costs of managing postoperative pain with similar outcomes between the RSG and LSG suggests a robotic approach for weight loss surgery may become cost-effective in the next few years. Though the median OR time in RSG was higher than in LSG, there was a trend towards decreased operation duration with increased number of procedure over time and correlated with increased experience acquired with each case (64).
Although several articles have been published related to the generally higher costs of robotic surgery (18,65), it is important to remember that its applications continue to broaden. Also, the updates in the robotic platforms (58,66) and their payment structure could reduce the financial investment associated with purchasing and maintaining the system. This has been a barrier to adopting robotic platforms, especially in developing countries (67). In the future, as the applications and advantages of robotic surgery become more established, the increased volume, reduced learning curve, judicious use of equipment, and reduced complications will make robotic platforms more economical. In addition, robotic surgery could reduce ergonomic injuries on the surgeon and provide an indirect cost-benefit.
Currently, scarce data is available regarding specific costs in robotic revisional bariatric surgery (RRBS). As surgical volume increases, more studies are needed to obtain accurate and current data that allow for better-informed recommendations regarding the value of this technology.
Learning curve
A learning curve can be quickly defined as an improvement in performance over time (68). It may also be measured as the time and ability to complete a task until failure is reduced to an acceptable point or altogether eliminated (69,70). Every surgical procedure has a multifactorial learning curve. The initial training period is usually characterized by continuous repetition of tasks until proficiency is achieved (69). During this phase of learning, longer procedure times and higher complication rates are common, and it is expected that these will diminish with acquired experience (70). Published evidence appears to point towards a shorter learning curve for robotic procedures when compared with the laparoscopic technique. However, it is worth noting that learning curve measurement is not entirely standardized in surgery and although some authors refer to OT as a parameter to measure the “learning” process, not every study reports OR time equally; some include docking time while others do not, so care must be taken when interpreting these results.
Schauer et al. proposed that the learning curve for laparoscopic RYGB was 100 cases (71). Studies from the US and Europe show a learning curve of 25 to 35 cases in the robotic RYGB cases (3,44,72). Vilallonga et al. reported a learning curve of 20 cases for RSG cases (73). Sudan et al. described the learning curve for RABPD-DS as 50 cases (70).
Buchs et al. identified two distinct phases of the learning process for RARYGB (72). The first 14 surgeries represent the initial learning curve phase, while the last 50 surgeries suggest a “mastery” phase where both potential complications and OT are reduced significantly (72).
Robot simulators have also been proposed as a viable technology to becoming proficient in technical skills before starting live cases (18). Instituting curricula such as the Fundamentals of Robotic Surgery program early in residency training will also reduce the learning curve. Learning both robotic and laparoscopic techniques at the same time might prove useful to develop skills simultaneously and in a way that may complement each other. Sanchez et al. reported significantly shorter OR times for RSG when the surgeon learned both techniques simultaneously (44). It is crucial to remember that the learning curve is not exclusive to the surgeon (3). Full robotic teams need to be trained (nurses, assistants, residents, anesthesiologists). The whole team learns with the surgeon and develops protocols about patient safety, OR set-up, and instruments required (55). This team effort may be reflected in reduced OR times with experience, better patient outcomes, less supply waste, and lower costs (55,74). This supports the idea of a standardized training program that will allow the whole team to achieve dexterity and become more proficient, with subsequent cost-effectiveness as the surgical volume increases (18).
Outcomes
Although robot-assisted primary bariatric surgery has not been shown to be superior to laparoscopic surgery (46-50,75), it is possible that robotic surgery can thrive in specialized circumstances. Weight loss surgery in super-obese patients and revisional bariatric surgery may be particular areas that benefit from a robotic approach in technically challenging patients (5,16,20,53,76,77). However, it is not easy to get to that stage without first developing the skills in primary cases (5). Some of the higher-impact published literature has been summarized in Table 1.
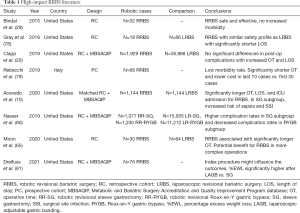
Full table
In a retrospective single-center report of revisional bariatric interventions, Beckmann et al. (76) analyzed 30 laparoscopic, four open, and 44 robotic procedures in terms of 30-day postoperative outcomes. After comparing revisional LRYGB with RRYGB, they found that robotic interventions lasted on average 37 minutes less, were associated with a significantly lower increase in postoperative C-reactive protein (CRP) levels, and had an overall lower rate of complications (7.3% in RRYGB vs. 22.2%in LRYGB). However, other series have failed to corroborate these results. Snyder et al. (77) published their 7-year experience with robotic-assisted conversion of failed restrictive procedures to RYGB, and even though outcomes were equivalent to the laparoscopic standard, they associated RRYGB with a significant immediate postoperative complications rate (17%); moreover, 24% of patients where readmitted 90 days after the procedure due to issues related to the operation. Likewise, Clapp et al. (20) retrospectively reviewed the MBSAQIP database from 2015 to 2016 to compare revisional laparoscopic weight loss surgery with revisional robotic weight loss surgery and failed to find a significant advantage of the robot in terms of postoperative complications.
Further, after comparing data from over 35,000 patients undergoing revisional weight-loss surgery (35,988 laparoscopic and 1,929 robotic), they concluded that robotic surgery is associated with an extended hospital stay and OR times without providing a significant benefit in outcomes. Interestingly, after comparing 220 RRBS vs. 220 LRBS patients in propensity-matched cohort analysis of the MBSAQIP database, El Chaar et al. (14) found a clinically, though not statistically, significant reduction in the incidence of 30-day serious adverse events (SAE), 30-day organ-specific infections (OSI), 30-day reoperation, and 30-day intervention following RRBS. However, in the subgroup analysis, this was found to be true for RYGB and not for SG, suggesting again that the robotic approach’s improved outcomes could be advantageous in the more technically challenging procedure.
Dreifuss et al. (81) found a significant difference in percentage excess weight loss (%EWL) when index procedure was LAGB vs. SG, and, although not statistically significant, Gray et al. (78) found a difference in the post-op complication rate between patients undergoing conversion after a stapled index procedure (27%) and non-stapled index procedure (17%). These two studies suggest there could be some influence from the type of index procedure in the outcomes though no causality has been established due to lack of prospective studies. Further research is necessary with subgroup analysis to determine the relevance of these findings.
Most available literature regarding RRBS is limited to retrospective cohorts (RCs) or case series, with no controlled or prospective studies to establish adequate statistical parameters. Also, even the large sample size studies with the MBSAQIP database are limited to 30-day outcomes due to the nature of the data collected. Some of the studies report inconsistent follow-up with the patients, hindering their ability to draw middle- and long-term conclusions, especially regarding weight management. Also, robotic-approach surgery may not be readily available in smaller centers or lower-income settings, with results not being applicable everywhere.
Conclusions
Revisional bariatric surgery appears to be one of the most promising areas for robotic development; the field is continuously and unequivocally evolving. The discrepancies reported in the literature may be explained by the lack of clear patient-selection guidelines for robot-assisted interventions and by the somewhat recent introduction of robotics in the field. In all likelihood, RRBS will have a substantial impact in the years to come. It is a safe alternative that achieves comparable outcomes to standard laparoscopy. Widespread use will likely bring costs down, and further research could show improved patient outcomes. It is our view that robotic surgery should be a part of every surgeon's armamentarium. Knowing when to choose the best approach on a patient-to-patient basis and offering the best technology can be extremely beneficial for future bariatric patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Advanced Laparoscopic Gastric Surgery”. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/dmr-21-6
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-6). The series “Advanced Laparoscopic Gastric Surgery” was commissioned by the editorial office without any funding or sponsorship. ADG served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Digestive Medicine Research from Dec 2019 to Nov 2021. ADG has received honoraria as a consultant for Levita and as a speaker for Gore and Medtronic. Dr. ADG reports personal fees from Levita, personal fees from Gore, personal fees from Medtronic, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guerron AD, Perez JE, Risoli T Jr, et al. Performance and improvement of the DiaRem score in diabetes remission prediction: a study with diverse procedure types. Surg Obes Relat Dis 2020;16:1531-42. [Crossref] [PubMed]
- Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016;151:1046-55. [Crossref] [PubMed]
- Jung MK, Hagen ME, Buchs NC, et al. Robotic bariatric surgery: a general review of the current status. Int J Med Robot 2017; [Crossref] [PubMed]
- American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011-2018. 2018. Available online: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
- Iranmanesh P, Bajwa KS, Felinski MM, et al. Robotic primary and revisional bariatric surgery. Surg Clin North Am 2020;100:417-30. [Crossref] [PubMed]
- Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg 2018;28:3783-94. [Crossref] [PubMed]
- Qiu J, Lundberg PW, Javier Birriel T, et al. Revisional bariatric surgery for weight regain and refractory complications in a single MBSAQIP accredited center: what are we dealing with? Obes Surg 2018;28:2789-95. [Crossref] [PubMed]
- Brethauer SA, Kothari S, Sudan R, et al. Systematic review on reoperative bariatric surgery American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis 2014;10:952-72. [Crossref] [PubMed]
- Park JY, Song D, Kim YJ. Causes and outcomes of revisional bariatric surgery: Initial experience at a single center. Ann Surg Treat Res 2014;86:295-301. [Crossref] [PubMed]
- Acevedo E, Mazzei M, Zhao H, et al. Outcomes in conventional laparoscopic versus robotic-assisted revisional bariatric surgery: a retrospective, case-controlled study of the MBSAQIP database. Surg Endosc 2020;34:1573-84. [Crossref] [PubMed]
- Clapp B, Harper B, Dodoo C, et al. Trends in revisional bariatric surgery using the MBSAQIP database 2015-2017. Surg Obes Relat Dis 2020;16:908-15. [Crossref] [PubMed]
- Switzer NJ, Karmali S, Gill RS, et al. Revisional bariatric surgery. Surg Clin North Am 2016;96:827-42. [Crossref] [PubMed]
- Inabnet WB, Belle SH, Bessler M, et al. Comparison of 30-day outcomes after non-LapBand primary and revisional bariatric surgical procedures from the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis 2010;6:22-30. [Crossref] [PubMed]
- El Chaar M, King K, Pastrana M, et al. Outcomes of robotic surgery in revisional bariatric cases: a propensity score-matched analysis of the MBSAQIP registry. J Robot Surg 2021;15:235-9. [Crossref] [PubMed]
- Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg 1994;4:353-7. [Crossref] [PubMed]
- Wilson EB, Sudan R. The evolution of robotic bariatric surgery. World J Surg 2013;37:2756-60. [Crossref] [PubMed]
- Sudan R, Puri V, Sudan D. Robotically assisted biliary pancreatic diversion with a duodenal switch: a new technique. Surg Endosc 2007;21:729-33. [Crossref] [PubMed]
- Jara RD, Guerrón AD, Portenier D. Complications of robotic surgery. Surg Clin North Am 2020;100:461-8. [Crossref] [PubMed]
- Veilleux E, Ponce J, Lutfi R. A review of the role of robotics in bariatric surgery: finding our future? J Laparoendosc Adv Surg Tech A 2020;30:36-9. [Crossref] [PubMed]
- Clapp B, Liggett E, Jones R, et al. Comparison of robotic revisional weight loss surgery and laparoscopic revisional weight loss surgery using the MBSAQIP database. Surg Obes Relat Dis 2019;15:909-19. [Crossref] [PubMed]
- Dykstra MA, Switzer NJ, Sherman V, et al. Roux en Y gastric bypass: how and why it fails. Surg Curr Res 2014;4:1000165
- Moon RC, Frommelt A, Teixeira AF, et al. Indications and outcomes of reversal of Roux-en-Y gastric bypass. Surg Obes Relat Dis 2015;11:821-6. [Crossref] [PubMed]
- Guerron AD, Sudan R. Evaluation and treatment of the patient who is regaining weight. In: Patti M, Di Corpo M, Schlottmann F. editors. Foregut surgery: achalasia, gastroesophageal reflux disease and obesity. Cham: Springer Nature Switzerland, 2020:295-307.
- Tran DD, Nwokeabia ID, Purnell S, et al. Revision of Roux-En-Y gastric bypass for weight regain: a systematic review of techniques and outcomes. Obes Surg 2016;26:1627-34. [Crossref] [PubMed]
- Ortega CB, Guerron AD, Portenier D. Endoscopic abscess septotomy: a less invasive approach for the treatment of sleeve gastrectomy leaks. J Laparoendosc Adv Surg Tech A 2018;28:859-63. [Crossref] [PubMed]
- Hallowell PT, Stellato TA, Yao DA, et al. Should bariatric revisional surgery be avoided secondary to increased morbidity and mortality? Am J Surg 2009;197:391-6. [Crossref] [PubMed]
- Chaar ME, Lundberg P, Stoltzfus J. Thirty-day outcomes of sleeve gastrectomy versus Roux-en-Y gastric bypass: first report based on Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database. Surg Obes Relat Dis 2018;14:545-51. [Crossref] [PubMed]
- Ikramuddin S, Kellogg TA, Leslie DB. Laparoscopic conversion of vertical banded gastroplasty to a Roux-en-Y gastric bypass. Surg Endosc 2007;21:1927-30. [Crossref] [PubMed]
- Bindal V, Gonzalez-Heredia R, Elli EF. Outcomes of robot-assisted Roux-en-Y gastric bypass as a reoperative bariatric procedure. Obes Surg 2015;25:1810-5. [Crossref] [PubMed]
- Sweeney JF, Goode SE, Rosemurgy AS. Redo gastric restriction: a higher risk procedure. Obes Surg 1994;4:244-7. [Crossref] [PubMed]
- Díaz R, Narvaez A, Welsh L, et al. Endoscopy after bariatric surgery: what the endoscopist must know. Rev Med Chil 2020;148:83-92. [PubMed]
- Lee Bion A, Le Roux Y, Alves A, et al. Bariatric revisional surgery: what are the challenges for the patient and the practitioner? J Visc Surg 2021;158:38-50. [Crossref] [PubMed]
- Ortega CB, Guerron AD, Yoo JS. The use of fluorescence angiography during laparoscopic sleeve gastrectomy. JSLS 2018;22:e2018.00005.
- Welsh L, Guerron AD. The use of near-infrared fluorescence in sleeve gastrectomy. In: Aleassa E, El-Hayek K. editors. Video atlas of intraoperative applications of near infrared fluorescence imaging. Cham: Springer Nature Switzerland, 2020:87-94.
- Ma P, Reddy S, Higa KD. Revisional bariatric/metabolic surgery: what dictates its indications? Curr Atheroscler Rep 2016;18:42. [Crossref] [PubMed]
- Sudan R, Nguyen NT, Hutter MM, et al. Morbidity, mortality, and weight loss outcomes after reoperative bariatric surgery in the USA. J Gastrointest Surg 2015;19:171-8; discussion 178-9. [Crossref] [PubMed]
- Sudan R, Kasotakis G, Betof A, et al. Sleeve gastrectomy strictures: technique for robotic-assisted strictureplasty. Surg Obes Relat Dis 2010;6:434-6. [Crossref] [PubMed]
- Himpens J. Strictures after sleeve gastrectomy. In: Gagner M, Cardoso A, Palermo M, et al. editors. The perfect sleeve gastrectomy. Cham: Springer Nature Switzerland, 2020:325-35.
- Chang PC, Tai CM, Hsin MC, et al. Surgical standardization to prevent gastric stenosis after laparoscopic sleeve gastrectomy: a case series. Surg Obes Relat Dis 2017;13:385-90. [Crossref] [PubMed]
- Cadiere GB, Himpens J, Vertruyen M, et al. The world’s first obesity surgery performed by a surgeon at a distance. Obes Surg 1999;9:206-9. [Crossref] [PubMed]
- Horgan S, Vanuno D. Technical report robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech 2001;11:415-9. [Crossref]
- Parini U, Fabozzi M, Brachet Contul R, et al. Laparoscopic gastric bypass performed with the Da Vinci Intuitive Robotic System: preliminary experience. Surg Endosc 2006;20:1851-7. [Crossref] [PubMed]
- Hubens G, Balliu L, Ruppert M, et al. Roux-en-Y gastric bypass procedure performed with the da Vinci robot system: is it worth it? Surg Endosc 2008;22:1690-6. [Crossref] [PubMed]
- Sanchez BR, Mohr CJ, Morton JM, et al. Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2005;1:549-54. [Crossref] [PubMed]
- Benizri EI, Renaud M, Reibel N, et al. Perioperative outcomes after totally robotic gastric bypass: a prospective nonrandomized controlled study. Am J Surg 2013;206:145-51. [Crossref] [PubMed]
- Köckerling F. Robotic vs. standard laparoscopic technique - what is better? Front Surg 2014;1:15. [PubMed]
- Acevedo E, Mazzei M, Zhao H, et al. Outcomes in conventional laparoscopic versus robotic-assisted primary bariatric surgery: a retrospective, case–controlled study of the MBSAQIP database. Surg Endosc 2020;34:1353-65. [Crossref] [PubMed]
- Beckmann JH, Bernsmeier A, Kersebaum JN, et al. The impact of robotics in learning Roux-en-Y gastric bypass: a retrospective analysis of 214 laparoscopic and robotic procedures: robotic vs. laparoscopic RYGB. Obes Surg 2020;30:2403-10. [Crossref] [PubMed]
- Lainas P, Kassir R, Benois M, et al. Comparative analysis of robotic versus laparoscopic Roux-en-Y gastric bypass in severely obese patients. J Robot Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Economopoulos KP, Theocharidis V, McKenzie TJ, et al. Robotic vs. laparoscopic Roux-En-Y gastric bypass: a systematic review and meta-analysis. Obes Surg 2015;25:2180-9. [Crossref] [PubMed]
- Bailey JG, Hayden JA, Davis PJB, et al. Robotic versus laparoscopic Roux-en-Y gastric bypass (RYGB) in obese adults ages 18 to 65 years: a systematic review and economic analysis. Surg Endosc. 2014;28:414-26. [Crossref] [PubMed]
- Beckmann JH, Aselmann H, Egberts JH, et al. Robot-assisted vs laparoscopic gastric bypass: first experiences with the DaVinci system in bariatric surgery. Chirurg 2018;89:612-20. [Crossref] [PubMed]
- Iranmanesh P, Fam J, Nguyen T, et al. Outcomes of primary versus revisional robotically assisted laparoscopic Roux-en-Y gastric bypass: a multicenter analysis of ten-year experience. Surg Endosc 2020; Epub ahead of print. [Crossref] [PubMed]
- Talamini MA, Chapman S, Horgan S, et al. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc 2003;17:1521-4. [Crossref] [PubMed]
- Bindal V, Bhatia P, Dudeja U, et al. Review of contemporary role of robotics in bariatric surgery. J Minim Access Surg 2015;11:16-21. [Crossref] [PubMed]
- Cadière GB, Himpens J, Germay O, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg 2001;25:1467-77. [Crossref] [PubMed]
- Sudan R, Desai S. Conversion of laparoscopic adjustable gastric band to robot-assisted laparoscopic biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis 2011;7:546-7. [Crossref] [PubMed]
- Protyniak B, Jorden J, Farmer R. Multiquadrant robotic colorectal surgery: the da Vinci Xi vs Si comparison. J Robot Surg 2018;12:67-74. [Crossref] [PubMed]
- Toro JP, Lin E, Patel AD. Review of robotics in foregut and bariatric surgery. Surg Endosc 2015;29:1-8. [Crossref] [PubMed]
- Esposito C, El Ghoneimi A, Yamataka A, et al. Work-related upper limb musculoskeletal disorders in paediatric laparoscopic surgery. A multicenter survey. J Pediatr Surg 2013;48:1750-6. [Crossref] [PubMed]
- Janki S, Mulder EEAP, IJzermans JNM, et al. Ergonomics in the operating room. Surg Endosc 2017;31:2457-66. [Crossref] [PubMed]
- Adair MJ, Alharthi S, Ortiz J, et al. Robotic surgery is more expensive with similar outcomes in sleeve gastrectomy: analysis of the NIS database. Am Surg 2019;85:39-45. [Crossref] [PubMed]
- Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg 2012;22:52-61. [Crossref] [PubMed]
- El Chaar M, Gacke J, Ringold S, et al. Cost analysis of robotic sleeve gastrectomy (R-SG) compared with laparoscopic sleeve gastrectomy (L-SG) in a single academic center: debunking a myth! Surg Obes Relat Dis 2019;15:675-9. [Crossref] [PubMed]
- Moon RC, Segura AR, Teixeira AF, et al. Feasibility and safety of robot-assisted bariatric conversions and revisions. Surg Obes Relat Dis 2020;16:1080-5. [Crossref] [PubMed]
- Niclauss N, Morel P, Jung MK, et al. A comparison of the da Vinci Xi vs. the da Vinci Si Surgical System for Roux-En-Y gastric bypass. Langenbecks Arch Surg 2019;404:615-20. [Crossref] [PubMed]
- Schraibman V, Macedo ALV, Epstein MG, et al. Comparison of the morbidity, weight loss, and relative costs between robotic and laparoscopic sleeve gastrectomy for the treatment of obesity in Brazil. Obes Surg 2014;24:1420-4. [Crossref] [PubMed]
- Cook JA, Ramsay CR, Fayers P. Statistical evaluation of learning curve effects in surgical trials. Clin Trials 2004;1:421-7. [Crossref] [PubMed]
- Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 2005;242:83-91. [Crossref] [PubMed]
- Sudan R, Bennett KM, Jacobs DO, et al. Multifactorial analysis of the learning curve for robot-assisted laparoscopic biliopancreatic diversion with duodenal switch. Ann Surg 2012;255:940-5. [Crossref] [PubMed]
- Schauer P, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc 2003;17:212-5. [Crossref] [PubMed]
- Buchs NC, Pugin F, Bucher P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc 2012;26:1116-21. [Crossref] [PubMed]
- Vilallonga R, Fort JM, Gonzalez O, et al. The initial learning curve for robot-assisted sleeve gastrectomy: a surgeon's experience while introducing the robotic technology in a bariatric surgery department. Minim Invasive Surg 2012;2012:347131 [Crossref] [PubMed]
- van der Schans EM, Hiep MAJ, Consten ECJ, et al. From Da Vinci Si to Da Vinci Xi: realistic times in draping and docking the robot. J Robot Surg 2020;14:835-9. [Crossref] [PubMed]
- Senellart P, Saint-Jalmes G, Mfam WS, et al. Laparoscopic versus full robotic Roux-en-Y gastric bypass: retrospective, single-center study of the feasibility and short-term results. J Robot Surg 2020;14:291-6. [Crossref] [PubMed]
- Beckmann JH, Mehdorn AS, Kersebaum JN, et al. Pros and cons of robotic revisional bariatric surgery. Visc Med 2020;36:238-45. [Crossref] [PubMed]
- Snyder B, Wilson T, Woodruff V, et al. Robotically assisted revision of bariatric surgeries is safe and effective to achieve further weight loss. World J Surg 2013;37:2569-73. [Crossref] [PubMed]
- Gray KD, Moore MD, Elmously A, et al. Perioperative outcomes of laparoscopic and robotic revisional bariatric surgery in a complex patient population. Obes Surg 2018;28:1852-9. [Crossref] [PubMed]
- Rebecchi F, Ugliono E, Allaix ME, et al. Robotic Roux-en-Y gastric bypass as a revisional bariatric procedure: a single-center prospective cohort study. Obes Surg 2020;30:11-7. [Crossref] [PubMed]
- Nasser H, Munie S, Kindel TL, et al. Comparative analysis of robotic versus laparoscopic revisional bariatric surgery: perioperative outcomes from the MBSAQIP database. Surg Obes Relat Dis 2020;16:397-405. [Crossref] [PubMed]
- Dreifuss NH, Mangano A, Hassan C, et al. Robotic revisional bariatric surgery: a high-volume center experience. Obes Surg 2021;31:1656-63. [Crossref] [PubMed]
Cite this article as: Ponce CJ, Guerron AD, Sudan R. Robot-assisted revisional bariatric surgery. Dig Med Res 2021;4:35.


