Role and timing of colonic stenting in colorectal cancer
Introduction
Large bowel obstruction is a common condition familiar to general surgeons and colorectal surgeons alike. Mechanical large bowel obstruction is primarily caused by malignancy with a relatively small number of cases being comprised of benign conditions. These include diverticular strictures, radiation related strictures, inflammatory bowel disease related strictures and sequalae of ischemia or prior surgery. Colorectal cancer may present by way of large bowel obstruction in as many as 8% to 29% of cases (1), although this will vary significantly depending on uptake of screening and ability to detect colorectal cancer prior to it becoming symptomatic. Thirty percent of cases that present with large bowel obstruction are stage IV based on radiology at time of diagnosis (2). This means that a third of the time, both treatment for the primary which is presenting symptomatically is required, but additionally systemic chemotherapy is required to treat the metastatic disease burden and in cases where the burden of disease is high in volume, there may be significant urgency around this. It is therefore desirable to be able to treat the symptoms associated with the primary cancer without creating morbidity that may either delay or impede the ability to provide systemic treatment. In general, the management of a symptomatic primary colorectal cancer can consist of either resecting the tumour and then either performing restorative surgery or constructing an ostomy, diverting the obstructing cancer by way of construction of an ostomy, performing intestinal bypass surgery to bypass the obstruction or by placing an endoluminal stent to relieve the obstruction. Performing surgery in the emergency context has historically been associated with morbidity rates of up to 60% and permanent stoma rates of 40% (3). Some historical studies have also suggested a death rate of up to 20% in this context (4). Stenting has been available since 1991 (5) and in appropriate cases affords patients the opportunity to have their obstruction relieved with a minimally invasive procedure without significant recovery periods, and where appropriate, the ability to then commence systemic chemotherapy treatment without delay. It may also allow the avoidance of an ostomy being created, which has the potential to impact on patient psychological well-being and quality of life (6). Nonetheless not all patients are best suited to this approach and even in patients who are suited to this approach this is not entirely free of risk and as such, all options need to be discussed with patients. This review gives an overview of the role and timing of stenting in colorectal cancer as well as details on how stents are placed, and potential complications and pitfalls associated with their use.
Indications
In the most absolute sense, colonic stenting is at least relatively indicated whenever there is malignant obstruction of the large bowel without an absolute indication for surgery such as peritonitis or perforation. Indications can broadly be divided into stenting in the setting of palliation or placing stents for bridging to more definitive surgery. Bridging is the placement of a stent where the assessment is that the colorectal cancer is to be treated with curative intent. Here the purpose of stenting is to allow decompression and relief of obstruction. This allows further work-up including colonoscopy, giving bowel preparation, and proceeding to resection in an urgent elective matter allowing the use of a minimally invasive technique for resection rather than performing a laparotomy. It also allows a single stage restorative procedure rather than performing multistage surgery with a stoma at the index procedure. They are also select instances where bridging may be appropriate because it is medically inappropriate or unsafe to perform surgery until further optimisation and assessment has taken place. In general assessment begins with determining if the patient presenting with large bowel obstruction is known to have colorectal cancer or if they are presenting de novo with an obstruction. In the de novo setting up to 25% of cases may be from non-malignant pathology such as diverticular disease or inflammatory bowel disease, and as such the impression that this is indeed due to malignancy requires endoscopic confirmation. Stenting is not indicated in the non-malignant context. It should also not be performed if there is peritonitis or concern for proximal colonic ischemia. Some lesions are more technically difficult to stent such as lesions at the flexures or in the right colon and these are relative contraindications. Patients who can be cured with single stage (restorative) surgery and are fit to undergo such surgery should generally be considered for a primary operative approach. Patients who would be undergoing non-curative surgery or would require multistage surgery or formation of an ostomy may be considered for stent placement as the initial intervention for their obstruction. One of the implications of this is that most right sided obstructions are not considered for stenting as they are very often amenable to single stage restorative surgery. They are also technically more challenging to stent. There may also be a higher risk of perforation related complications owing to the colon being thinner than on the left (7). Nonetheless these lesions can be stented if single stage surgery with anastomosis is not likely to be feasible. An ostomy will frequently be needed for left sided resections done in the setting of peritonitis or ischaemia. A diverting ostomy will be needed in lesions that cannot be stented and are not resectable.
Settings where stenting may be considered are:
- Palliative: SEMS can be used as a palliative measure in patients with incurable malignant large bowel obstruction with either chronic or acute co-morbidities with or without metastatic disease;
- Bridging: patients current condition precludes surgery but this is expected to improve after stenting allowing subsequent surgical management
Insertion technique
Insertion begins with appropriate case selection and then selection of an appropriate stent design and size. Although there are a range of stents, in practice placing a through the scope (TTS) uncovered stent with a stent length of approximately 9 cm and a diameter of 25 mm will allow the majority of malignant strictures causing large bowel obstruction to be adequately treated endoscopically. It is important that the chosen stent will overlap the stricture by at least 2cm at each end. The majority of large bowel obstructions will be diagnosed by means of a computed tomography (CT) scan. An initial assessment of the location and approximate length of the area of malignant stricturing can be made by carefully assessing the scan. If further detail in relation to this is required to make a decision about stenting suitability, or indeed the choice of stent, this information can be obtained by obtaining rectal contrast enhanced imaging either by means of a gastrografin enema and fluoroscopy study or a rectal contrast enhanced CT scan. Some obtain these studies as a matter of routine, but often it is possible to stent adequately and appropriately in the absence of these additional studies. Obtaining these additional studies increases one overall likelihood of success but stenting can be performed successfully in their absence. Our preference is to place the stent in the operating theatre. This means that if a stent complication arises that requires surgery, or the stent cannot be placed, and surgery is then required this can take place immediately. The procedure can typically be performed under conscious sedation without the need for general anaesthesia. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) is a very helpful adjunct for oxygenating patients. The patient is positioned on a fluoroscopically capable operating table either in the left lateral position or in the Lloyd-Davies position according to operator preference. Both positions are quite satisfactory, and the patient can indeed be repositioned if a better fluoroscopic image can be obtained by doing so. An adult colonoscope is then inserted and advanced to the point of obstruction and the lesion identified. If required, endoscopic biopsies can be taken at this time and this should be done prior to stent placement. A flexible guidewire (our preference is to use a 0.035-inch Jagwire™ with a 5cm hydrophilic tip) can then be placed through the working channel of the colonoscope and used to negotiate the stricture. If the wire is being placed around a corner and this is difficult an ERCP cannula can be used to insert the wire through. Negotiation can be confirmed with fluoroscopy. If there is any doubt or difficulty encountered, contrast can be injected via an ERCP catheter through the stricture to assess it further, and also to aid in confirmation that it has been traversed by the wire. Once the wire is in placed through the stricture, the stent delivery catheter can be placed TTS working channel and over the wire and advanced through the stricture. There are markings on the delivery catheter and these and fluoroscopy are used to guide how far to advance the delivery catheter. Once in place the stent can then be deployed from the delivery catheter. There is a tendency when this occurs for the stent to spring forward and so allowance for this and adjustment of the scope and catheter position should be made continuously to ensure the stent deploys optimally. On occasion if the stricture is not overly tight and in a favourable position within the colon, the delivery catheter can be placed through the stricture without prior placement of a guidewire. If the stent is deployed and it is noted that the positioning is not idea, up to a certain point the stent can be returned to the delivery catheter and further adjustment made as the stent will be still constrained. The point at which the stent becomes unconstrained does vary somewhat from delivery system to delivery system and note should be made of this at the start of the procedure. Once fully deployed, there will often be a sudden rush of gas or stool signifying relief of the obstruction. Fluoroscopically, ideal placement will show a “diablo sign”, with proximal and distal flaring of the stent and narrowing in the centre which is symmetrical and centred over the centre of the strictured area. If the area of stricturing is very long or the stent for some reason has not captured the proximal extent of the stricture, it is possible to place a second stent within the first stent to extend the area of stenting and deal with that. If this is the case, the 2 stents should overlap one another by 2 cm. Our practise is typically to place the patient on a liquid diet initially, obtain a formal X-ray post procedurally to confirm expansion of the stent (typically the next day) and generally patients are kept in the hospital for one night. Patients are placed on a bowel regimen of osmotic laxatives to keep the bowel actions on the looser and softer side to avoid stool impaction in the stent. Although the stent creates a patent lumen, that segment of the colon is generally functionally adynamic. Overall the most critical aspects of the procedure are ensuring safe passage of the guidewire and then when deploying the stent having the stent centred on the lesion and fully traversing it and the distal flange being proximal to the rectum in left sided stenting to avoid tenesmus from the stent.
Types of stents currently commercially available
Colonic stents are generally uncovered stents and typically have a flared or flanged region proximately and distally. They have historically been manufactured from nitinol or from elgiloy. Such stents are termed SEMS (self-expanding metallic stents). These alloys possess the mechanical property of super elasticity which means that they can be compressed into a delivery device and then become unconstrained and expand when released from the delivery device. They are also able to be relatively sharply angulated to allow them to be placed across lesions at areas of angulation such as the recto-sigmoid junction or the hepatic or splenic flexure. Comparing the two materials, nitinol is comprised of nickel and titanium whereas elgiloy is comprised of cobalt, chromium and nickel. It is more corrosion resistant and capable of generating greater radial forces than nitinol is. Nitinol has greater elasticity. The stents currently commercially available are all made of nitinol. Stents placed in Australia are almost invariably uncovered stints however covered designs do exist. Generally speaking, the benefit of the uncovered stent is it is less liable to migrate. A covered stent may have reduced tissue ingrowth which may improve the duration of stent patency. Stents that are placed by surgeons or endoscopists tend to have a TTS design whereby they are constrained within a delivery catheter that is passed through the working channel of a colonoscope. There are however over the wire designs that are intended to be used by radiologists and placed solely under fluoroscopy guidance. Table 1 gives a list of TGA approved stents and their specifications. In terms of commercial use, the Boston Wallflex and Cook Evolution represent the majority of usage in Australia and not all TGA approved stents are readily commercially available.
Timing of stent placement
Obstruction of the large bowel can be described as being either endoscopic, radiologic or clinical in nature. In the majority of patients there will be an orderly progression of this whereby endoscopic obstruction will precede radiological obstruction which will in turn precede clinical obstruction. That is to say a patient who is given about preparation which they tolerate and then has a colonoscopy which demonstrates a lesion in the colon with a narrow lumen and an inability to pass any type of endoscope, will typically not have a CT scan That demonstrates obstruction but the scan typically will demonstrate thickening or an abnormality consistent with the lesion. Similarly, a patient can have a CT scan that shows obstruction but remain clinically free of symptoms. Patients with clinical symptoms invariably have radiological obstruction and endoscopic obstruction. It is self-evident that if a patient presents with clinical obstruction and the most appropriate way to deal with the obstruction is by endoluminal stenting the timing of this would be of an emergent nature. However, some patients will have endoscopic or radiological obstruction and no clinical obstruction and have a metastatic disease burden. These patients will need to commence systemic therapy and the question then becomes should I stand be placed prior to the onset of any clinical symptoms. Certainly, in cases where there is evidence of radiological obstruction this is prudent. In cases where there is no radiological obstruction and only endoscopic obstruction, a case by case discussion is required. In general cases where the lumen is already very tight, and progression may render stenting not possible or cases where the metastatic disease burden is very high (>50% of liver volume for e.g.) and interruptions to chemotherapy may spell the end of any further active oncological treatment, stenting should be strongly considered. All such cases should be discussed in a multidisciplinary tumour board meeting. In case where a stent has been placed in a bridging capacity the optimal timing is thought to be around 10 days post as this allows for optimal decompression, and also optimisation of medical conditions and nutrition without allowing such time to pass as scarring and adhesions related to any reaction to the stent causing technical issues. This is reflected in the ESGE guidelines (9).
Potential complications and safety profile
From a safety perspective, several level II and III studies have examined periprocedural mortality comparing stenting with surgery to relief obstruction, typically either diversion, Hartmann’s resection or intestinal bypass and generally not resection with restoration of continuity. Those studies show very low overall mortality rates in the immediate post-procedure setting without meaningful differences between the 2 groups (7,10-12). Complications arising from stenting may be divided into early complications and late complications. In terms of general success rates in achieving the relief of obstruction, several reviews have looked at this matter and the generally quoted technical success rate of stenting is in the order of 94% and the clinical success rate of stenting in the immediate sense is 91% (13). Case selection will obviously have some bearing on this and in general terms stenting for extrinsic compression of the colon rather than a luminal disease process is associated with a lower rate of success, around 75% radiologically and 50% clinically respectively. However, dealing with extrinsic compression surgically is much more likely to result in the formation of an ostomy and this should also be borne in mind when deciding what to initially attempt treatment with. Early complications are principally either failure to relieve obstruction, due to an inability to place the stent or a failure for the obstruction to clinically resolve despite adequate stent placement, and perforation as a complication of stent placement. Perforation rates in the literature vary and many of the studies are small case series but the generally quoted rate is around 3–5% (13). Late complications include delayed perforation, tumour ingrowth with recurrent obstruction, faecal impaction causing obstruction, bleeding and stent migration. Delayed perforation has been cited as a concern if patients receive systemic therapy with bevacizumab with some studies suggesting a relative risk factor of 3.6 increased perforation risk. However, the data on this is conflicting and a recent publication looking at 104 patients receiving bevacizumab and receiving colonic stents showed an overall perforation rate of 1% 3 times lower than in the 95 patients in a control group (14). In clinical practice stenting should not be overlooked for this reason in elderly and co-morbid patients and overall cannot be made entirely as a data-based decision as the available data is limited, conflicting and controversial (15). Tenesmus can occur if stents extend distal to the rectosigmoid junction. Of these complications, stent migration is the most common in many series and in others re-obstruction due to tumour progression is most common. The actual clinical significance of these complications is obviously quite variable and will also vary depending on the individual clinical situation. For instance, if a patient is stented with high volume metastatic disease, goes on to receive systemic chemotherapy and has an excellent response with marked tumour regression, resulting in the stent migrating distally and being passed per rectum, this is a good clinical outcome. This stent has served its purpose, and the decision now is whether to continue with the systemic treatment or to interrupt it and consider resecting the primary tumour. On the other hand, if a patient develops tumour perforation and becomes markedly septic, and this is in the context of being mid cycle of chemotherapy with profound neutropenia, this carries a significant mobility and mortality. With respect to tumour regrowth and re-obstruction several studies have shown that if a stent is placed and despite best supportive care there is disease progression, stents will remain patent for at least 90 days (16). As such if a patient presents with high volume metastatic disease, it is probable that if they fail to respond to treatment a stent will maintain bowel lumen patency for the duration of their life. If they have a good response to systemic treatment one would anticipate a good response to the primary tumour as well and in that setting would not anticipate obstruction. If, however, it appears that the volume of metastatic disease is such that even with a poor response to treatment there may be a significant period of time the patient may continue to survive for, the potential for obstruction to occur before the patient is overwhelmed by metastatic disease burden bears consideration. In that setting, consideration should either be given to performing surgery as the initial endeavour to deal with the primary tumour or counselling the patient that re intervention may be required. This may include operating, or it is described that stenting can be re-performed.
Case examples
We here present 3 case examples. One where a patient is believed to be at high risk of impending obstruction and has an urgent need for chemotherapy, another where a bridging stent is placed in a patient who needs medical optimization, and a third where patient presents acutely with a malignant large bowel obstruction.
Case 1
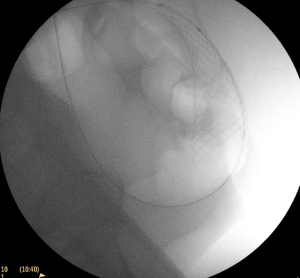
A 54-year-old previously medically well women was investigated with a CT scan for vague right upper quadrant pain that had been present for 2 months. Her BMI was 19 and she had lost 5 kg over the same period. The CT scan demonstrated hypodense lesions consistent with GI metastases involving all 8 liver segments and approximately 65% of the liver parenchyma. Thickening was present in the mid to distal sigmoid colon. A colonoscopy done to determine a primary source for malignancy demonstrated a large tumour corresponding to the site of thickening on the CT scan with a pinhole lumen. Endoscopic biopsies confirmed adenocarcinoma and molecular testing showed bRAF mutant, k-ras wildtype. Concern was expressed that the patient may develop clinical obstruction in the early phases of commencing chemotherapy and in the setting of high-volume disease pre-emptive treatment of the primary lesion was recommended at a multidisciplinary tumour board meeting. A 9 cm/25 mm Wallflex stent was placed without difficulty and with a good radiological (see Figure 1) and clinical result. The patient spent 1 night in hospital for observation and was discharged home eating and without obstruction on an aperient regimen. She commenced systemic therapy in the form of triplet chemotherapy FOLFOXIRI and Avastin. Two months later in the midst of her 4th cycle she presented acutely with fever and left sided abdominal pain. CT scan demonstrated free gas in the abdomen and small volume free fluid in the left paracolic gutter. CT scan also confirmed significant reduction in the size of all the liver lesions and the primary colonic tumour could no longer be seen. She proceeded to an emergent laparoscopy where she underwent resection of the sigmoid colon with the stent in situ and removal of the specimen and stent via planned colostomy site and formation of an end colostomy. Recovery from this was rapid with 4 days in hospital and chemotherapy being able to be recommenced 2 weeks from the time of the laparoscopy without issue. Histopathology suggested a grade 3 tumour response to the chemotherapy. Ultimately disease control was achieved for 18 months at which point the patient developed increasing metastatic disease burden in the liver as well as peritoneal disease and rising CEA levels.
Case 2
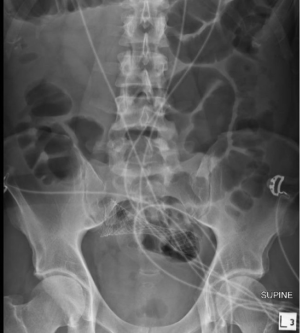
A 44-year-old male presented acutely with 10 days of vomiting, abdominal pain and progressive abdominal distension. CT scanning demonstrated large bowel obstruction with transition point in the mid sigmoid colon and the impression of an apple core lesion at this level. He had a competent ileocecal valve but no clinical or radiological evidence of caecal ischaemia. Imaging did not suggest metastatic disease and a CT scan of the chest also did not suggest this. CEA was 8. There was a significant medical history of arrhythmogenic right ventricular cardiomyopathy with a prior cardiac arrest, an implanted cardioverter defibrillator device (AICD), difficult to control atrial fibrillation, cardiomyopathy with poor ejection fraction documented on most recent echocardiogram and amiodarone induced thyrotoxicosis, as well as significant renal impairment, a seizure disorder and prior cerebrovascular accidents. The patient was anticoagulated with apixaban. He had not been able to take any of his medications for 3 days due to his GI symptoms. At presentation he had atrial fibrillation with rapid ventricular response at a rate of 140–160. Perioperative medical input was sought, and it was felt it would be ideal to place a bridging stent and then seek to medically optimise his co-morbidities and formally re-evaluate his cardiac function prior to attempting resection. A 9 cm/25 mm Wallflex stent was placed without difficulty and with a good radiological and clinical result. This is shown in Figure 2. The patient was initially recovery in ICU to allow his cardiac rhythm to be optimally medically managed. He was able to go home after 3 days with this well controlled. He underwent uncomplicated elective resection of his tumour by means of a robotically assisted high anterior resection with primary anastomosis 14 days later. This allowed adequate time for optimal colonic decompression, comprehensive medically assessment and optimisation. Pathology demonstrated high risk stage 2 disease (T3N0 with perineural invasion presenting with obstruction) and he was adjuvant systemic therapy in the form of capecitabine in spite of his significant co-morbidities in lieu of his young age. He was well and free of recurrence at follow up 9 months later.
Case 3
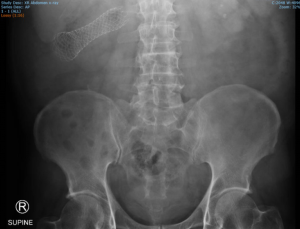
A 63-year-old man presented with a large bowel obstruction. This was in the context of having previously been diagnosed with Stage 4 colorectal cancer with a primary tumour at the hepatic flexure. At the time of original diagnosis there was high volume disease in the liver with all segments effected, peritoneal and omental disease, ascites and marked nodal disease. ECOG performance status was 1. Biopsy confirmed K-ras mutant adenocarcinoma and systemic therapy with capecitabine was initiated. This was initially well tolerated but the patient then developed skin toxicity requiring the chemotherapy to be temporarily discontinued. During the chemotherapy free period the patient had a myocardial infarction resulting in an ejection fraction of 25% and then developed obstruction from the primary tumour. This was treated with a 9 cm/25 mm Wallflex stent without difficulty and with a good clinical and radiological result, show in Figure 3. The patient was then recommenced on chemotherapy in the form of reduced dose 5-FU, irinotecan and Avastin. This was reasonably well tolerated and resulted in stable, slightly reduced volume metastatic disease with no issues with the stent or symptoms from the primary tumour at follow up 12 months later.
Summary
Overall colonic stenting plays an important role in the management of a significant number of patients with malignant large bowel obstruction. It is an especially important technique when patients present with Stage IV disease and the treatment priority is systemic chemotherapy or palliative treatment. There may be a select role for placing stents in a bridging capacity, but this is on a more select basis and is overall an area of more controversy unlike in the palliative context where the role is clearly established.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Eva Segelov) for the series “Colorectal Cancer” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-133). The series “Colorectal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg 1994;81:1270-6. [Crossref] [PubMed]
- Nguyen DA, Mai-Phan TA, Do PTT, et al. Emergency surgery for obstructed colorectal cancer in Vietnam. Asian J Surg 2020;43:683-9. [Crossref] [PubMed]
- Mauro MA, Koehler RE, Baron TH. Advances in Gastrointestinal Intervention: The Treatment of Gastroduodenal and Colorectal Obstructions with Metallic Stents. Radiology 2000;215:659-69. [Crossref] [PubMed]
- Iversen LH, Bülow S, Christensen IJ, et al. Postoperative medical complications are the main cause of early death after emergency surgery for colonic cancer. Br J Surg 2008;95:1012-9. [Crossref] [PubMed]
- Dohmoto M. New method-endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig 1991;3:1507-12.
- Karadağ A, Menteş BB, Üner A, et al. Impact of stomatherapy on quality of life in patients with permanent colostomies or ileostomies. Int J Colorectal Dis 2003;18:234-8. [Crossref] [PubMed]
- Baik SH, Kim NK, Cho HW, et al. Clinical outcomes of metallic stent insertion for obstructive colorectal cancer. Hepatogastroenterology 2006;53:183-7. [PubMed]
- Sachdev A, Packey C, Gress F. Gastrointestinal Stenting: A Review. Gastroenterol Endosc News. 2018;(February).
- Kye BH, Kim JH, Kim HJ, et al. The optimal time interval between the placement of self-expandable metallic stent and elective surgery in patients with obstructive colon cancer. Sci Rep 2020;10:9502. [Crossref] [PubMed]
- Fiori E, Lamazza A, De Cesare A, et al. Palliative Management of Malignant Rectosigmoidal Obstruction. Colostomy vs. Endoscopic Stenting. A Randomized Prospective Trial. Anticancer Res 2004;24:265-8. [PubMed]
- Nagula S, Ishill N, Nash C, et al. Quality of Life and Symptom Control after Stent Placement or Surgical Palliation of Malignant Colorectal Obstruction. J Am Coll Surg 2010;210:45-53. [Crossref] [PubMed]
- Osman Rashid. The cost effectiveness of self-expanding metal stents in the management of malignant left-sided large bowel obstruction. Colorectal Dis 2000;2:233-7. [Crossref] [PubMed]
- Sebastian S, Johnston S, Geoghegan T, et al. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 2004;99:2051-7. [Crossref] [PubMed]
- Lee JH, Emelogu I, Kukreja K, et al. Safety and efficacy of metal stents for malignant colonic obstruction in patients treated with bevacizumab. Gastrointest Endosc 2019;90:116-24. [Crossref] [PubMed]
- van Halsema EE, van Hooft JE. Bevacizumab in patients treated with palliative colonic stent placement: Is it safe? Gastrointest Endosc 2019;90:125-6. [Crossref] [PubMed]
- Johnson R, Marsh R, Corson J, et al. A comparison of two methods of palliation of large bowel obstruction due to irremovable colon cancer. Ann R Coll Surg Engl 2004;86:99-103. [Crossref] [PubMed]
Cite this article as: Buxey K, Chouhan H. Role and timing of colonic stenting in colorectal cancer. Dig Med Res 2021;4:36.