
A close view on histopathological changes in inflammatory bowel disease, a narrative review
Introduction
The histological examination of surgical specimens or endoscopic biopsies is a central element in establishing a diagnosis of inflammatory bowel disease (IBD). Ulcerative colitis (UC) and Crohn’s disease (CD) encompasses the two major forms of IBD (1,2). Both are chronic remittent diseases, with an increasing incidence worldwide, that are causing severe morbidity and reduced quality of life in affected individuals (3,4). UC and CD differs in pathophysiology, affected parts of the gastrointestinal (GI) tract, symptoms, complications and treatment regimens. Nevertheless, some overlap in clinical and histopathological manifestations can indeed be seen, which can lead to diagnostic doubt and mix-up of the correct diagnosis. In general, UC affects the colon only and spreads continuously from rectum to more proximal colonic segments. The inflammation is mostly superficial and complicated by erosions, ulcers and bloody diarrhea with mucinous stool (1). CD can in contrast affect any part of the GI tract, and is characterized by discontinued inflammation (skip lesions) with transmural inflammation that leads to fibrosis, fistula and strictures, causing symptoms such as chronic abdominal pain, diarrhea, obstruction or perianal lesions (2). The pathophysiology underlying IBD is complex and not completely understood. It includes a leaky epithelial barrier and a dysregulated immune response towards the intestinal luminal content. Several factors such as genetics, environment, nutrition, and composition of the intestinal microbiome all contribute to susceptibility for IBD (5-7).
An accurate and timely diagnosis of IBD is important in order to start appropriate treatment. As no single golden-standard modality for the diagnosis of IBD exists, the diagnostic procedure should involve a multidisciplinary approach, including specialists in the fields of gastroenterology, pathology and radiology. The diagnosis is based on a combination of clinical symptoms, endoscopy, histopathology, imaging and laboratory testing whenever appropriate to rule out any differential diagnoses, such as infection or other type of colitis (8). The diagnostic criteria for IBD have not changed much over several decades. This also applies to the histopathological features of IBD, which have been well-known for many years and described in comprehensive textbooks (9). Some guidelines and consensus reports on the histopathology examination of IBD specimens do exists, of which one of the most comprehensive was published by the European Society of Pathology (ESP) and the European Crohn’s and Colitis Organization (ECCO) in 2013 (10,11) with an ECCO position paper update in 2020 (12). In addition, recent studies points to the importance of applying histopathological assessment not only to establish the diagnosis, but also as a tool to assess responses to therapy (the concepts of “histological remission” and “treat-to-target”) and to serve as a predictive factor for disease- and treatment outcome (13).
A basic knowledge of histopathology is important for all clinicians working with IBD in order to interpret and act on the information provided in the pathology report. Here we review the histopathological changes in IBD with an emphasis on typical features that aids in establishment of the diagnosis. We cover the histopathologic approach to the diagnostic specimens and pinpoint diagnostic challenges the pathologist faces. Furthermore, the usage of histological indexes for evaluation of disease activity is discussed. We present the following article in accordance with the narrative review reporting checklist (available at http://dx.doi.org/10.21037/dmr-21-1).
Procedures for the histological diagnosis of IBD
When IBD is suspected, the diagnostic biopsies should ideally be obtained before initiation of medical therapy because drugs can induce changes in histological morphology (11). For optimal interpretation of a histological specimens, access to detailed clinical information is essential (11). The progression of the histological findings that are suitable for the diagnosis of IBD is dependent of disease-activity and time. Thus, initial onset and later stages of disease show different microscopic characteristics. At least two endoscopic biopsies from a given bowel segment is recommended in order to detect minimal changes (14) and should represent the most affected lesions, including the edge of ulcers. Biopsies from each bowel segment should be sent in separate containers rather than pooling different segments in the same container (13). Biopsies should immediately be fixed in buffered formalin followed by paraffin embedment for proper histological examination. However, surgical specimens should preferentially be grossly evaluated before fixation, as macroscopic changes are best studied on fresh tissue. This can with benefit include photographic documentation. Awareness of lesions suspicious for dysplasia or cancer is important. Tissue sampling for microscopy of surgical specimens should include terminal ileum, appendix, lymph nodes and multiple samples from affected and normal appearing mucosa whenever feasible. An optimal number of sections have not been established. Though, it is recommended that sections should be acquired for every 10 cm of resected bowel, in addition to sections from relevant focal changes. The distance from areas of tissue sampling to resections margins should be noted (11,15).
Because the histological changes in IBD can be focal, serial sections in the tissue block increases diagnostic accuracy and should be performed in order to identify subtle changes (10,16,17). There is no definite number of sections required, but the ESP state that two-three tissue levels is preferred, and if feasible step-section each consisting of five or more sections can be applied (10-12). Routine staining with hematoxylin and eosin (H&E) is appropriate for diagnostic purpose. Whereas special-stains or immunohistochemistry are not needed routinely, they can be used as supplement is selected cases, e.g., a stain for cytomegalovirus (CMV) might be of relevance (11).
Gross pathology
UC
Classically, gross examination of a UC surgical resection specimen reveals diffuse and continuous inflammation with no skip lesions. UC affects the rectum with variable, but continuous, involvement of the more proximally colon (Table 1). The shift between the inflamed and normal mucosa is distinct (Figure 1A) (11). The extent of UC can be classified according to the Montreal classification (18) as (I) ulcerative proctitis (only rectum is affected), (II) left-sided or distal UC (colonic involvement distal for the left flexure), and (III) extensive UC or pancolitis (colonic involvement proximal to the left flexure). However, skip lesions can been seen in UC in some cases, and it is important to be aware of this matter in order to avoid misinterpretations as CD. In about 20% of cases of extensive UC, involvement of the terminal ileum with inflammation known as “backwash ileitis” occurs (19). Inflammation surrounding the appendiceal orifice, can be found in left-sided colitis, which is known as “cecal patch” (Figure 1B) (11,20). Medical therapy can affect the extend of disease and result in patchiness, especially in the form of rectal sparing after topical treatment with glucocorticoids.
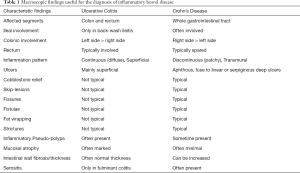
Full table
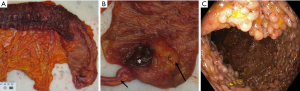
In active disease, the mucosal surface typically become edematous, granular and vulnerable. Ulcers are, if present, frequently minor and oriented longitudinally in relation to the teniae coli. In severe disease ulcers undermining the nearby mucosa may lead to mucosal denudation. Inflammatory pseudo-polyps may arise as a consequence of widespread ulceration with sparing of areas of remaining mucosa (Figure 1C). In severe colitis, serositis may be present and changes of the mucosa is not sufficiently distinct to macroscopically differentiate UC from CD. A severe complication to fulminant disease is toxic megacolon, where the intestinal wall becomes thin and dilated (9).
In the quiescent stage of UC, the mucosa may seem totally normal, or exhibit diffuse granularity and inflammatory pseudo-polyps. After a long-standing UC, the intestinal wall may become thick and contracted with an atrophic mucosal surface (9).
CD
CD can affect any part of the GI tract, from mouth to anus, but most commonly involves the terminal ileum. At time of diagnosis approximately one third of patients with CD have small bowel involvement only, one third large bowel involvement only and one third have ileo-colonic disease (2). Typically, macroscopic examination of a resection specimen shows a patchy (discontinuous) pattern of inflammation with areas of normal mucosa interspersed between inflamed areas. Transition from involved to normal areas is usually sharp (Figure 2A). The serosal surface of an involved intestinal segment may be hyperemic and covered with inflammatory exudate. In long-standing disease serositis and adhesions can arise. In CD of the small intestine, and less commonly also in the colon, mesenteric adipose tissue expands towards the anti-mesenteric surface of inflamed segments. This is termed “fat wrapping” or “creeping fat”, which is a valuable diagnostic feature for the diagnosis of CD (Figure 2B). The underlying mechanism is still not fully understood, but recently it has been suggested that translocation of the gut microbiota to mesenteric adipose tissue is involved, so the fat creeps in order to incapsulate the bacteria to prevent systemic spread (21).
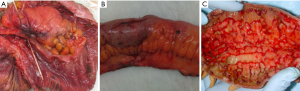
The earliest mucosal changes are minor aphthous ulcers that often arise near lymphoid follicles of the intestinal wall and are surrounded by relatively normal appearing mucosa. When the small ulcers fuse, they form larger and deeper linear ulcers with overhanging mucosal edges, giving rise to the characteristically cobblestone appearance (Figure 2C). With time, healing of ulcers can leave depressed scars and as in UC inflammatory polyps can occur (11).
At segments of transmural inflammation, the intestinal wall can be thick and stiff due to fibrosis and fibromuscular proliferation. In long-standing disease, strictures sometimes arise, which more often is observed in the small than in the large intestine. In complex cases, fissures and fistulous tracts, and intermural- or abdominal abscesses can develop, which is a typical hallmark of CD. Perforations to the abdominal cavity are uncommon but can be seen as a complication of fissures or fistulas, or as a result of superimposed ischemia or infection. Fibrous adhesions may close off sites of perforation, so they may not be visible upon gross examination (9). Another hallmark of CD is perianal manifestations, which at some point develop in a large proportion of patients with CD. This includes skin tags, ulcers, blind sinus tracs, fissures, fistulae, abscesses and strictures (11).
Microscopy
The histological diagnosis of IBD is based upon assessment of four main features: (I) mucosal architecture, (II) lamina propria and submucosal cellularity, (III) neutrophil granulocyte infiltration, and (IV) epithelial abnormality (10). Thus, knowledge of the normal histology of GI-mucosa is essential for optimal interpretation of biopsy specimens.
- The morphologic features of mucosal architecture changes are termed crypt architectural distortion. Normal crypts are straight, parallel and extend from the surface to nearly above the muscularis mucosae. The crypt architectural distortion observed in IBD is characterized by irregularly arranged, dilated, branched, and/or shortened crypts. This can both be a sign of ongoing inflammation or the process of regeneration (11). However, the crypts in the normal rectum can show minor abnormalities resembling architectural distortion (9).
- Abnormal cellularity of the lamina propria refers to an increased and altered distribution of cell types that are normally present. Lymphocytes and plasma cells are always found in the colorectal lamina propria. In the normal colon, the number of inflammatory cells varies depending on anatomical site. In general, the cecum and right colon are most cellular with a progressive decrease in the cellularity from the right to the left side (9). Also, a focally increased number of lymphoid cells are present in close relation to the lymphoid tissues of the normal gut associated lymphoid tissue (GALT) (22). The abundance of eosinophil granulocytes varies a lot between normal individuals (9,12).
- Neutrophil granulocyte infiltration is the hallmark of active disease. Neutrophils can be found in the lamina propria or they can invade the crypt surface epithelium (termed cryptitis) and the lumen of crypts forming crypt abscesses. Neutrophils are in general not found in normal mucosa (12).
- The epithelial abnormalities found in IBD includes mucin depletion, metaplastic changes and surface epithelial damage. Mucin depletion can be defined as a decreased number of goblet cells or decreased amount of intracellular mucin. Metaplastic changes are seen in the form of pyloric gland metaplasia (a typical sign of chronicity in ileal involvement of CD) or Paneth cell metaplasia. The damage of the surface epithelium, e.g., focal cell loss, flattening, erosions, and ulcers reflects the activity of disease. None of these findings are for sure disease specific and might be observed in UC, CD and other types of colitis as well (10).
The histologic findings in IBD differ depending on the clinical phase of disease and the grade of inflammatory activity. IBD can be categorized as “Chronic active”, “Chronic inactive” or “Active” (without features of chronicity) (9). The histological features that define chronicity (regardless of “activity”), are crypt architectural distortion, crypt atrophy, diffuse mixed lamina propria inflammation, basal plasmacytosis, basally located lymphoid aggregates, and Paneth cell metaplasia.
Inflammatory activity is defined by the presence of neutrophils. Neutrophilic cryptitis, crypt abscesses, hemorrhage, erosions, ulceration and necrosis are all features of active inflammation. The pathology report should describe the histological features of chronicity and activity and preferably also grade the degree of activity as mild/moderate/severe. The grading should be performed in the most affected biopsy, and if feasible a separate grade should be assigned to biopsies from different bowel segments. The need for grading has led to introduction of several scoring systems or histological indexes. There is no golden standard for grading of activity, but one example is the Nancy Index (23), which in 2020 was recommended as suitable for daily clinical grading of UC by ECCO (12). The Nancy Index has five grades, from 0 to 4. Grade 0 represents a normal biopsy with no (or only a little) chronic inflammation and no active inflammation. Grade 1 (“chronic inflammation”) means that chronic inflammation, but no active inflammation is present. Grade 2 (“mild activity”) means only a few neutrophils that are difficult to see and no ulceration. Grade 3 (moderate-severe activity”) indicates a moderate or severe neutrophil infiltrate that is easy to see but no ulceration. Grade 4 (“ulceration”) indicates ulceration of the surface epithelium (24).
UC
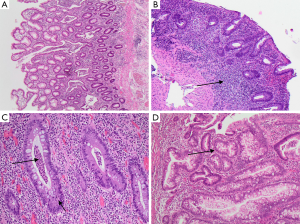
In UC, the mucosa is characteristically involved in a continuous manner. Sometimes also the superficial submucosa is involved. The changes are often most distinct in the distal colon and rectum. The inflammatory infiltrate diffusely expands the lamina propria and is mainly lymphoplasmacytic with superimposed neutrophils (Figure 3A). The number of eosinophils is variable. The density of plasma cells is usually highest in the basal parts of the lamina propria, which is termed basal plasmacytosis (Figure 3B). Crypt abscesses (Figure 3C) are more common in UC than CD (Table 2). Epithelioid granulomas which is a hallmark of CD, are not identified in UC, where only foreign body granulomas evolved as a response to mucin from ruptured crypts (termed cryptolytic granulomas) are observed (11). Lymphoid aggregates, that may show germinal centers, are common in UC. These are situated basally between crypt bases and the muscularis mucosae (9). Expansion of the lamina propria by the inflammatory cells and the presence of basal lymphoid aggregates contributes to the irregular spacing of the crypts. The architectural distortion is usually more pronounced in UC than CD.
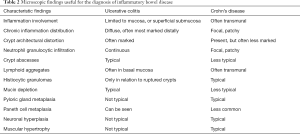
Full table
In early stages of UC, the diagnosis can be challenging. A normal crypt architecture and absence of diffuse lamina propria inflammatory cell infiltration do not necessarily exclude early-stage disease. Basal plasmacytosis might be an initial finding with the highest predictive value for the diagnosis of UC and is identified in up to 38% of patients within 2 weeks after presentation of symptoms (25). In contrast, in a recent study of pre-clinical UC, that were discovered incidentally in “asymptomatic” individuals undergoing endoscopy in a colorectal-screening program basal plasmacytosis were rarely observed (26).
The classical features of longstanding UC are distortion of the crypt architecture and increased lamina propria cellularity. Though, over time this pattern can change, both as a consequence of treatment or natural disease course. During medical treatment, the extent of involvement often declines, resulting in rectal sparing with restoration of the rectal mucosa in about 30–40% of patients. Areas of normal mucosa and discontinuous inflammation can also evolve with time, changing the distribution pattern from the classical UC continuous pattern to a more discontinuous pattern (27,28). This is important to keep in mind to avoid incorrectly changing a diagnosis of UC into CD. Furthermore, longstanding inflammatory activity may increase the risk of dysplasia, which requires extra awareness (Figure 3D).
In clinical remission, histological features reflecting preceding inflammatory activity, such as architectural abnormalities and reduced numbers of crypts may still be seen. During mucosal regeneration, the mucin stores of goblet cells normalize. The inflammatory infiltrate may show variable density, so the lamina propria may be either hyper- or hypocellular. With time, as basal plasmacytosis declines, a normal cellularity and architecture can be restored (13).
CD
CD is characterized by a patchy inflammatory pattern, so that endoscopic biopsies can show varying degree of inflammation, both within a single biopsy and between biopsies form the same bowel segment. Thus, focal microscopic changes such as chronic inflammation and focal crypt architectural distortion next to normal crypts is a diagnostic clue to CD (11). The inflammatory infiltrate in CD can be transmural. Transmural lymphoid aggregates and inflammatory cells that invades the submucosa and even lamina muscularis and may give rise to deep fissures, ulcers and fibrosis (Figure 4A). Transmural changes can only be identified in resection specimens, as biopsies are more superficial. The presence of non-caseating epithelioid granulomas favors a diagnosis of CD, but only if they are found without any relation to ruptured crypts, and identification of a granuloma is not strictly required for establishment of the diagnosis (11). An epithelioid cell granuloma is defined as a collection of at least five epithelioid cells (activated histiocytes) with or without multinucleated giant cells. Granulomas of CD are often vaguely bordered, and necrosis is unusual (Figure 4B,C). Furthermore, granulomas are more frequently found in children than in adults (29).

In biopsies from the terminal ileum an irregular villous architecture is indicative of CD, but if it lies in continuity with a proximal colitis, this can be a sign of backwash ileitis in UC as well (11). Pyloric gland metaplasia may evolve (Figure 4D) and is observed in up to 25% of ileal biopsies from patients with CD (and very commonly in ileal specimens after surgical resection) but only rarely in UC (11).
Pouchitis
The most common surgical procedure for patients with UC requiring colectomy is a proctocolectomy with creation of an ileal pouch anal anastomosis (IPAA) (1). A common complication after this procedure is pouchitis, which constitutes a non-specific idiopathic inflammation of the neo-rectal ileal mucosa. The diagnosis of pouchitis is based on a combination of symptoms, endoscopy and histological findings. If the duration of the inflammation is less than 4 weeks and responsive to treatment with antibiotics, the term acute pouchitis is used. Whereas, longstanding inflammation refractory to treatment is known as chronic pouchitis (30). The changes are often patchy and can be more prominent in the lower and posterior regions of the pouch, therefore several biopsies are often required to confirm the diagnosis (10,12).
In up to 85% patients with a normal functioning pouch, histological signs of chronic inflammatory changes, such as architectural distortion and lymfo-histocytic infiltration of the lamina propria, is present (11). Neutrophils are rarely seen. Furthermore, villous atrophy and crypt hyperplasia can be found as adaptive or regenerative changes of colonic metaplasia. In cases of symptomatic chronic pouchitis one should rule out CMV re-activation as a contribution factor. In acute pouchitis, neutrophilic infiltrates become more numerous and cryptitis, crypt abscesses and ulcerations develop (11). The findings of chronic pouchitis are often patchy and can in some cases resemble typical hallmarks of CD with deeply located submucosal lymphoid aggregates and fistulous tracts. Furthermore, as some patients develop complications typical of CD, such as perianal fistulas and stenoses or fistulas in the pouch, this may cast doubt on the initial diagnosis, but do not disprove a diagnosis of UC. The term “CD in pouch” is not fully defined (31). Changing a diagnosis from UC to CD due to CD-like findings after IPAA surgery should only be considered if reexamination of the original surgical specimen shows typical features of CD (10,11).
Indeterminate and unclassified colitis
In up to 5% of IBD cases, a definite diagnosis of either UC or CD cannot be established (32). Diagnostic uncertainty is usually an issue in children. However, disease involvement limited to the colon with a histological pattern of patchy acute and chronic inflammation with architectural changes, not consenting a final diagnosis, may be an issue in adults as well. In the literature several different terms have been used to cover such cases, including “indeterminate colitis” (IC), “inflammatory bowel disease unclassified” (IBDU), “chronic inflammatory bowel disease unclassified” and “chronic idiopathic inflammatory bowel disease not otherwise specified”. In order to allow comparison between different studies, the ECCO/ESP consensus report from 2013 (10) supported an alinement on terminology that suggest the term IC should be limited to diagnoses based on histological examination of surgical specimens. Thus, the term IBDU is preferred in case of a patient with chronic colitis with clear signs of IBD based upon the clinical history, but where endoscopy and histology biopsies are inconclusive. Both IC and IBDU should be considered a temporary diagnosis, as disease progression over time often enables a classification of either UC or CD.
Children and adolescents
About 10–20% of IBD patients are diagnosed during childhood. Pediatric-onset of IBD can show less characteristic symptoms and histological findings than in adult-onset (33). In general, diagnostic biopsies from children with UC often show less severe inflammation, less architectural abnormalities and less epithelial injury despite extensive disease (10,11). Backwash ileitis occurs with the same frequency as in adults, but children more often present with patchy inflammation and rectal sparing at time of debut (10,11). In children, CD is more often restricted to the colon and less frequently to the ileum. Biopsies from the entire colon can show chronic inflammatory changes, causing troubles to distinct CD from UC. Nevertheless, the upper GI-tract is more often involved than in adult CD. Due to this atypical presentation of UC and CD, clinical work-up for the diagnosis of IBD in children should include a total colonoscopy with ileal intubation, esophagogastroduodenoscopy, multiple biopsies, and a complete small bowel exploration (e.g., a capsule endoscopy) as diagnostic procedures (34).
Infectious colitis and other differential diagnoses
The differential diagnoses to CD and UC include chronic and acute intestinal infections, drug induced colitis and gastritis, graft-versus-host-reactions, ischemic colitis, vasculitis, intestinal lymphoma and other causes of chronic diarrhea such as microscopic colitis. Some of these have specific histological characteristics (e.g., lymphoma, microscopic colitis and vasculitis). Whereas others, such as infectious and drug induced colitis can show the same histological changes of acute and chronic inflammation as seen in IBD.
The interaction between IBD and intestinal infection (parasites, fungi, bacteria and viruses) is complex and still not fully understood. Intestinal infections have been associated with both initial onset of IBD and flare-ups of disease, whereas superinfection might complicate the disease course (35-37). From the pathologist view, intestinal infection should always be considered a differential diagnosis, especially in areas with a high prevalence of chronic pathogenic infections, and some situations requires extra cautiousness. In the early phase of acute onset IBD there might be an overlap with the histopathological features seen in acute intestinal infection. Histology does not reliably detect superinfection, so here the clinical time course and a stool sample for pathogens is essential. The presence of caseating granulomas, or endemic areas in general, should lead to investigations for intestinal tuberculosis (38,39). A Ziehl–Neelsen staining can be used to identify acid-fast organism which can be supplemented by a PCR analysis for Mycobacterium tuberculosis in the tissue block. Treatment failure of immunosuppressive drugs or the presence of larger ulcers with granulation tissue should lead to investigations for reactivation of a latent CMV infection, that might require antiviral treatment (40). In HE stained slides CMV can usually be identified in granulation tissue as large (cytomegalic) cells of mostly mesenchymal origin such as fibroblast and endothelial cells. They are up to four times larger than normal with large intranuclear inclusions surrounded by a clear halo and small cytoplasmatic inclusions, though some infected cells are less characteristic. A pitfall is that the morphology of apoptotic cells and ganglion cells might look like CMV inclusions. Immunohistochemistry or PCR analysis can increase the diagnostic accuracy for CMV (11,41).
The diseases of microscopic colitis, that is lymphocytic colitis and collagenous colitis, are also considered a subtype of IBD. They cause chronic diarrhea, but are in contrast to UC and CD, characterized by a macroscopic normal mucosa, with some characteristic microscopic findings. Lymphocytic colitis is diagnosed by an increased number of intraepithelial lymphocytes (more than 20 per 100 epithelial cell), whereas a thickened subepithelial collagenous band of more than 10 µm defines collagenous colitis (42,43). Crypt architectural distortion is uncommon, and the distinction from UC and CD is often unproblematic. Drug-induced inflammation in the GI-tract can also be a histopathological differential diagnosis to IBD, sharing many of the same microscopic features. Colitis can be caused by a variety of drugs (44) including but not limited to: non-steroid-anti-inflammatory drugs (NSAID) (44), checkpoint-inhibitors (45) and chemotherapeutics. Here knowledge of clinical history is extremely important to avoid a misdiagnosis of IBD.
Upper GI inflammation, esophagitis, gastritis and duodenitis (H. pylori negative) is more common in IBD than the background population, especially in early-onset IBD (46). Upper GI involvement is a part of the natural disease course in CD but can also occur in UC (47). Whether or not the GI inflammation is part of IBD can be a diagnostic problem. Typical differential diagnosis includes gastro-esophageal reflux, H. pylori gastritis, reactive gastritis and peptic-duodenitis, that should be ruled out before assigning the symptoms as a part of IBD. Entities such as lymphocytic esophagitis, focally enhanced gastritis and duodenal cryptitis are all considered possible manifestations of IBD, especially in children and more often in CD than UC (48). However, apart from the occurrence of a classical granuloma of CD, there are no disease specific histological changes indicative of upper GI involvement of IBD.
IBD, dysplasia and cancer
The risk of colorectal cancer (CRC) is increased in some patients with IBD compared to the background population, and is related to longstanding disease, level of activity and pediatric-onset (1,2,49-53). The prognosis of CRC in IBD may be worse than CRC in the general population and shows a higher mortality (49). In general CRC develops from dysplastic precursor lesions. The presence of dysplasia in endoscopic biopsies is the most reliable marker of cancer risk (10,11). This makes the histopathological evaluation of dysplasia in endoscopic biopsies crucial for the surveillance of IBD patients, as it is the pathologist’s interpretation of biopsy specimens that distinguish between high-risk and low-risk, and thereby guide the follow-up regime towards continued endoscopic surveillance or surgical resection (54). Two distinct macroscopic types of dysplastic lesions are recognized: flat and elevated. The flat dysplasia refers to lesions that are not visible by endoscopy and is often found incidentally in biopsy specimens. Whereas, elevated lesions refer to visible lesion and cover a broad range of entities, including adenoma-like lesions and non-adenoma-like lesions such as, elevated plaques, wart like-lesions and large sessile polyps (54). The dysplastic lesions develop after longstanding inflammation and can be patchy and widespread, thus, chromoendoscopy is recommended as a screening procedure in order to detect flat or small lesions (8).
Histological, dysplasia is defined as an “histological unequivocal intraepithelial neoplasia without evidence of tissue invasion” (in the intestine this accounts for invasion through the lamina muscularis mucosa) and is classified according to the Vienna classification system into five categories: (I) negative for neoplasia/dysplasia, (II) indefinite for neoplasia/dysplasia, (III) non-invasive low grade neoplasia/dysplasia, (IV) non-invasive high-grade neoplasia/dysplasia (V) invasive neoplasia/dysplasia (cancer). As inter-observatory agreement, especially on the evaluation of indefinite- and low-grade dysplasia, is not optimal (55), it is recommended that a diagnosis of dysplasia is confirmed by an independent specialist in GI pathology (8). The microscopical features used to evaluate dysplasia in IBD are based on a combination of cytological and architectural aberrations of the crypt epithelium and are identical to those used in the general assessment of intestinal dysplasia (54). This includes an increased nucleus/cytoplasmic ratio, hyperchromasia, loss of cell polarity, atypical mitosis and lack of stratification and maturation. The architectural changes include crypt-branching and crowding (known as “back-to-back” configuration) as well as villiform changes (56). Diagnostic uncertainty may be supported by immunohistochemistry for p53 which marks dysplastic cells, however, also can be expressed in regenerating non-dysplastic epithelium (11).
Role of histology in assessing disease activity in the clinical setting
Assessment of disease activity and severity is important for making therapeutic decisions in the clinical management of IBD, as well as for documentation of treatment effects in clinical trials. The most obvious goals for treatment are complete remission of symptoms and control of inflammation. In daily clinical practice inflammation is measured by symptoms, laboratory tests (e.g., CRP and fecal calprotectin) and endoscopy. Currently histology is not recommended for routine evaluation of treatment responses and disease activity (8).
The term “histological remission”, though not unifying defined, is used to describe the complete histological normalization of the mucosa with cessation of neutrophil infiltration and inflammatory activity, including architectural abnormalities. Whereas “histological improvement” describes a progress towards less inflammatory activity in the biopsies (13,57). There is a well-known discordance between clinical symptoms, endoscopic findings and histological disease activity. Especially in UC, evaluation of histological activity has turned out to predict clinical relapse, use of corticosteroids, and hospitalization better than endoscopy (58-61).
The role of histology as a predictive factor has been the subject of several studies in CD as well (62). In both pediatric patients (63,64) and adults (29) with CD, the presence of granulomas are associated with a higher risk of hospitalization and a shorter time to start of immune modulating therapy. In patients with CD undergoing ileocecal surgery, two recent metanalysis (65,66) investigated histological evaluation of resections margins in surgical specimens. They found that positive resection margins (active inflammation in the resection margin), granulomas, and plexitis (involvement of inflammation in the myenteric plexus situated between the circular and longitudinal muscularis propria layer of the intestinal wall) are predictive of post-operative CD recurrence.
Due to the need of a standardized measure of histological activity in clinical trials, several different histological scoring indexes have been proposed during the last 40 years. The principle of all these indexes, is to assign a score/points according to the presence of different histological features (such as mucosal architecture distortion, neutrophil granulocyte infiltration, epithelial abnormality, ulceration etc.), and to sum up to a final score or grade reflecting the degree of inflammatory activity and damage. Ideally a scoring index should be reproducible, reflect clinically disease activity and be easy to use. Furthermore, it should remain unchanged in stable patients and detect changes in disease activity over time. For UC more than 30 histological indexes has been described (59). Of these the “Geboes score” (67,68) developed in 2000 has been the most widely used and can serve as an independent risk-factor for disease progression in UC (69). The more recent “Nancy histological index” (23) and “Robarts histopathological index” (70) both from 2016 have proven feasible, easy to use, and are the most proper validated (57,71). Validation, in the sense of testing inter- and intra-observer reproducibility and alignment with clinical symptoms over time, is important before implementation of a histological index. This can be difficult in CD due to the patchy nature of disease. Fourteen different histological indexes have been suggested for CD (72), of which the “Global histologic disease activity score” and the “Naini and Cortina index” is the most widely used, though they have not been proper validated (62,72).
Reporting on histological remission has turned out to serve as a good endpoint in clinical trials and might be a future key treatment-goal in a “treat-to-target” strategy in the clinical setting (57). Unfortunately, there are currently no general agreement on which index should be used. This can make direct comparison between studies difficult, though one could expect a good correlation between the well validated indexes (71). The 2020 ECCO position paper (12), concluded that the Nancy index can be recommended for daily clinical practice. For clinical trials both the Nancy and Robarts histopathology index are feasible, though both of them are solely validated for UC. This have led to the development of a new, consensus-based scoring index in 2020 (13). The IBD-DCA score, which can be applied to both UC and CD, is intended for both clinical practice and clinical trials, though this score still needs validation in a prospective cohort.
Discussion and conclusions
The histopathological features of UC and CD are well-described, and microscopic assessment of endoscopic biopsy specimens is crucial for establishing a diagnosis of IBD. As none of the histological features of IBD are truly disease specific, a definite diagnosis of UC or CD should never be based on histological findings alone. This implicates that a well-functioning collaboration between the clinicians, radiologists and pathologists is central for optimal management of IBD, which could be structured in the setting of a “multidisciplinary team meeting” (MDT) (73). The histopathological evaluation is furthermore an important part of detecting early signs of dysplasia to prevent cancer development. For the purpose of sign-out, the pathology report should include findings suggestive of chronic colitis (basal plasmacytosis, basal lymphoid aggregates, crypt architectural/atrophic changes, mucin depletion and metaplasia), and further give an indication of disease activity based on the extend of neutrophil infiltration (in lamia propria, cryptitis and crypt abscesses) and epithelial damage (erosions and ulcerations), which should be graded if present. This can guide clinicians in decision making when establishing the diagnosis and selecting treatment, though histological remission is not (yet) implemented as a treatment-target in routine clinical practice. For optimal implementation of a histological index and assessment of histological remission in clinical settings, a consensus agreement involving both the clinicians/endoscopist and the pathologist is needed. Preferably, a standardized endoscopy biopsy procedure should be implemented, so specimens for histological examination is uniform between patients and medical centers. Further, agreement on using a particular scoring index would allow easier comparison between centers. In ileocecal resection specimens of CD, recent advances suggest that it might be beneficial to routinely report on active inflammation, plexitis and granulomas in the resection margins in order to guide clinical follow up after surgery (74). Also, there is a need for a more standardized reporting of IBD resection specimens, e.g., a synopsis report form and checklist as currently used in the evaluation of CRC resection specimens, in order to fully implement histology as a predictive factor after surgery.
The recent progress of use of histological indexes in clinical trials and their capability to serve as a predictive factor to guide disease management, also after the diagnosis has been established, clearly points to the fact that histopathology remains an ever important factor in the management of IBD.
Acknowledgments
We acknowledge Tine Plato Kuhlmann for support with photographic images.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the narrative review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-21-1
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-1). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Prim 2020;6:74. [Crossref] [PubMed]
- Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Prim 2020;6:1-19. [Crossref]
- Radford SJ, McGing J, Czuber-Dochan W, et al. Systematic review: the impact of inflammatory bowel disease-related fatigue on health-related quality of life. Frontline Gastroenterol 2020;12:11-21. [Crossref] [PubMed]
- Hammer T, Langholz E. The epidemiology of inflammatory bowel disease: balance between East and West? A narrative review. Dig Med Res 2020;3:48. [Crossref]
- Piovani D, Danese S, Peyrin-Biroulet L, et al. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019;157:647-59.e4. [Crossref] [PubMed]
- Kellermann L, Jensen KB, Bergenheim F, et al. Mucosal vitamin D signaling in inflammatory bowel disease. Autoimmun Rev 2020;19:102672 [Crossref] [PubMed]
- Chang JT. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med 2020;383:2652-64. [Crossref] [PubMed]
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144-64. [Crossref] [PubMed]
- Patil DT, Greenson JK, Odze RD. Inflammatory Disorders of the Large Intestine. In: Goldblum R, Odze J. editors. Surgical Pathology of the GI Tract Liver Biliary Tract and Pancreas. Amsterdam, Netherlands: Elsevier, 2014:433-511.
- Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827-51. [Crossref] [PubMed]
- Langner C, Magro F, Driessen A, et al. The histopathological approach to inflammatory bowel disease: A practice guide. Virchows Arch 2014;464:511-27. [Crossref] [PubMed]
- Magro F, Doherty G, Peyrin-Biroulet L, et al. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J Crohns Colitis 2020;14:1503-11. [Crossref] [PubMed]
- Lang-Schwarz C, Agaimy A, Atreya R, et al. Maximizing the diagnostic information from biopsies in chronic inflammatory bowel diseases: recommendations from the Erlangen International Consensus Conference on Inflammatory Bowel Diseases and presentation of the IBD-DCA score as a proposal for a new i. Virchows Arch 2021;478:581-94. [Crossref] [PubMed]
- Battat R, Vande Casteele N, Pai RK, et al. Evaluating the optimum number of biopsies to assess histological inflammation in ulcerative colitis: a retrospective cohort study. Aliment Pharmacol Ther 2020;52:1574-82. [PubMed]
- Burroughs SH, Williams GT. Examination of large intestine resection specimens. J Clin Pathol 2000;53:344-9. [Crossref] [PubMed]
- Surawicz CM, Meisel JL, Ylvisaker T, et al. Rectal biopsy in the diagnosis of Crohn’s disease: Value of multiple biopsies and serial sectioning. Gastroenterology 1981;80:66-71. [Crossref] [PubMed]
- Surawicz CM. Serial sectioning of a portion of a rectal biopsy detects more focal abnormalities - A prospective study of patients with inflammatory bowel disease. Dig Dis Sci 1982;27:434-6. [Crossref] [PubMed]
- Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19: Suppl A 5A-36A.
- Haskell H, Andrews CW, Ready SI, et al. Pathologic features and clinical significance of backwash ileitis in ulcerative colitis. Am J Surg Pathol 2005;29:1472-81. [Crossref] [PubMed]
- Ladefoged K, Kristian Munck L, Jorgensen F, et al. Skip inflammation of the appendiceal orifice: A prospective endoscopic study. Scand J Gastroenterol 2005;40:1192-6. [Crossref] [PubMed]
- Ha CWY, Martin A, Sepich-Poore GD, et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020;183:666-83.e17. [Crossref] [PubMed]
- Fenton TM, Jørgensen PB, Niss K, et al. Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity 2020;52:557-70.e6. [Crossref] [PubMed]
- Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut 2017;66:43-9. [Crossref] [PubMed]
- Marchal-Bressenot A, Scherl A, Salleron J, et al. A practical guide to assess the Nancy histological index for UC. Gut 2016;65:1919-20. [Crossref] [PubMed]
- Schumacher G, Kollberg B, Sandstedt B. A prospective study of first attacks of inflammatory bowel disease and infectious colitis: Histologic course during the 1st year after presentation. Scand J Gastroenterol 1994;29:318-32. [Crossref] [PubMed]
- Rodríguez-Lago I, Ramírez C, Merino O, et al. Early microscopic findings in preclinical inflammatory bowel disease. Dig Liver Dis 2020;52:1467-72. [Crossref] [PubMed]
- Kleer CG, Appelman HD. Ulcerative Colitis: Patterns of involvement in colorectal biopsies and changes with time. Am J Surg Pathol 1998;22:983-9. [Crossref] [PubMed]
- Kim B, Barnett JL, Kleer CG, et al. Endoscopic and Histological Patchiness in Treated Ulcerative Colitis. Am J Gastroenterol 1999;94:3258-62. [Crossref] [PubMed]
- Hong SW, Yoon H, Shin CM, et al. Clinical significance of granulomas in Crohn’s disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2020;35:364-73. [Crossref] [PubMed]
- Akiyama S, Rai V, Rubin DT. Pouchitis in inflammatory bowel disease: a review of diagnosis, prognosis, and treatment. Intest Res. 2021;19:1-11. [Crossref] [PubMed]
- Lightner AL, Pemberton JH, Loftus EJ. Crohnʼs Disease of the Ileoanal Pouch. Inflamm Bowel Dis 2016;22:1502-8. [Crossref] [PubMed]
- Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol 2003;16:347-58. [Crossref] [PubMed]
- Wilson DC, Russell RK. Overview of paediatric IBD. Semin Pediatr Surg 2017;26:344-8. [Crossref] [PubMed]
- Oliveira SB, Monteiro IM. Diagnosis and management of inflammatory bowel disease in children. BMJ 2017;357:j2083. [Crossref] [PubMed]
- Lin WC, Chang CW, Chen MJ, et al. Challenges in the diagnosis of ulcerative colitis with concomitant bacterial infections and chronic infectious colitis. PLoS One 2017;12:e0189377 [Crossref] [PubMed]
- Ng SC, Chan FK. Infections and inflammatory bowel disease: Challenges in Asia. J Dig Dis 2013;14:567-73. [Crossref] [PubMed]
- Banerjee R, Pal P, Mak JWY, et al. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol 2020;5:1076-88. [Crossref] [PubMed]
- Sato R, Nagai H, Matsui H, et al. Ten cases of intestinal tuberculosis which were initially misdiagnosed as inflammatory bowel disease. Intern Med 2019;58:2003-8. [Crossref] [PubMed]
- Kedia S, Das P, Madhusudhan KS, et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J Gastroenterol 2019;25:418-32. [Crossref] [PubMed]
- Gilmore RB, Taylor KM, Morrissey CO, et al. Cytomegalovirus in inflammatory bowel disease: a clinical approach. Intern Med J 2020; Epub ahead of print. [Crossref] [PubMed]
- Sager K, Alam S, Bond A, et al. Review article: Cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther 2015;41:725-33. [Crossref] [PubMed]
- Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015;66:613-26. [Crossref] [PubMed]
- Fiehn AK, Miehlke S, Aust D, et al. Distribution of histopathological features along the colon in microscopic colitis. Int J Colorectal Dis 2021;36:151-9. [Crossref] [PubMed]
- Goldstein NS, Cinenza AN. The histopathology of nonsteroidal anti-inflammatory drug-associated colitis. Am J Clin Pathol 1998;110:622-8. [Crossref] [PubMed]
- Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: Pathologists’ perspective. J Clin Pathol 2018;71:665-71. [Crossref] [PubMed]
- Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:39-44. [Crossref] [PubMed]
- Harpaz N, Polydorides AD. Upper Gastrointestinal Manifestations of Inflammatory Bowel Disease. Surg Pathol Clin 2020;13:413-30. [Crossref] [PubMed]
- Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: A pathological review. World J Gastroenterol 2019;25:1928-35. [Crossref] [PubMed]
- Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet 2020;395:123-31. [Crossref] [PubMed]
- Wang YN, Li J, Zheng WY, et al. Clinical characteristics of ulcerative colitis-related colorectal cancer in Chinese patients. J Dig Dis 2017;18:684-90. [Crossref] [PubMed]
- Li J, Zhou WX, Liu S, et al. Similarities and differences in clinical and pathologic features of inflammatory bowel disease-associated colorectal cancer in China and Canada. Chin Med J (Engl) 2019;132:2664-9. [Crossref] [PubMed]
- Kjærgaard VS, Jensen CB, Elmahdi R, et al. Cancer Risk in Pediatric-Onset Inflammatory Bowel Disease: A Population-Based Danish Cohort Study. Gastroenterology 2020;159:1609-11. [Crossref] [PubMed]
- Yamamoto-Furusho JK, Parra-Holguín NN. Narrative review of colorectal cancer risk in patients with inflammatory bowel disease. Dig Med Res 2020;3:56. [Crossref]
- Bressenot A, Cahn V, Danese S, et al. Microscopic features of colorectal neoplasia in inflammatory bowel diseases. World J Gastroenterol 2014;20:3164-72. [Crossref] [PubMed]
- Allende D, Elmessiry M, Hao W, et al. Inter-observer and intra-observer variability in the diagnosis of dysplasia in patients with inflammatory bowel disease: Correlation of pathological and endoscopic findings. Colorectal Dis 2014;16:710-8. [Crossref] [PubMed]
- Odze RD. Pathology of Dysplasia and Cancer in Inflammatory Bowel Disease. Gastroenterol Clin North Am 2006;35:533-52. [Crossref] [PubMed]
- Chateau T, Feakins R, Marchal-Bressenot A, et al. Histological remission in ulcerative colitis: Under the microscope is the cure. Am J Gastroenterol 2020;115:179-89. [Crossref] [PubMed]
- Park S, Abdi T, Gentry M, et al. Histological Disease Activity as a Predictor of Clinical Relapse among Patients with Ulcerative Colitis: Systematic Review and Meta-Analysis. Am J Gastroenterol 2016;111:1692-701. [Crossref] [PubMed]
- Mosli MH, Parker CE, Nelson SA, et al. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev 2017;5:CD011256 [Crossref] [PubMed]
- Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: Histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016;65:408-14. [Crossref] [PubMed]
- Pai RK, Hartman DJ, Rivers CR, et al. Complete Resolution of Mucosal Neutrophils Associates With Improved Long-Term Clinical Outcomes of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2020;18:2510-7.e5. [Crossref] [PubMed]
- Pai RK, Jairath V. What is the role of histopathology in the evaluation of disease activity in Crohn’s disease? Best Pract Res Clin Gastroenterol 2019;38-39:101601 [Crossref] [PubMed]
- Rothschild B, Rinawi F, Herman Y, et al. Prognostic significance of granulomas in children with Crohn’s disease. Scand J Gastroenterol 2017;52:716-21. [Crossref] [PubMed]
- Ideström M, Rubio CA, Onelöv E, et al. Pediatric Crohn’s disease from onset to adulthood: Granulomas are associated with an early need for immunomodulation. Scand J Gastroenterol 2014;49:950-7. [Crossref] [PubMed]
- Ryan JM, Rogers AC, O’Toole A, et al. Meta-analysis of Histological Margin Positivity in the Prediction of Recurrence After Crohn’s Resection. Dis Colon Rectum 2019;62:882-92. [Crossref] [PubMed]
- Tandon P, Malhi G, Abdali D, et al. Active margins, Plexitis, and Granulomas Increase Post-operative Crohn’s Recurrence: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2021;19:451-62. [Crossref] [PubMed]
- Geboes K, Riddell R, Öst A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404-9. [Crossref] [PubMed]
- Jauregui-Amezaga A, Geerits A, Das Y, et al. A Simplified Geboes Score for Ulcerative Colitis. J Crohns Colitis 2017;11:305-13. [PubMed]
- Magro F, Alves C, Lopes J, et al. Histologic Features of Colon Biopsies (Geboes Score) Associated With Progression of Ulcerative Colitis for the First 36 Months After Biopsy. Clin Gastroenterol Hepatol 2020; Epub ahead of print. [Crossref] [PubMed]
- Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut 2017;66:50-8. [Crossref] [PubMed]
- Magro F, Lopes J, Borralho P, et al. Comparison of the Nancy Index With Continuous Geboes Score: Histological Remission and Response in Ulcerative Colitis. J Crohns Colitis 2020;14:1021-5. [Crossref] [PubMed]
- Novak G, Parker CE, Pai RK, et al. Histologic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev 2017;7:CD012351 [Crossref] [PubMed]
- Ferman M, Lim AH, Hossain M, et al. Multidisciplinary team meetings appear to be effective in inflammatory bowel disease management: an audit of process and outcomes. Intern Med J 2018;48:1102-8. [Crossref] [PubMed]
- Ananthakrishnan AN, Deshpande V. It Is All in the Fine Print: A Call for a Histopathology Checklist for IBD. Clin Gastroenterol Hepatol 2021;19:446-7. [Crossref] [PubMed]
Cite this article as: Kellermann L, Riis LB. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig Med Res 2021;4:3.


