A narrative review of recent progress and current perspectives on video capsule endoscopy
Introduction
Capsule endoscopy (CE) has evolved since the first report of small-bowel CE (SBCE) by Iddan et al. in 2000 (1). Several SBCE types are in current use worldwide, and colon CE (CCE) approaches have also been developed. The evolution of CE has improved the ability to inspect the entire gastrointestinal tract in a noninvasive manner. SBCE has been reported to be useful in obscure gastrointestinal bleeding (OGIB) and Crohn’s disease (CD), whereas CCE has demonstrated utility in polyp/colon cancer detection and assessment of disease severity in patients with ulcerative colitis (UC). Numerous recent studies on artificial intelligence (AI)-based reading systems for CE suggest that these new technologies may reduce the burden of reading on clinicians. This review summarizes the current status of SBCE and CCE and the recently emerging role of AI in CE. We conducted a survey based on articles searchable in English since 2000 by PubMed. We present the following article in accordance with the narrative review checklist (available at http://dx.doi.org/10.21037/dmr-20-162).
SBCE
SBCE, which was introduced into clinical practice in 2000 for the diagnosis of small intestinal diseases, has been used for the diagnosis of various gastrointestinal diseases (1). It is swallowed and passes through the gastrointestinal tract passively via peristalsis and that the images are downloaded from the data recorder to a computer for later review. In those patients unable to swallow the capsule, or in those with gastroparesis, the capsule can be placed endoscopically into the duodenum. The two capsules approved for SBCE in Japan are PillCam® (Medtronic, Yokneam, Israel) and EndoCapsule® (Olympus, Tokyo, Japan).
The first SBCE was performed using M2A® by Given Imaging (Yokneam, Israel), which was later renamed as PillCam® SB (2), which is 11 mm ×26 mm in size and can capture two images/second with a 140-degree angle of view. The captured images are sequentially transmitted to a recorder mounted outside the body. PillCam® SB2 is the second-generation small-bowel capsule with improved features, including 156-degrees angle of view, automatic light control, and higher-resolution camera, although it is similar in size to PillCam® SB (3,4). In addition, the battery life has been improved from about eight hours in PillCam® SB to about 12 hours in PillCam® SB2, leading to improvement in the rate of total small bowel observation. However, the limited ability to diagnose diseases in the proximal small bowel is a limitation of SBCE (5-7); the third-generation PillCam® SB3 was developed to resolve this issue. PillCam® SB3 has the same size and angle of view as PillCam® SB2 but contains a higher-resolution camera and an adaptive frame rate (AFR) function, which evaluates the movement speed of the capsule and changes the shooting speed between 2 and 6 frames/second (fps). However, in a retrospective study comparing PillCam® SB2 with PillCam® SB3, the completion rate (93.6% vs. 96.2%, P=0.27) and overall endoscopic findings (73.4% vs. 78.8%, respectively, P=0.23) were not significantly different, reflecting that the diagnostic performance was comparable between the two capsule endoscopes (8).
The first-generation EndoCapsule® was introduced in 2008 (9), and the second-generation EndoCapsule (EndoCapsule® 10) is currently in use (10). EndoCapsule® 10 is 11 mm ×26 mm in size, has a 160-degree angle of view, 12 hours of battery life, and sequentially transmits the captured images to the recorder outside the body.
Other capsule endoscopes for the small bowel used across the globe are MiroCam® (IntroMedic, Seoul, Korea) (11,12), OMOM Capsule2® (Jinshan Science and Technology, Chongqing, China) (13,14), and CapsoCam® (CapsoVision, Saratoga, CA, USA) (15-17).
In Japan, the indications for CE were initially limited to patients with OGIB who were had negative findings by esophagogastroduodenoscopy and colonoscopy, due to the risk of capsule retention (18). In 2012, patency capsules (PillCam® patency) were approved by the Ministry of Health, Labor, and Welfare of Japan and the indications of CE were expanded to include patients with known or suspected small bowel disease based on a previous gastrointestinal patency test using PillCam® patency capsules in patients with suspected gastrointestinal strictures. However, even in use of patency capsule, the indication of CE should be evaluated. Contraindication of CE are severe gastrointestinal stenosis, intestinal obstruction, pacemaker implantation, dysphagia, history of abdominal radiation, pregnant women, and patients who do not consent to undergo capsule endoscopic retrieval in case of retention.
Utility of SBCE in OGIB
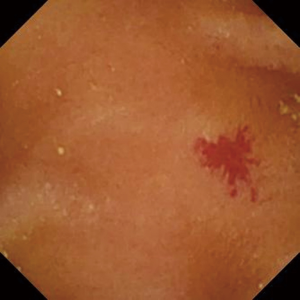
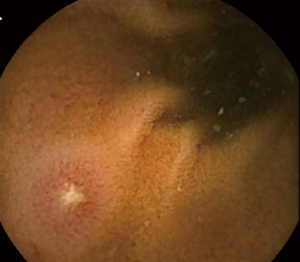
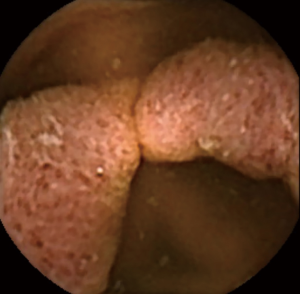
OGIB is defined as gastrointestinal bleeding from a source that cannot be identified by upper and lower gastrointestinal endoscopy. Small-bowel bleeding is suspected in most cases of OGIB and accounts for about 5% of all gastrointestinal bleeding cases (19,20). The most frequently reported source of bleeding is small intestinal angioectasia (Figure 1), followed by erosive/ulcerative lesions (Figure 2), neoplastic lesions, and CD. The most frequently reported vascular lesions are histopathologically classified into three types: lesions with venous and capillary characteristics (angioectasia), lesions with arterial characteristics (Dieulafoy’s lesions), and lesions with both arterial and venous characteristics [arteriovenous malformations (AVMs)] (21). Based on this classification, Yano et al. categorized small intestinal vascular lesions into six types, taking into account the presence of pulsatility and lesion size on endoscopic findings (21). This classification is also useful in selecting the appropriate intervention to achieve endoscopic hemostasis because argon plasma coagulation, clipping, and surgery or arterial embolization are appropriate for angioectasia (Type 1), Dieulafoy’s lesions (Type 2), and AVMs (Type 3), respectively. Because this classification is designed for enteroscopy, establishing a diagnosis based on the presence of pulsatility is difficult with CE, which does not allow continuous observation of a certain area. However, it is important to understand this classification based on pathology. Any vascular lesion detected by SBCE should be evaluated by subsequent balloon-assisted enteroscopy (BAE).
The reported diagnostic yield of SBCE in identifying the source of bleeding in OGIB ranges between 30% and 70% (22-31). Additionally, various factors have been reported to be associated with diagnostic yield in CE. Specifically, capsule administration within 48 hours after overt OGIB was reported to be associated with a favorable diagnostic yield (32). Further, Sidhu et al. reported that anti-coagulation therapy, hepatic comorbidity, and increasing age were associated with a positive yield (23). Lepileur et al. also found that age over 60 years, male sex, history of overt bleeding, and recent hospitalization were associated with improved diagnosis of OGIB using SBCE (33).
Different diagnostic algorithms for OGIB have been proposed in different countries across the globe, with most algorithms recommending SBCE as the first-line diagnostic modality for OGIB (34-38). The OGIB diagnostic algorithm proposed by the Japanese Society of Gastrointestinal Endoscopy in 2017 recommends that contrast-enhanced computed tomography (CT) from the chest to the pelvic region should be considered as first-line modality and that BAE should be considered in the presence of a bleeding source identified by CT (18). SBCE is recommended in the absence of an overt bleeding source on CT, and oral or trans-anal BAE is selected from the SBCE findings in patients with positive SBCE findings.
Utility of SBCE in CD
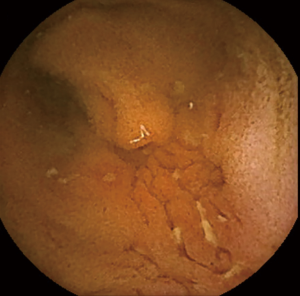
CD is a chronic inflammatory bowel disease characterized by longitudinal ulcers and cobblestone appearance throughout the gastrointestinal tract from the oral cavity to anus. Histologically, CD is characterized by non-cavitary epithelioid granulomas. Longitudinal aphthoid lesions/ulcers (Figure 3) and discrete ulcers, which are observed in the early stages of CD’s natural history, fuse and develop into longitudinal ulcers and cobblestone appearance, and many patients eventually require surgical treatment due to complications such as strictures, fistulas, and abscesses (39). Patients with CD who undergo active intervention with ileal resection based on the results of endoscopic monitoring were reported to have a favorable prognosis (40), and also Takabayashi et al. reported that endoscopic evaluation of mucosal healing in the deep small intestine is important for CD prognosis (41); therefore, the evaluation of small-bowel lesions is considered to be an important component in improving the diagnosis and treatment of patients with CD who often harbor small-bowel lesions.
In Japan, SBCE was initially contraindicated in CD due to the risk of capsule retention resulting from intestinal stenosis. Following the introduction of PillCam® patency capsule in 2012, SBCE can currently be performed only in patients with CD and confirmed small-bowel patency. Mucosal CD lesions in jejunum, which were previously considered to occur in low frequency, are increasingly identified with the advent of SBCE (42). Esaki et al. compared the SBCE findings of 63 patients with CD to those of 45 patients without CD and reported that the rates of longitudinal ulcers, cobblestone appearance, irregular ulcers, and irregular or linear erosions were significantly higher in those with CD than in those without CD (43). In addition, longitudinal and cycloid arrangements of small lesions such as aphthae and erosions were frequently observed in the duodenum and jejunum of patients with CD, suggesting that SBCE might be useful for the early diagnosis of CD by focusing on the distribution and arrangement of small intestinal mucosal lesions.
PillCam Crohn’s (Medtronic, Israel) is a recently designed capsule endoscope designed to view the small bowel and colon. PillCam Crohn’s has two cameras, each with a 168-degree angle of view, allowing for a 336-degree view. The capsule endoscope has an AFR that can be adjusted to 4 or 35 fps and is designed to operate in a mode that provides complete coverage of the small intestine as well as the colon. Leighton et al. conducted a study to compare the diagnostic yield of ileocolonoscopy to SBCE with PillCam Crohn’s (44) and found that the per-patient diagnostic yield in active CD was better with SBCE compared to ileocolonoscopy (83.3% vs. 69.7%). There were 12 and 3 patients whose lesions were detected by SBCE alone and ileocolonoscopy alone, respectively. In addition, it is difficult to determine the diagnosis of CD based on the presence of ulcers alone, and it is also important to interview patients about their history of use of NSAIDs and other drugs that may cause mucosal damage. Therefore, further studies are needed to determine the utility of CE in CD.
In addition, two scoring methods, capsule endoscopy CD activity index (CECDAI) (45) and the Lewis score (46), have been used to evaluate CD severity using SBCE. In CECDAI, first proposed for small-bowel lesions in CD by Gal et al. in 2008 (45), the small intestine is divided into the proximal and parenchymal segments based on the transit time and is evaluated based on inflammation, extent, and stricture scores. In the study, CECDAI correlates well between readers but not with CD activity index (CDAI) or inflammatory bowel disease questionnaire (IBDQ). Future studies are expected to compare the recently proposed CECDAIic, which includes colorectal score (47) in addition to the other variables included in CECDAI, with other scoring modalities. The Lewis score, introduced by Gralnek et al. in 2008 (46), evaluates inflammatory changes in the small intestinal mucosa based on three parameters: edematous changes in villi, ulceration, and stenosis. The Lewis score was reported to have a diagnostic sensitivity of 89.5% and specificity of 78.9% in patients with suspected CD. Meanwhile, the correlation of biomarkers such as fecal calprotectin and C-reactive protein with the Lewis score and CECDAI is controversial and should be further investigated (48-52).
CCE
CCE was first reported by Eliakim et al. in 2006 (53). The improved second-generation capsule for CCE [PillCam® Colon2 (CCE-2), Medotronic] is 11.6 mm ×31.5 mm in size, 5 mm larger than SBCE, and has cameras on both sides. Since the viewing angle of the cameras at both ends is 172°, the total viewing angle is 344°, very close to 360°. The first-generation CCE could only capture 4 fps, and the results of large-scale clinical trials on first-generation CCE in Europe and the United States were not satisfactory, based on a sensitivity of 64% for CCE in diagnosing colorectal polyps >6 mm (54). The CCE-2 was launched after improvements were made to resolve the short transverse colon passage time of few seconds. To address this oversight, the CCE-2 is equipped with an AFR function that can automatically recognize the movement speed of the capsule and change the frame rate between 4 and 35 fps by communicating with the data recorder attached to the body. With this feature, the number of captured images can be increased in places where the capsule moves quickly and can be reduced in places where it stagnates. Using a software for reading coupled with a data recorder allows the estimation of polyp size and real-time monitoring of capsule images. Contraindications of CCE-2 are suspected gastrointestinal obstruction, stenosis, or fistula; implantation of a medical electrical device such as a cardiac pacemaker; dysphagia; and allergies or known contraindications to the medications and preparation agents used in the procedure.
Utility of CCE in polyp detection
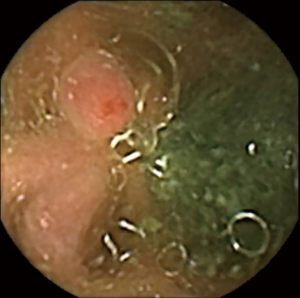
Multicenter studies in Europe and the United States have demonstrated that CCE-2 is effective in screening for colorectal cancer and polyps (Figure 4) (55). The first-generation capsules for CCE, which did not have the AFR function, had a sensitivity of 39–79% for colorectal polyps >6 mm (54,56-59). However, CCE-2 is associated with improved rate of diagnosis, with a reported sensitivity of 81–94% and specificity of 64–94% for colorectal polyps >6 mm (60-66). In 2009, Eliakim et al. conducted a study comparing CCE-2 and CS in 104 patients and reported that the sensitivity and specificity of CCE-2 were 89% [95% confidence interval (CI): 70–97%] and 76% (95% CI: 72–78%), respectively, for polyps >6 mm (60). Subsequently, in a large study of 695 patients, Rex et al. reported good results with CCE-2 based on a sensitivity of 81% (95% CI: 77–84%) and specificity of 93% (95% CI: 91–95%) for polyps >6 mm (61). CCE-2 was also compared with CT colonography for polyp detection. Rondonotti et al. reported that the sensitivity and specificity of CCE-2 were 88.2% (95% CI: 62.2–97.9%) and 87.8% (95% CI: 70.8–96.0%), respectively, for polyps >6 mm; the authors also found that the sensitivity and specificity of CT colonography (88.2% [95% CI: 62.2–97.9%] and 84.8% [95% CI: 67.0–94.0%]) were closely comparable to those of CCE-2 (65). In non-polypoid tumors, the sensitivity of CCE-2 was 87%, which was higher than that of CT colonography (67%) (66).
As mentioned above, CCE has demonstrated good efficacy in polyp detection; however, one major issue with CCE is the low overall colorectal observation rate. For example, Kobaek-Larsen et al. and Ota et al. reported CCE completion rates of 54% and 75%, respectively (67,68). One study reported that the sensitivity of CCE in advanced colorectal cancer was 85%; all undetectable cases were those in which the CCE did not reach the lesion (68). Therefore, future efforts should include the development of better pretreatment protocols that will increase total colorectal observation rate to improve the lesion detection rate by CCE.
Utility of CCE in UC
The efficacy of CCE-2 in inflammatory bowel disease has been reported in several studies. UC is a colonic inflammatory condition of unknown etiology that is characterized by repeated remissions and exacerbations (Figure 5). Since repeated colorectal examinations are required for the diagnosis and treatment of UC, CCE is a suitable monitoring tool due to its noninvasiveness in these patients. Endoscopy plays several important roles in the treatment of UC, including diagnosis at the time of initial diagnosis, determination of treatment efficacy, monitoring of inflammation, and surveillance for colorectal cancer. Importantly, endoscopic mucosal healing has been recently implicated in the long-term prognosis of long-term clinical remission, avoidance of bowel resection, and steroid-free clinical remission, further highlighting the importance of endoscopic diagnosis (69). The risk of colorectal cancer increases with increasing disease duration and more extensive inflammation, especially in patients with UC (70).A meta-analysis reported an overall colorectal cancer incidence rate of 3.7% (95% CI: 3.1–4.2%) in patients with UC (71), therefore, a colonoscopy every 1–2 years is generally recommended. CS, the gold standard to evaluate disease status in patients with UC, allows the determination of the nature, extent, and extent of diffusely spreading colorectal lesions. On the other hand, the invasiveness of CS and patient compliance may have a negative impact; the reported testing rate in long-term patients with inflammatory bowel disease is only 54% after four years of observation (72). In addition, in patients with clinically severe UC, total colonoscopy may not be possible due to the risk of worsening disease or perforation. Since CCE does not cause pain and does not require sedation or insufflation, the intestinal mucosa can be observed noninvasively, moreover the lesion site is limited to the colon, the application of CCE was considered in UC. In addition, the follow-up of patients with UC for confirmation of the treatment effect and evaluation of disease severity, among other aspects, can be performed with a lighter pretreatment, as only the evaluation of the degree of inflammation and the extent of disease are necessary in diffusely spreading lesions. A representative study using first-generation CCE in patients with UC is by Sung et al. (73), who reported that the sensitivity and specificity of first-generation CCE to detect active UC lesions were 89% (95% CI: 80–95%) and 75% (95% CI: 51–90%), respectively. Therefore, the authors did not recommend first-generation CCE as an alternative to colonoscopy, although CCE has been currently shown to be a safe approach. In 2013, in a study comparing first-generation CCE with CS in patients with UC, Ye et al. reported that both CCE assessment of active lesion severity (κ=0.751, P<0.001) and the lesion extent (κ=0.522, P<0.001) were significantly correlated to CS assessment of them (74). Additionally, San et al. reported that first-generation CCE findings about lesion severity (κ=0.79; 95% CI: 0.62–0.96) and inflammation extent (κ=0.71; 95% CI: 0.52–0.90) exhibited good correlation with colonoscopy findings (75).For CCE-2, we first reported it in 2013 (10). In that study on Japanese patients with UC, we also explored pretreatment methods, including 2L PEG and prokinetics (mosapride citrate and metoclopramide), which is the standard pretreatment method for enterography in Japan. However, the total colon observation rate of 69% was unsatisfactory and good colon cleansing was not observed in the 4-step evaluation of colon cleansing (76). Conversely, there was a high correlation between the Matts’ endoscopy score based on the evaluation of CCE-2 images and the CS findings (P=0.797) (77). In a study of 108 patients with UC (78), although the number of patients in that study who had a complete examination was somewhat low at 67%, Shi et al. reported that the Mayo endoscopic subscore (79) determined by CCE-2 was highly correlated to that determined by CS (intraclass correlation coefficient 0.69, 95%CI 0.46–0.81; P<0.001). The study also reported a high correlation of CCE-2 and CS in the UC endoscopic index of severity (UCEIS) (80) (intraclass correlation coefficient 0.64, 95% CI: 0.38–0.78; P<0.001) and concluded that the CCE-2 was a reliable tool for monitoring UC.
The UCEIS (81) and the Mayo endoscopy score (79) are used to evaluate the severity of UC in CS. Due to the lack of scoring systems to assess UC-related inflammation and given that CCE has several distinct features compared to CS, we have recently developed a new UC severity score termed capsule scoring of UC (CSUC) to specifically assess inflammation associated with UC by CCE and verified its utility (81). The correlations observed between CSUC and various tests, such as fecal calprotectin, C-reactive protein, and Lichtiger clinical score (82), were closely comparable to those observed with UCEIS determined by CS. Future studies are expected to evaluate the utility of CSUC in real-world clinical practice.
AI systems in CE
The development of CE has facilitated the noninvasive evaluation of lesions in the small intestine and colon. However, since CE captures as many as 50,000 images per patient, image evaluation is an extremely time-consuming and difficult task for clinicians. In addition, the risk of oversight should be fully considered in cases where rare abnormal findings need to be identified among a large number of images. Therefore, various reading software, such as the QuickView mode and Suspected blood indicator (SBI) in the PillCam® system and the Omni mode in the EndoCapsule® system, have been developed to reduce reading time. Recent studies have evaluated AI-based image detection of CE (83,84).
Convolutional neural network (CNN), a deep-learning model that mimics the visual cortex of living organisms has high pattern recognition capability for images based on training using many images. Therefore, to automatically detect lesions in CE, the CNN should be trained on a large number of images to build an image diagnosis system with high diagnostic accuracy. However, collecting endoscopic images from many patients with the same disease is challenging due to the variety of lesions and the rarity of many diseases. In addition to the low resolution and light intensity, the images obtained by CE are affected by intestinal contents such as food residue, bile, and foam, which hinder the collection of a large number of good-quality images suitable for training and creation of a highly accurate system.
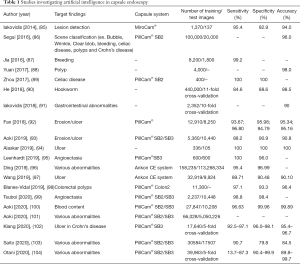
Most of the currently reported AI reading systems for CE are for SBCE, and models have been reported to detect small intestinal mucosal disorders (i.e., erosions and ulcers), angioectasia, blood, polyps, celiac disease, and parasites (Table 1). Aoki et al. developed a reading system for mucosal disorders (i.e., erosions and ulcers) by training a CNN model with 5,360 images. The authors tested the system’s ability to detect erosions and ulcers using 10,440 images and reported a sensitivity of 88.2%, specificity of 90.9%, and accuracy of 90.8% (93). In a study on angioectasia, Tsuboi et al. reported that a CNN model trained on 2,237 images had a sensitivity of 98.8% and a specificity of 98.4% (99).Ding et al. trained a CNN model on 158,235 capsule images, including abnormal findings such as inflammation, ulcers, polyps, lymphangiectasia, blood, lymphatic follicles, diverticula, and parasites, as well as normal images, and reported that the sensitivity and specificity of their AI-based detection system were 95.4% and 96.9%, respectively (96).
Full table
More recently, several studies were performed to verify the detection power of reading models for full videos. Ding et al. compared the lesion detection rate and reading time between CNN-extracted field images and normal images, both analyzed by an endoscopist, in 5,000 CE cases, including 3280 cases with abnormal findings, using a model developed to detect abnormalities in CE (96). The authors found that the endoscopist read an average of 578 CNN-extracted images, which was significantly less than the 22,654 images with regular reading. The mean reading time for CNN-extracted images was significantly shorter than that for the regular reading (96.9 vs. 5.9 minutes). The study also demonstrated that the detection sensitivity of reading only CNN-extracted images was significantly higher than that of normal reading (sensitivity per lesion, 99.9% vs. 76.89%; sensitivity per video, 99.88% vs. 74.57%), suggesting that the rate of detecting abnormal findings was higher and the reading time was shorter with the reading of CNN-extracted images, which may reduce the reading cost of CE.
Future perspectives
SBCE and CCE are useful for the diagnosis of various diseases, with recent studies focusing on the utility of AI-based methods in the analysis of images captured by CE. there are currently no CNN-based image reading systems in practice although numerous studies suggest its utility in reading capsule endoscope images. The versatility of CNN-based image reading systems should be evaluated using full videos of multiple endoscopic systems. In addition, since previous studies were retrospective in design and may contain various biases, randomized-prospective studies are necessary to verify its utility.
Acknowledgments
The authors would like to thank Enago (
Funding: This work was supported by JSPS KAKENHI Grant Number 19K08402.
Footnote
Reporting Checklist: The authors have completed the narrative review checklist. Available at http://dx.doi.org/10.21037/dmr-20-162
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-162). NH serves as an unpaid editorial board member of Digestive Medicine Research from Aug 2020 to Jul 2022. NH reports grants from Olympus, grants from Covidien, during the conduct of the study, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417. [Crossref] [PubMed]
- Meron GD. The development of the swallowable video capsule (M2A). Gastrointest Endosc 2000;52:817-9. [Crossref] [PubMed]
- Park S, Chun HJ, Keum B, et al. Capsule Endoscopy to Detect Normally Positioned Duodenal Papilla: Performance Comparison of SB and SB2. Gastroenterol Res Pract 2012;2012:202935 [Crossref] [PubMed]
- Rahman M, Akerman S, DeVito B, et al. Comparison of the diagnostic yield and outcomes between standard 8 h capsule endoscopy and the new 12 h capsule endoscopy for investigating small bowel pathology. World J Gastroenterol 2015;21:5542-7. [Crossref] [PubMed]
- Honda W, Ohmiya N, Hirooka Y, et al. Enteroscopic and radiologic diagnoses, treatment, and prognoses of small-bowel tumors. Gastrointest Endosc 2012;76:344-54. [Crossref] [PubMed]
- Leenhardt R, Li C, Koulaouzidis A, et al. Nomenclature and semantic description of vascular lesions in small bowel capsule endoscopy: an international Delphi consensus statement. Endosc Int Open 2019;7:E372-9. [Crossref] [PubMed]
- Koulaouzidis A, Plevris JN. Detection of the ampulla of Vater in small bowel capsule endoscopy: experience with two different systems. J Dig Dis 2012;13:621-7. [Crossref] [PubMed]
- Xavier S, Monteiro S, Magalhães J, et al. Capsule endoscopy with PillCamSB2 versus PillCamSB3: has the improvement in technology resulted in a step forward? Rev Esp Enferm Dig 2018;110:155-9. [PubMed]
- Ogata H, Kumai K, Imaeda H, et al. Clinical impact of a newly developed capsule endoscope: usefulness of a real-time image viewer for gastric transit abnormality. J Gastroenterol 2008;43:186-92. [Crossref] [PubMed]
- Hosoe N, Matsuoka K, Naganuma M, et al. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol 2013;28:1174-9. [Crossref] [PubMed]
- Dolak W, Kulnigg-Dabsch S, Evstatiev R, et al. A randomized head-to-head study of small-bowel imaging comparing MiroCam and EndoCapsule. Endoscopy 2012;44:1012-20. [Crossref] [PubMed]
- Choi EH, Mergener K, Semrad C, et al. A multicenter, prospective, randomized comparison of a novel signal transmission capsule endoscope to an existing capsule endoscope. Gastrointest Endosc 2013;78:325-32. [Crossref] [PubMed]
- Liao Z, Xu C, Li ZS. Completion rate and diagnostic yield of small-bowel capsule endoscopy: 1 vs. 2 frames per second. Endoscopy 2010;42:360-4. [Crossref] [PubMed]
- Liao Z, Gao R, Li F, et al. Fields of applications, diagnostic yields and findings of OMOM capsule endoscopy in 2400 Chinese patients. World J Gastroenterol 2010;16:2669-76. [Crossref] [PubMed]
- Friedrich K, Gehrke S, Stremmel W, et al. First clinical trial of a newly developed capsule endoscope with panoramic side view for small bowel: a pilot study. J Gastroenterol Hepatol 2013;28:1496-501. [Crossref] [PubMed]
- Zwinger LL, Siegmund B, Stroux A, et al. CapsoCam SV-1 Versus PillCam SB 3 in the Detection of Obscure Gastrointestinal Bleeding: Results of a Prospective Randomized Comparative Multicenter Study. J Clin Gastroenterol 2019;53:e101-6. [Crossref] [PubMed]
- Tontini GE, Wiedbrauck F, Cavallaro F, et al. Small-bowel capsule endoscopy with panoramic view: results of the first multicenter, observational study (with videos). Gastrointest Endosc 2017;85:401-8.e2. [Crossref] [PubMed]
- Yamamoto H, Ogata H, Matsumoto T, et al. Clinical Practice Guideline for Enteroscopy. Dig Endosc 2017;29:519-46. [Crossref] [PubMed]
- Rockey DC. Occult and obscure gastrointestinal bleeding: causes and clinical management. Nat Rev Gastroenterol Hepatol 2010;7:265-79. [Crossref] [PubMed]
- Briley CA Jr, Jackson DC, Johnsrude IS, et al. Acute gastrointestinal hemorrhage of small-bowel origin. Radiology 1980;136:317-9. [Crossref] [PubMed]
- Yano T, Yamamoto H, Sunada K, et al. Endoscopic classification of vascular lesions of the small intestine (with videos). Gastrointest Endosc 2008;67:169-72. [Crossref] [PubMed]
- Estévez E, González-Conde B, Vázquez-Iglesias JL, et al. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol 2006;18:881-8. [Crossref] [PubMed]
- Sidhu R, Sanders DS, Kapur K, et al. Factors predicting the diagnostic yield and intervention in obscure gastrointestinal bleeding investigated using capsule endoscopy. J Gastrointestin Liver Dis 2009;18:273-8. [PubMed]
- van Turenhout ST, Jacobs MA, van Weyenberg SJ, et al. Diagnostic yield of capsule endoscopy in a tertiary hospital in patients with obscure gastrointestinal bleeding. J Gastrointestin Liver Dis 2010;19:141-5. [PubMed]
- Albert JG, Schülbe R, Hahn L, et al. Impact of capsule endoscopy on outcome in mid-intestinal bleeding: a multicentre cohort study in 285 patients. Eur J Gastroenterol Hepatol 2008;20:971-7. [Crossref] [PubMed]
- Esaki M, Matsumoto T, Yada S, et al. Factors associated with the clinical impact of capsule endoscopy in patients with overt obscure gastrointestinal bleeding. Dig Dis Sci 2010;55:2294-301. [Crossref] [PubMed]
- Robinson CA, Jackson C, Condon D, et al. Impact of inpatient status and gender on small-bowel capsule endoscopy findings. Gastrointest Endosc 2011;74:1061-6. [Crossref] [PubMed]
- Lecleire S, Iwanicki-Caron I, Di-Fiore A, et al. Yield and impact of emergency capsule enteroscopy in severe obscure-overt gastrointestinal bleeding. Endoscopy 2012;44:337-42. [Crossref] [PubMed]
- Yamada A, Watabe H, Kobayashi Y, et al. Timing of capsule endoscopy influences the diagnosis and outcome in obscure-overt gastrointestinal bleeding. Hepatogastroenterology 2012;59:676-9. [PubMed]
- Wang A, Banerjee S, Barth BA, et al. Wireless capsule endoscopy. Gastrointest Endosc 2013;78:805-15. [Crossref] [PubMed]
- Cañas-Ventura A, Márquez L, Bessa X, et al. Outcome in obscure gastrointestinal bleeding after capsule endoscopy. World J Gastrointest Endosc 2013;5:551-8. [Crossref] [PubMed]
- Gomes C, Pinho R, Rodrigues A, et al. Impact of the timing of capsule endoscopy in overt obscure gastrointestinal bleeding on yield and rebleeding rate - is sooner than 14 d advisable? World J Gastrointest Endosc 2018;10:74-82. [Crossref] [PubMed]
- Lepileur L, Dray X, Antonietti M, et al. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol 2012;10:1376-80. [Crossref] [PubMed]
- Shim KN, Moon JS, Chang DK, et al. Guideline for capsule endoscopy: obscure gastrointestinal bleeding. Clin Endosc 2013;46:45-53. [Crossref] [PubMed]
- Fisher L, Lee Krinsky M, Anderson MA, et al. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc 2010;72:471-9. [Crossref] [PubMed]
- Gerson LB, Fidler JL, Cave DR, et al. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol 2015;110:1265-87; quiz 88. [Crossref] [PubMed]
- Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology 2007;133:1694-6. [Crossref] [PubMed]
- Rondonotti E, Spada C, Adler S, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2018;50:423-46. [Crossref] [PubMed]
- Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet 2017;389:1741-55. [Crossref] [PubMed]
- Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis 2009;15:1295-301. [Crossref] [PubMed]
- Takabayashi K, Hosoe N, Kato M, et al. Significance of endoscopic deep small bowel evaluation using balloon-assisted enteroscopy for Crohn's disease in clinical remission. J Gastroenterol 2021;56:25-33. [Crossref] [PubMed]
- Petruzziello C, Onali S, Calabrese E, et al. Wireless capsule endoscopy and proximal small bowel lesions in Crohn's disease. World J Gastroenterol 2010;16:3299-304. [Crossref] [PubMed]
- Esaki M, Matsumoto T, Ohmiya N, et al. Capsule endoscopy findings for the diagnosis of Crohn's disease: a nationwide case-control study. J Gastroenterol 2019;54:249-60. [Crossref] [PubMed]
- Leighton JA, Helper DJ, Gralnek IM, et al. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn's disease: a feasibility study. Gastrointest Endosc 2017;85:196-205.e1. [Crossref] [PubMed]
- Gal E, Geller A, Fraser G, et al. Assessment and validation of the new capsule endoscopy Crohn's disease activity index (CECDAI). Dig Dis Sci 2008;53:1933-7. [Crossref] [PubMed]
- Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008;27:146-54. [Crossref] [PubMed]
- Niv Y, Gal E, Gabovitz V, et al. Capsule Endoscopy Crohn's Disease Activity Index (CECDAIic or Niv Score) for the Small Bowel and Colon. J Clin Gastroenterol 2018;52:45-9. [Crossref] [PubMed]
- Niv E, Fishman S, Kachman H, et al. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn's disease. J Crohns Colitis 2014;8:1616-23. [Crossref] [PubMed]
- Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn's Disease Activity Index. Dig Dis Sci 2012;57:987-93. [Crossref] [PubMed]
- Koulaouzidis A, Sipponen T, Nemeth A, et al. Association Between Fecal Calprotectin Levels and Small-bowel Inflammation Score in Capsule Endoscopy: A Multicenter Retrospective Study. Dig Dis Sci 2016;61:2033-40. [Crossref] [PubMed]
- Höög CM, Bark L, Broström O, et al. Capsule endoscopic findings correlate with fecal calprotectin and C-reactive protein in patients with suspected small-bowel Crohn's disease. Scand J Gastroenterol 2014;49:1084-90. [Crossref] [PubMed]
- He C, Zhang J, Chen Z, et al. Relationships of capsule endoscopy Lewis score with clinical disease activity indices, C-reactive protein, and small bowel transit time in pediatric and adult patients with small bowel Crohn's disease. Medicine (Baltimore) 2017;96:e7780 [Crossref] [PubMed]
- Eliakim R, Fireman Z, Gralnek IM, et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy 2006;38:963-70. [Crossref] [PubMed]
- Van Gossum A, Munoz-Navas M, Fernandez-Urien I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009;361:264-70. Erratum in: N Engl J Med 2009;361:1220PubMed</jrn>. [Crossref] [PubMed]
- Spada C, Hassan C, Munoz-Navas M, et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc 2011;74:581-9.e1. Erratum in: Gastrointest Endosc 2011;74:1174. [Crossref] [PubMed]
- Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy 2006;38:971-7. [Crossref] [PubMed]
- Gay G, Delvaux M, Frederic M, et al. Could the colonic capsule PillCam Colon be clinically useful for selecting patients who deserve a complete colonoscopy?: results of clinical comparison with colonoscopy in the perspective of colorectal cancer screening. Am J Gastroenterol 2010;105:1076-86. [Crossref] [PubMed]
- Sacher-Huvelin S, Coron E, Gaudric M, et al. Colon capsule endoscopy vs. colonoscopy in patients at average or increased risk of colorectal cancer. Aliment Pharmacol Ther 2010;32:1145-53. [Crossref] [PubMed]
- Pilz JB, Portmann S, Peter S, et al. Colon Capsule Endoscopy compared to Conventional Colonoscopy under routine screening conditions. BMC Gastroenterol 2010;10:66. [Crossref] [PubMed]
- Eliakim R, Yassin K, Niv Y, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy 2009;41:1026-31. [Crossref] [PubMed]
- Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015;148:948-57.e2. [Crossref] [PubMed]
- Saito Y, Saito S, Oka S, et al. Evaluation of the clinical efficacy of colon capsule endoscopy in the detection of lesions of the colon: prospective, multicenter, open study. Gastrointest Endosc 2015;82:861-9. [Crossref] [PubMed]
- Parodi A, Vanbiervliet G, Hassan C, et al. Colon capsule endoscopy to screen for colorectal neoplasia in those with family histories of colorectal cancer. Gastrointest Endosc 2018;87:695-704. [Crossref] [PubMed]
- Spada C, Pasha SF, Gross SA, et al. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1533-43.e8. [Crossref] [PubMed]
- Rondonotti E, Borghi C, Mandelli G, et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol 2014;12:1303-10. [Crossref] [PubMed]
- Utano K, Katsuki S, Matsuda T, et al. Colon Capsule Endoscopy versus CT Colonography in Patients with Large Non-Polypoid Tumours: A Multicentre Prospective Comparative Study (4CN Study). Digestion 2020;101:615-23. [Crossref] [PubMed]
- Kobaek-Larsen M, Kroijer R, Dyrvig AK, et al. Back-to-back colon capsule endoscopy and optical colonoscopy in colorectal cancer screening individuals. Colorectal Dis 2018;20:479-85. [Crossref] [PubMed]
- Ota Y, Yamada A, Kobayashi Y, et al. Diagnostic capability of colon capsule endoscopy for advanced colorectal cancer: A pilot study. Dig Endosc 2017;29:695-701. [Crossref] [PubMed]
- Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785-94. [Crossref] [PubMed]
- Eaden JA, Mayberry JF. Colorectal cancer complicating ulcerative colitis: a review. Am J Gastroenterol 2000;95:2710-9. [Crossref] [PubMed]
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526-35. [Crossref] [PubMed]
- Vienne A, Simon T, Cosnes J, et al. Low prevalence of colonoscopic surveillance of inflammatory bowel disease patients with longstanding extensive colitis: a clinical practice survey nested in the CESAME cohort. Aliment Pharmacol Ther 2011;34:188-95. [Crossref] [PubMed]
- Sung J, Ho KY, Chiu HM, et al. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy 2012;44:754-8. [Crossref] [PubMed]
- Ye CA, Gao YJ, Ge ZZ, et al. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis 2013;14:117-24. [Crossref] [PubMed]
- San Juan-Acosta M, Caunedo-Alvarez A, Arguelles-Arias F, et al. Colon capsule endoscopy is a safe and useful tool to assess disease parameters in patients with ulcerative colitis. Eur J Gastroenterol Hepatol 2014;26:894-901. [Crossref] [PubMed]
- Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy 2011;43:123-7. [Crossref] [PubMed]
- Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med 1961;30:393-407. [PubMed]
- Shi HY, Chan FKL, Higashimori A, et al. A prospective study on second-generation colon capsule endoscopy to detect mucosal lesions and disease activity in ulcerative colitis (with video). Gastrointest Endosc 2017;86:1139-46.e6. [Crossref] [PubMed]
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625-9. [Crossref] [PubMed]
- Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012;61:535-42. [Crossref] [PubMed]
- Hosoe N, Nakano M, Takeuchi K, et al. Establishment of a Novel Scoring System for Colon Capsule Endoscopy to Assess the Severity of Ulcerative Colitis-Capsule Scoring of Ulcerative Colitis. Inflamm Bowel Dis 2018;24:2641-7. [Crossref] [PubMed]
- Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330:1841-5. [Crossref] [PubMed]
- Iakovidis DK, Koulaouzidis A. Software for enhanced video capsule endoscopy: challenges for essential progress. Nat Rev Gastroenterol Hepatol 2015;12:172-86. [Crossref] [PubMed]
- Dray X, Iakovidis D, Houdeville C, et al. Artificial intelligence in small bowel capsule endoscopy - current status, challenges and future promise. J Gastroenterol Hepatol 2021;36:12-9. [Crossref] [PubMed]
- Iakovidis DK, Koulaouzidis A. Automatic lesion detection in capsule endoscopy based on color saliency: closer to an essential adjunct for reviewing software. Gastrointest Endosc 2014;80:877-83. [Crossref] [PubMed]
- Seguí S, Drozdzal M, Pascual G, et al. Generic feature learning for wireless capsule endoscopy analysis. Comput Biol Med 2016;79:163-72. [Crossref] [PubMed]
- Jia X, Meng MQ. A deep convolutional neural network for bleeding detection in Wireless Capsule Endoscopy images. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:639-42. [Crossref] [PubMed]
- Yuan Y, Meng MQ. Deep learning for polyp recognition in wireless capsule endoscopy images. Med Phys 2017;44:1379-89. [Crossref] [PubMed]
- Zhou T, Han G, Li BN, et al. Quantitative analysis of patients with celiac disease by video capsule endoscopy: A deep learning method. Comput Biol Med 2017;85:1-6. [Crossref] [PubMed]
- He JY, Wu X, Jiang YG, et al. Hookworm Detection in Wireless Capsule Endoscopy Images With Deep Learning. IEEE Trans Image Process 2018;27:2379-92. [Crossref] [PubMed]
- Iakovidis DK, Georgakopoulos SV, Vasilakakis M, et al. Detecting and Locating Gastrointestinal Anomalies Using Deep Learning and Iterative Cluster Unification. IEEE Trans Med Imaging 2018;37:2196-210. [Crossref] [PubMed]
- Fan S, Xu L, Fan Y, et al. Computer-aided detection of small intestinal ulcer and erosion in wireless capsule endoscopy images. Phys Med Biol 2018;63:165001 [Crossref] [PubMed]
- Aoki T, Yamada A, Aoyama K, et al. Automatic detection of erosions and ulcerations in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest Endosc 2019;89:357-63.e2. [Crossref] [PubMed]
- Alaskar H, Hussain A, Al-Aseem N, et al. Application of Convolutional Neural Networks for Automated Ulcer Detection in Wireless Capsule Endoscopy Images. Sensors (Basel) 2019;19:1265. [Crossref] [PubMed]
- Leenhardt R, Vasseur P, Li C, et al. A neural network algorithm for detection of GI angiectasia during small-bowel capsule endoscopy. Gastrointest Endosc 2019;89:189-94. [Crossref] [PubMed]
- Ding Z, Shi H, Zhang H, et al. Gastroenterologist-Level Identification of Small-Bowel Diseases and Normal Variants by Capsule Endoscopy Using a Deep-Learning Model. Gastroenterology 2019;157:1044-54.e5. [Crossref] [PubMed]
- Wang S, Xing Y, Zhang L, et al. A systematic evaluation and optimization of automatic detection of ulcers in wireless capsule endoscopy on a large dataset using deep convolutional neural networks. Phys Med Biol 2019;64:235014 [Crossref] [PubMed]
- Blanes-Vidal V, Baatrup G, Nadimi ES. Addressing priority challenges in the detection and assessment of colorectal polyps from capsule endoscopy and colonoscopy in colorectal cancer screening using machine learning. Acta Oncol 2019;58:S29-36. [Crossref] [PubMed]
- Tsuboi A, Oka S, Aoyama K, et al. Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images. Dig Endosc 2020;32:382-90. [Crossref] [PubMed]
- Aoki T, Yamada A, Kato Y, et al. Automatic detection of blood content in capsule endoscopy images based on a deep convolutional neural network. J Gastroenterol Hepatol 2020;35:1196-200. [Crossref] [PubMed]
- Aoki T, Yamada A, Kato Y, et al. Automatic detection of various abnormalities in capsule endoscopy videos by a deep learning-based system: a multicenter study. Gastrointest Endosc 2021;93:165-173.e1. [Crossref] [PubMed]
- Klang E, Barash Y, Margalit RY, et al. Deep learning algorithms for automated detection of Crohn's disease ulcers by video capsule endoscopy. Gastrointest Endosc 2020;91:606-13.e2. [Crossref] [PubMed]
- Saito H, Aoki T, Aoyama K, et al. Automatic detection and classification of protruding lesions in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest Endosc 2020;92:144-51.e1. [Crossref] [PubMed]
- Otani K, Nakada A, Kurose Y, et al. Automatic detection of different types of small-bowel lesions on capsule endoscopy images using a newly developed deep convolutional neural network. Endoscopy 2020;52:786-91. [Crossref] [PubMed]
Cite this article as: Hayashi Y, Hosoe N, Limpias Kamiya KJ, Takabayashi K, Ogata H, Kanai T. A narrative review of recent progress and current perspectives on video capsule endoscopy. Dig Med Res 2021;4:5.