How multimodal treatment improves surgery for oesophageal cancer—a narrative review
Introduction
The outcome of treatment of oesophageal cancer like all solid cancers is biologically pre-determined by the presence or absence of putative micrometastases (1). Thus, surgical treatment is loco-regional control only but potential cure entails in addition adjuvant chemotherapy to destroy the putative micrometastases. The reason for poor survival is the late presentation as the tumour has usually invaded muscularis propria, or beyond once symptoms present. In addition, the patients are often elderly with multiple co-morbidities. Therefore, cure by surgery alone may only occur in early cancer when the disease has not spread. A knowledge of the surgical anatomy of the oesophagus and surgical pathology of oesophageal carcinoma which entails the modes of spread is mandatory to understanding the different therapeutic surgical procedures adopted for tumours at each site (2). Over the last 30 years, the marked increase in incidence of gastroesophageal junction (GOJ) tumours of 3–4% per annum (3,4) was associated with the downward migration of oesophageal tumours and proximal shift of gastric tumours (2,5). The epidemiological profile suggests a similar aetiology and is consistent with the two cancers having a similar phenotype and p53 gene mutation and may thus be treated as a separate entity (6,7). Only specialist oesophagogastric specialist surgical centres can accurately classify the tumour of GOJ as arising in the distal oesophagus (type I), gastric cardia (type II), or subcardinal stomach (type III) (2,3). The staging of the cancer needs to be accurate and thorough so that therapeutic strategies can be planned appropriately and potential curative therapy targeted to those likely to benefit. Oesophageal cancer is one of the most challenging pathological conditions because of the magnitude of the surgical procedure, dealing with wide areas of the neck, mediastinum and abdomen and the versatility required in surgical reconstruction (2,8,9). Survival is related to the stage of the disease, and with stage 1 disease, 5-year survival of greater than 80% have been achieved (10) emphasizing the importance of early detection. The reasons for improved results with falling morbidity and mortality over the past 20 years for oesophageal resection for all stages of tumour are the increase in specialist units with the influence of surgeon case volume (>20 cases/year) (11), multidisciplinary approach with multimodality treatment, earlier diagnosis, better patient selection and improved perioperative management. Surgery is the only treatment modality that has consistently been shown to prolong survival despite only in about 20% of cases (2). This is because of the natural history of an increasing obstructive symptom (dysphagia) that has been present for several months as the tumour evolves over many months or years. Therefore, patients with oesophageal cancer are considered either for radical treatment or for palliative therapy in those who are too elderly, unfit or have advanced tumours. The three main combined modality approaches are (I) preoperative chemo or chemo-radiotherapy, (II) oesophagectomy with adjuvant chemotherapy or chemo-radiotherapy, and (III) primary definitive chemo-radiotherapy with or without salvage oesophagectomy. The remarkable advances in optical technology which optimizes the view of the surgical field by using up to 10× magnification has brought forward minimally invasive oesophagectomy with endoscopic instruments to oesophageal cancer surgery. However, the initial results with the thoracoscopic approach did not show a real benefit over the open approach, in particular due to a high number of pulmonary complications (12,13). Although no improvement has been determined in relation to cancer survival, both malnourished and non-malnourished patients could benefit from nutritional support during multimodality treatment (2). We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-142).
Discussion
Patient pathway, staging and selection for oesophageal surgery
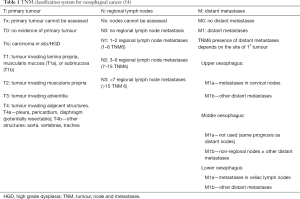
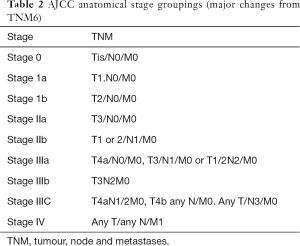
The patients who should undergo staging are (I) patients diagnosed with malignancy, (II) patients diagnosed with high grade dysplasia (HGD) to exclude co-existing malignancy or focus of malignancy and (III) patients who have undergone neo-adjuvant therapy for re-staging. The diagnosis and biopsy is by oesophagogastroduodenoscopy (OGD). OGD would elucidate the site, size, proximal and distal extent of the tumour. Pre-operative dilatation or stenting if appropriate with preferable biodegradable stent would improve nutrition. Clinical [tumour, node and metastases (TNM)] staging determines the anatomical extent of the tumour prior to treatment (Tables 1,2) (14). Assessment includes physical examination, endoscopic biopsies, laboratory studies and imaging. The location of the tumour, depth of invasion and evidence of nodal and distant spread is documented. A minimum of eight biopsies is taken to diagnose malignancy. An interobserver diagnosis of neoplasia is greater for endoscopic mucosal resection (EMR) than biopsy as the submucosa is present in 88% of samples in EMR as opposed to 1% of biopsies. EMR is thus superior to endoscopic ultrasound scan (EUS) in staging early T1 cancers. Barium swallow may show irregular filling defect but may miss a proportion of smaller tumours which has made it now largely defunct. OGD may be repeated by surgeons at time of laparoscopy to plan the operative approach (2). A spiral IV contrast-enhanced computed tomography (CT) scan of thorax/abdomen/pelvis would assess local spread, exclude distant and unresectable disease. The poor delineation of layers of oesophageal wall may render it difficult to differentiate T1 and T2 tumours but volumetric analysis would improve this. Microscopic invasion in T3 tumours may be elusive but multi-planar reformats delineate T3/4. It predicts mediastinal invasion in >80% of cases and involvement of aorta, tracheobronchial tree, and crura are easily identified. EUS assess tumour size, depth of invasion and local lymph nodes. Thus, in addition to distinguishing layers of oesophageal wall it is superior to CT for local tumour staging. EUS alone or in combination with CT has a sensitivity of 91% for detecting local nodal disease, and thus, all candidates for curative resection are considered for EUS ± fine needle aspiration (FNA) cytology if indicated (2). Local resectability by EMR/endoscopic submucosal dissection (ESD) is established at specialist centres. Combination of metabolic assessment with 18-fluorodeoxyglucose positron emission tomography (18F-FDG) PET and CT provides integrated functional and anatomical data. It would exclude distant metastases such as bone and delineate significance of equivocal lesions, such as pulmonary radio-opacities. Staging adjuncts include abdominal triple phase CT, ultrasound scan (USS), or magnetic resonance imaging (MRI) for equivocal hepatic lesions; MRI thorax for those who cannot undergo CT or for additional investigation following CT/EUS; bronchoscopy ± USS if tracheobronchial invasion; thoracoscopy for suspicious nodes not amenable to biopsy or assessment by CT or other image-guided techniques and neck imaging with EUS or CT in patients with cervical tumours (2). Laparoscopy primarily assesses peritoneal spread especially in oesophageal tumours with a gastric component. Additional information is derived from 17% GOJ tumours and 28% gastric tumours. A positive peritoneal washout cytology from oesophageal and junctional cancers have a poor prognosis with a median survival of between 3 and 23 months (2). It would exclude colonic disease which may be required as a conduit and a feeding jejunostomy may be placed at this stage. The clinico-pathological staging is when the preoperative and intra-operative findings are refined after histopathological examination of the resected specimen. This would allow the cancer to be classified into one of four stages which will predict prognosis (Table 2). The N factor in the staging system is not the same as the extent of lymphadenectomy. The pathological evaluation is performed to determine the presence/absence of residual tumour post-neoadjuvant chemo-radiation, depth of invasion (pT stage), tumour regression grade and nodal metastasis (pN) (15). The additional information about host response such as biological markers and acute phase proteins may allow a more accurate prediction of prognosis when combined with conventional histopathological staging data (7,16) and tailor treatment appropriately.
Full table
Preoperative assessments
The patient requires an adequate cardiopulmonary reserve to be able to meet the metabolic demands of oesophageal surgery (2,17-19). A recent study showed that cardiopulmonary fitness predicts post-operative major morbidity after oesophagectomy for patients with cancer (20). In addition, a postoperative systemic inflammatory response syndrome (SIRS) following oesophagectomy is predictive of subsequent pulmonary complications (17-19). The preoperative assessment is preferably achieved at a multidisciplinary clinic with surgeons, anaesthetists, dietician and physiotherapists. Formal assessment of performance status although largely subjective, exercise testing and cardiopulmonary exercise testing (CPET) are an agreed protocol. Exercise capacity is surrogate for functional cardio-respiratory reserve. Poor exercise tolerance correlates with perioperative risks independent of age and other factors. Exercise-induced hypotension suggestive of ventricular impairment secondary to coronary artery disease is ominous mandating investigation. CPET is a dynamic non-invasive objective test of the cardio-respiratory system to adapt to sudden increase in oxygen demand. However, the peri-operative risk stratification by CPET is debated because limitations can occur in patients with reduced lower limb function related to osteoarthritis or limb dysfunction, and malnutrition would reduce exercise tolerance. Oesopagogastric cancer-associated malnutrition may be tumour, patient or treatment (surgery, radiotherapy, chemotherapy)-related. Severe malnutrition in patients undergoing curative treatment of oesophageal cancer is associated with increased mortality and morbidity, reduced treatment efficacy and increased length of hospital stay (21). Nutrition assessment ± hyperalimentation using a validated nutritional risk tool will identify at risk patients who are offered advice and considered for pre-operative nutrition. Those with BMI <18.5 or >20% weight loss have increased risk of post-surgical complications. Obesity also increased risk of complications, but rare in these patients. Specific evidence is lacking for the routine prolonged jejunal feeding with regard to the mounting evidence of complications (2,21-23). The patients are psychologically prepared following counseling about treatment options and detailed description of the perioperative period. Smoking is stopped and thromboembolic prophylaxis rendered with anti-thromboembolic stockings, low molecular weight heparin, and pre-operative pneumatic calf compression. Four units blood is cross-matched but use is avoided if possible due to risks associated with transfusion. High dependency unit (HDU) or intensive therapy unit (ITU) bed is made available. The colon is prepared if required as conduit. The stomach may not be available as a first choice conduit or an extra-long graft may be required to reach the hypopharynx in instances such as prior gastrectomy, concomitant gastrectomy for synchronous gastric tumours, proximal squamous cell carcinoma (SCC) or failures of previous gastric pull-up. An epidural is placed for post-operative analgesia. Intravenous broad spectrum antibiotics administered immediately pre-operatively or at induction. A double lumen endotracheal tube would allow exclusion of one lung (2).
Operative settings, indications, rationale and results
All patients should have been discussed in a multidisciplinary setting and surgery only undertaken if it is general consensus of the team. The surgical decisions are taken based upon predicted prognosis and effect of intervention upon quality of life. Where radical surgery is based upon histology alone, results should be confirmed by a second pathologist. Surgery should ideally take place in high volume centres with sufficient surgical and anaesthetic experience. Laparoscopic and thoracoscopic techniques should take place in specialist centres, by experienced surgeons, with full informed consent and local clinical governance committee support. Operative indications for malignancy are (I) with curative intent for patients fit with early lesions (T1–3/N0/M0), (II) for HGD in a long Barrett’s segment or endoscopic treatments in short segments (Table 3). Surgery has no place when haematogenous spread has occurred. Radical surgery and lymphadenectomy aim for R0 resection with proximal, distal, and circumferential margin clearance. The rationale is to achieve optimal staging, control local disease and improve cure rates. There are excellent results for early squamous and adenocarcinoma with 5-year survival >80% when tumour is confined to mucosa and 50% when submucosa involved. Immediate postoperative nutrition following oesophagectomy is now favoured because nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve (23). Overall surgical treatment gives 5-year survival of 5–20%, in-hospital mortality <10%, clinical anastomotic leak rates <5%, and curative resection rates (R0) exceed 30% (2).

Full table
Types of oesophagectomy
The type of surgical procedure is determined by (I) the site and type of tumour, (II) the extent of lymphadenectomy needed, (III) the surgeon’s expertise which will determine the type of reconstruction, and the use of pyloric drainage procedures would depend on surgical preference. Upper third tumours require a cervical incision in transhiatal or three-stage (McKeown) oesophagectomy for neck anastomosis (24). Transhiatal oesophagectomy (25) is advocated for intraepithelial SCC and HGD in Barrett’s with very low incidence of nodal disease and surgeon’s preference. There are fewer pulmonary complications with transhiatal oesophagectomy than transthoracic routes, but often achieve sub-optimal lymphadenectomy. It can have major blood loss due to surrounding structures and a low threshold for thoracotomy is required in this eventuality (26). The three-stage oesophagectomy (McKeown) has same abdominal and thoracic phases as Ivor-Lewis technique (27) classically used for mid-oesophageal tumours/lower third tumours, although the thoracic phase precede abdominal and cervical. It is therefore used for proximal tumours where slightly more dissection and resection is required to achieve a safe proximal margin. The radical curative surgery (Akiyama technique) (28) performed in Japan because of higher incidence of SCC entails an initial Ivor-Lewis abdominal and thoracic phases, and then bilateral cervical incision with extensive lymphadenectomy in neck, mediastinum and abdomen. Recurrent laryngeal nerve injury is an additional morbidity. The 5-year survival is over 50% in those undergoing curative resection. Pharyngo-laryngo-oesophagectomy for carcinoma of the upper cervical oesophagus or hypopharynx with a free interposition jejunal graft and microvascular anastomosis in the neck is usually performed by head and neck surgeons (29). Carcinoma above the diaphragm requires thoracotomy for formal lymph node dissection and the Ivor-Lewis procedure is the most widely used (8,27,30,31). A third cervical stage can be added to improve clearance and anastomosis performed in the neck or a left thoraco-abdominal approach for a distal tumour. A transhiatal approach for Siewert type I (distal oesophagus) and II (true junctional) GOJ tumours in which tumour is mobilized under direct vision is an alternative (8,30). Ivor-Lewis procedure entails an initial laparotomy which would first assess fixity and nodal involvement for suitability for resection. If resection is possible the stomach is mobilized with preservation of right gastric and gastroepiploic vessels for blood supply to the gastric conduit. The diaphragmatic crus is incised to enlarge the hiatus and the lower oesophagus mobilize up into the thorax by blunt and finger dissection. The abdomen is then closed and the thoracic phase commenced. Following a right thoracotomy at level of 5th or 6th rib, the collapsed right lung is pulled down and forward to expose the mediastinal pleura which is incised to allow ligation of the oesophageal aortic branches over the anterior border of the aorta. The azygos vein is doubly ligated with 2/0 vicryl as it arches over the root of the right lung and, the thoracic duct is ligated and divided just above the diaphragm at around T10 to prevent chylothorax. The thoracic oesophageal mobilization with en bloc lymphadenectomy is continued until it meets the abdominal mobilization and, all pleural attachments divided to allow stomach to pass into thorax. The nasogastric tube is withdrawn, proximal oesophageal stay sutures inserted and oesophagus transected at level of the apex of the thorax. The stomach is divided along lesser curve with linear stapler and the specimen with lymph nodes removed en bloc. A gastro-oesophageal anastomosis is performed within the thorax. Basal and apical underwater seal chest drains are inserted, the ribs re-approximated with strong, absorbable, interrupted sutures, lung re-expanded, chest muscles closed in layers and clips to skin.
Tumours below the diaphragm require radical excision of lower thoracic oesophagus and gastric cardia or entire stomach if the stomach is largely involved. A left thoraco-abdominal approach provides excellent access to lower thoracic oesophagus and upper stomach. A left thoraco-abdominal oesophagectomy is also used for bulky tumours of GOJ, lower and middle third oesophagus, but contraindicated for malignancy above aortic arch due to poor access. The oesophagus is mobilized from the hiatus upwards to aortic arch, transected and an intra-thoracic oesophagogastric anastomosis performed following the fashioning of the gastric conduit. It has relatively poor access to infracolic abdomen and thoracic duct and historically high levels of R1/R2 resections reported (32). The Japan clinical Oncology group Trial largely on SCC showed increased complications and no survival benefit and its appropriateness for cardiac tumours only (33). Gillies et al. (34) reported an in hospital mortality of 5.7% following left thoraco-abdominal oesophagectomy on 211 patients, 52% single complication, 7% anastomotic leak, 71% R0 (negative resection margin) and a 1- and 5-year survival of 70% and 21% respectively. Davies et al. (35) demonstrated oncological equivalence between the left thoraco-abdominal approach and the Ivor-Lewis approach, but possible short-term advantages to the former.
Alternative conduits for oesophageal replacement
As a long graft colon interposition is a second-line reconstructive option after accurate patient selection and investigation of the colon. The left colon vascularized by the ascending branch of the left colic artery and with a highly dependable venous drainage provided by the marginal Riolan’s arcade is the best oesophageal substitute in most circumstances (36,37). It is suitable for an upper intrathoracic or neck anastomosis but the location of the anastomosis depends on the route of colon interposition. A retrosternal route and a cervical oesophago-colic anastomosis is preferred in patients with ‘hostile’ mediastinum from previous thoracotomy or radiotherapy, or as a second-stage ‘bypass’ procedure following emergency oesophagectomy or oesophageal exclusion and diversion. The colonic graft can be placed antesternally through a subcutaneous tunnel when the retrosternal route is not viable due to previous sternotomy (36,38). As free jejunal grafts are a suitable alternative to long-pedicled grafts for reconstruction of the cervical oesophagus even in patients who received previous chemo-radiotherapy, it may also be used by a tailored surgical approach (substernal, presternal, median sternotomy route) to repair the failed colon interposition (39). Despite the intrinsic reliable blood supply, the pattern of the vascular arcade and loop redundancy does not render the jejunum reaching the hypopharynx easily. Thus, long-pedicled jejunal interposition requires good surgical judgement and superior technical skills (39,40).
Resection margins, specimen and extent of lymphadenectomy
Extensive studies show resection margins should be 10 cm proximal to macroscopic tumour and 5 cm distal when oesophagus is in the natural state (41-44). Adenocarcinoma of lower oesophagus commonly invades gastric cardia, fundus and lesser curve. Some degree of gastric excision is essential for adequate resection and lymphadenectomy in the abdomen. Adequate radial margins also need to be considered and contiguous excision of the crura and diaphragm needs to be considered particularly for junctional tumours. Not infrequently, primary tumours with multicentric lesions commonly in SCC require more extensive lengths for safe surgical margins. This explains the finding of positive resection margins in nearly 40% of specimens when the oesophageal resection margin is limited to only 4 cm and still of 17% when the margin is 10 cm (45,46). A 10 cm margin on both sides of a tumour measuring an average of 5.5 cm would require an overall length of specimen exceeding that of the normal human oesophagus (~25 cm). Tumours with an upper margin of less than 10 cm from the cricopharyngeus would require a resection of the distal pharynx and larynx with or not preserving the patient’s voice. Thus, if resection margins are less than 4 cm consideration should be given to adjuvant chemo-radiotherapy (47). Although a tumour-free surgical margin may be the main goal of surgical resection, patients with microscopically cancer-positive margins may die of other manifestations before clinical evidence of loco-regional recurrence (1,2,48,49) This is an argument for neo-adjuvant chemotherapy as corroborated in studies which confirmed that neoadjuvant therapy and en bloc transthoracic oesophagectomy show favourable results for achievement of negative circumferential resection margin (CRM) (50,51). Most authors would agree that in order to make allowance for intramural submucosal spread of squamous and adenocarcinomas a subtotal oesophagectomy should be carried out in patients with tumours at any site (1,2). The pathology report on the resection specimen should include as a minimum (I) type of tumour, (II) grade of tumour, depth of invasion, (III) involvement of resection margins, (IV) vascular invasion, (V) presence of Barrett’s metaplasia and (VI) number of nodes resected and the number containing metastatic tumour (15). The extent of lymphadenectomy continues to be an area of considerable controversy (52-54) especially as distant lymphatic spread is frequent in early SCC (7,15). Many surgeons do not practice a formal lymphadenectomy during either transhiatal or transthoracic approaches. However, there is little justification for oesophagectomy to be performed with intent to cure without any attempt to clear the first tier of lymph nodes. The description of tiers of lymph nodes in oesophageal cancer has been designed according to the anatomy of the lymphatic drainage system of the oesophagus (2,55-57). The strong arguments for extensive surgery including lymphadenectomy are (I) it contributes to the accurate staging of the disease with a minimum of 15 excised lymph nodes (28,58-60), (II) reduces loco-regional recurrence and produces prolonged tumour-free survival and, (III) by increasing the number of patients undergoing R0 resection (no residual disease left behind), the 5-year survival rate is improved (61).
Strategies to minimize loco-regional recurrence
Pre-operative treatment is currently recommended for all but the earliest resectable carcinomas of the oesophagus for both SCC and adenocarcinoma. The Medical Research Council (MRC) OE02 study of two cycles of cisplatin and fluorouracil (CF) chemotherapy of 802 patients with carcinoma of the oesophagus prior to definitive surgery set the standard of for neo-adjuvant therapy for oesophageal cancer in the UK (62). The median survival improved from 13.3 to 16.8 months, and the 2-year survival from 34% to 43%. Meta-analyses of randomized trials have supported the use of neoadjuvant chemotherapy before surgical resection of locally advanced oesophageal cancer to improve survival without serious systemic adverse events (62,63). Neoadjuvant therapy using combinations of chemotherapy and/or radiotherapy is being increasingly used in protocols for the multimodality management of oesophageal cancer. Chemotherapy is administered concurrently with radiotherapy and act as a radio-sensitizer. Several studies have shown that tumour regression grade and nodal metastasis after assessment of pathological response to neoadjuvant therapy are independent prognostic factors for both adenocarcinoma and SCC (64-67). In the USA, the current National Comprehensive Cancer Network 2010 guidelines (68) recommend neo-adjuvant chemo-radiotherapy based on several studies (69-74). However, the evidence of benefit over neo-adjuvant chemotherapy is unknown (75-77). Individual randomized trials of neo-adjuvant chemo-radiotherapy against surgery alone have not all been positive, but meta-analysis have suggested there is an improvement in median survival and overall survival at 3 and 5 years. Gebski meta-analysis (78) of 10 randomized control trials (RCTs) comparing neo-adjuvant chemo-radiotherapy to surgery alone showed a 13% absolute difference in survival at 3 years. The Dutch trial of paclitaxel and carboplatin and surgery compared to surgery alone in 74% distal oesophageal and 12% GOJ showed a mean survival of 49 months compared to 26 months for the surgery alone arm with the benefit more pronounced in the SCC arm (79). The FFCD 9901 trial consisted of fluorouracil (FU)/cisplatin plus radiotherapy and surgery compared to surgery alone on 195 patients with localized stage I and II oesophageal SCC (70%) and adenocarcinoma (29%). This was aborted because of no advantage to chemo-radiation and significantly increased operative mortality of 7.3% (80). There was a higher frequency of loco-regional relapse in the chemo-radiotherapy alone group proving the importance of surgery. Survival rates were however similar in both groups. Neo-adjuvant chemo-radiotherapy is considered for tumours above T2 or N1 (excluding T1 N0 tumours) and in patients who are medically fit for both resection and chemo-radiotherapy (81,82). Although trials are ongoing to determine optimum radiation dose and most effective chemotherapeutic agents, neo-adjuvant chemo-radiotherapy as an option is included in British and European guidelines (2). Standard chemotherapy agents are cisplatin and FU but newer agents, paclitaxel, carboplatin, and capecitabine have shown promise in trials although nutritional support via jejunostomy is often required. Surgery should not take place for at least 4 weeks after radiotherapy to allow inflammation to settle and maximum response to occur. The CROSS study of paclitaxel and carboplatin with radiotherapy showed a median survival of 49 months as compared to 26 months in surgery alone arm and the 3-year survival rate was increased from 48% to 59%. The R0 resection rate was 90% compared to 65% in the surgery alone arm (83). Complete pathological response was seen in 25% of cases associated with a significantly better prognosis and a 5-year survival rate of up to 60%. A Cochrane meta-analysis (84) of preoperative radiotherapy for patients with resectable oesophageal carcinoma of any histological subtype showed only 3–4% absolute improvement in overall survival. Thus, it is not recommended for resectable oesophageal SCC or adenocarcinoma. The combination of radiotherapy and surgery on early-stage tumours may be an overkill or over-treatment as they are both loco-regional treatments. The dose to normal tissues particularly the spinal cord and lung should be kept within internationally accepted dose limits (40–50 Gy in 20–30 daily fractions) which is lower than doses for definitive chemoradiotherapy as primary treatment. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) [2006] trial of perioperative chemotherapy consisting of three cycles of epirubicin, cisplatin and infusional FU (ECF) before and after surgery produced impressive results in GOJ oesophageal and stomach adenocarcinoma, and the regime is currently recommended for lower third and junctional adenocarcinoma in Europe and USA (85). An international phase 3 randomized clinical trial using monoclonal anti human epidermal growth factor receptor (HER2 antibody), trastuzumab, was found to prolong survival in adenocarcinomas of stomach and GOJ that expressed HER2 (7,86).
Conclusions
Various combined-modality approaches have been attempted to improve outcome of surgical resection of oesophageal cancer. Selection of patients for the appropriate combination treatment is important to avoid the serious adverse events in over treatment with cytotoxic chemo- and radiotherapy. Patients with complete pathological response to surgery or chemo-radiation alone may be considered to be managed expectantly. The future may lie on biological/genomic markers that will determine the prognosis of each oesophageal cancer, and render the optimum tailored treatment modality for this aggressive disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-142
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-142). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B. Biological research in the evolution of cancer surgery: a personal perspective. Cancer Res 2008;68:10007-20. [Crossref] [PubMed]
- Allum WH, Griffin SM, Watson A, et al. Guidelines for the management of oesophageal and gastric cancer. Gut 2002;50:v1-23. [Crossref] [PubMed]
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9. [Crossref] [PubMed]
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012;23:3155-62. [Crossref] [PubMed]
- Kuwano H, Kato H, Miyazaki T, et al. Genetic alterations in esophageal cancer. Surg Today 2005;35:7-18. [Crossref] [PubMed]
- Bartley AN, Christ J, Fitzgibbons PL, et al. Template for reporting results of HER2 (ERBB2) biomarker testing of specimens from patients with adenocarcinoma of the stomach or esophagogastric junction. Arch Pathol Lab Med 2015;139:618-20. [Crossref] [PubMed]
- Law S, Wong J. Current management of esophageal cancer. J Gastrointest Surg 2005;9:291-310. [Crossref] [PubMed]
- Wright CD. Esophageal cancer surgery in 2005. Minerva Chir 2005;60:431-44. [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Steele RJC. The influence of surgeon case volume on outcome in site-specific cancer surgery. Eur J Surg Oncol 1996;22:211-3. [Crossref] [PubMed]
- Cuschieri A. Endoscopic subtotal oesophagectomy for cancer using the right thoracoscopic approach. Surg Oncol 1993;2:3-11. [Crossref] [PubMed]
- Biere SS, van Berge Hene Gouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomized controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC. editors. AJCC Cancer staging manual. 7th ed. New York: Springer, 2010.
- Jain S, Dhingra S. Pathology of esophageal cancer and Barrett’s oesophagus. Ann Cardiothorac Surg 2017;6:99-109. [Crossref] [PubMed]
- Wayman J, O’ Nanlon D, Hayes N, et al. Fibrinogen levels correlate with stage of disease in patients with oesophageal cancer. Br J Surg 1997;84:185-8. [PubMed]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009;9:418-28. [Crossref] [PubMed]
- Weledji EP, Verla V. Failure to rescue patients from early critical complications of oesophagogastric cancer surgery. Ann Med Surg (Lond) 2016;7:34-41. [Crossref] [PubMed]
- D'Journo XB, Michelet P, Marin V, et al. An early inflammatory response to oesophagectomy predicts the occurrence of pulmonary complications. Eur J Cardiothorac Surg 2010;37:1144-51. [Crossref] [PubMed]
- Patel N, Powell AG, Wheat JR, et al. Cardiopulmonary fitness predicts post-operative major morbidity after oesophagectomy for patients with cancer. Physiol Rep 2019;7:e14174 [Crossref] [PubMed]
- Mariette C, Alves A, Beroist S, et al. Perioperative care in in digestive surgery. J Chir (Paris) 2005;142:14-28. [Crossref] [PubMed]
- Luyer MD, Greve JW, Hadfoune M, et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med 2005;202:1023-9. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Immediate postoperative oral nutrition following oesophagectomy: a multicentre clinical trial. Ann Thorac Surg 2016;102:1141-8. [Crossref] [PubMed]
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg 1976;63:259-62. [Crossref] [PubMed]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [Crossref] [PubMed]
- Barreto JC, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World J Gastroenterol 2010;16:3804-10. [Crossref] [PubMed]
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Ancona E, Pianalto S, Merigliano S, et al. Free jejunal transfer for the reconstruction of pharyngoesophagus. Dis Esoph 1995;8:40-3.
- Rentz J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg 2003;125:1114-20. [Crossref] [PubMed]
- Visbal AL, Allen MS, Miller DL, et al. Ivor Lewis esophagogastrectomy for esophageal cancer. Ann Thorac Surg 2001;71:1803-8. [Crossref] [PubMed]
- Forshaw MJ, Gossage JA, Ockrim J, et al. Left thoracoabdominal esophagogastrectomy: still a valid operation for carcinoma of the distal esophagus and esophagogastric junction. Dis Esophagus 2006;19:340-5. [Crossref] [PubMed]
- Kurokawa Y, Sasako M, Sano T, et al. Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg 2015;102:341-8. [Crossref] [PubMed]
- Gillies RS, Simpkin A, Sgromo B, et al. Left thoracoabdominal esophagectomy: Results from a single specialist centre. Dis Esophagus 2011;24:138-44. [Crossref] [PubMed]
- Davies AR, Zylstra J, Baker CR, et al. A comparison of the left thoracoabdominal and Ivor-Lewis esophagectomy. Dis Esophagus 2018;31: [Crossref] [PubMed]
- Bakshi A, Sugarbaker DJ, Burt BM. Alternative conduits for oesophageal replacement. Ann Cardiothorac Surg 2017;6:137-43. [Crossref] [PubMed]
- Peters JH, Kronson JW, Katz M, et al. Arterial anatomic considerations in colon interposition for esophageal replacement. Arch Surg 1995;130:858-62; discussion 862-3. [Crossref] [PubMed]
- Bonavina L, Chirica M, Skrobic O, et al. Foregut caustic injuries: results of the world society of emergency surgery consensus conference. World J Emerg Surg 2015;10:44. [Crossref] [PubMed]
- Horvath OP, Abedini N, Papp A, et al. Free jejunal flap esophagoplasty for ischemic colon conduit replacement. Ann Esophagus 2019;2:15. [Crossref]
- Bonavina L. Reoperative surgery for colon conduit failure: a major challenge in esophageal reconstruction. Ann Esophagus 2020;3:2. [Crossref]
- Skinner DB. En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg 1983;85:59-71. [Crossref] [PubMed]
- Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann Surg 1986;203:173-6. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Watonabe G, et al. Development of surgery for carcinoma of the oesophagus. Am J Surg 1984;147:9-16. [Crossref] [PubMed]
- Tam PC, Siu KF, Cheung HC, et al. Local recurrences after subtotal oesophagectomy for squamous cell carcinoma. Ann Surg 1987;205:189-94. [Crossref] [PubMed]
- Hill S, Cahill J, Wastell C. The right approach to carcinoma of the cardia, preliminary results. Eur J Surg Oncol 1992;18:282-6. [PubMed]
- Giuli R. Surgery for squamous carcinoma of the oesophagus- an overview. In: Jamieson GG. editor. Surgery of the oesophagus. Edinburgh: Churchil Livingstone, 1988:585-95.
- Wong J. Esophageal resection for cancer: the rationale of cuurent practice. Am J Surg 1987;153:18-24. [Crossref] [PubMed]
- Mandard AM, Chasle J, Marney J, et al. Autopsy findings in 111 cases of oesophageal cancer. Cancer 1981;48:329-35. [Crossref] [PubMed]
- Sons HU, Borchard F. Cancer of the distal oesophagus and cardia. Incidence, tumourous infiltration and metastatic spread. Ann Surg 1986;203:188-95. [Crossref] [PubMed]
- Karstens KF, Izbicki JR, Reeh M. Does the margin matter in esophageal cancer? Dig Surg 2018;35:196-203. [Crossref] [PubMed]
- Sujendran V, Wheeler J, Baron R, et al. Effect of neoadjuvant chemotherapy on circumferential margin positivity and its impact on prognosis in patients with resectable oesophageal cancer. Br J Surg 2008;95:191-4. [Crossref] [PubMed]
- Goldminc M, Maddern G, Le Prise E, et al. Oesophagectomy by a transhiatal approach or thoracotomy: a prospective randomized study Br J Surg 1993;80:367-70. [Crossref] [PubMed]
- Kato H, Watanabe H, Tachimore Y, et al. Evaluation of neck lymph dissection for thoracic carcinoma. Ann Thorac Surg 1991;51:931-5. [Crossref] [PubMed]
- Hagen JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993;106:850-8; discussion 858-9. [Crossref] [PubMed]
- Haagensen DC. The lymphatics in cancer. Philadelphia: WB Saunders, 1972:245-9.
- Sato T, Sacamoto K. Illustrations and photographs of surgical oesophageal anatomy, specially prepared for lymph node dissection, In Color atlas of surgical anatomy for oesophageal cancer. Tokyo and Berlin: Springer-Verlag, 1992:25-90.
- Tanabe G, Baba M, Kuroshima K, et al. Clinical evaluation of the esophageal lymph flow system based on RI uptake of dissected regional lymph nodes following lymphoscintigraphy. Nihon Geka Gakkai Zasshi 1986;87:315-23. [PubMed]
- Stein HJ, Bjorn MF, Bruecher LDM, et al. Early esophageal cancer: Pattern of lymphatic spread and prognostic features for long-term survival after surgical resection. Ann Surg 2005;242:566-73, discussion 573-5. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Siewert JR, Hoelscher AH, Adolf J, et al. Oesophageal carcinoma en bloc oesophagectomy with mediastinal lymphadenectomy in oesophageal reconstruction with delayed urgency. In: Siewert JR, Hoelscher AH. editors. Disease of the oesophagus. Berlin: Springer-Verlag, 1987:427-32.
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomized controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Ronellenfitsch U, Scwarzbach M, Hofheinx R. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data Eur J cancer 2013;49:3149-58. [Crossref] [PubMed]
- Wu TT, Chirieac LR, Abraham SC, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol 2007;31:58-64. [Crossref] [PubMed]
- Donohoe CL, O'Farrell NJ, Grant T, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg 2013;258:784-92; discussion 792. [Crossref] [PubMed]
- Karamitopoulou E, Thies S, Zlobec I, et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am J Surg Pathol 2014;38:1551-6. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines). Esophageal and Esophagogastric junction Cancers. Version 1.2014. Available online: https://www.nccn.org/patients/
- Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut 2004;53:925-30. [Crossref] [PubMed]
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2003;185:538-43. [Crossref] [PubMed]
- Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery 2005;137:172-7. [Crossref] [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [Crossref] [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [Crossref] [PubMed]
- Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg 2001;88:338-56. [Crossref] [PubMed]
- Malthaner RA, Wong RK, Rumble RB, et al. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med 2004;2:35. [Crossref] [PubMed]
- Kaklamanos IG, Walker GR, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol 2003;10:754-61. [Crossref] [PubMed]
- Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2002;183:274-9. [Crossref] [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [Crossref] [PubMed]
- van Meerten E, Muller K, Tilanus HW, et al. Neoadjuvant concurrent chemoradiation with weekly paclitaxel and carboplatin for patients with oesophageal cancer: a phase II study. Br J Cancer 2006;94:1389-94. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the oesophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Hironaka S, Ohtsu A, Boku N, et al. Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T(2–3)N(any)M(0) squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2003;57:425-33. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer. Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev 2005;CD001799 [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
Cite this article as: Weledji EP. How multimodal treatment improves surgery for oesophageal cancer—a narrative review. Dig Med Res 2021;4:10.