
Thermal ablation of intrahepatic cholangiocarcinoma: a narrative review
Introduction
Intrahepatic cholangiocarcinoma (ICC) represents approximately 5–20% of liver malignancies and carries a poor 5-year overall survival rate of 5% (1). While many cases are sporadic, known risk factors include primary sclerosing cholangitis, metabolic syndrome, alcohol abuse, viral hepatitis, and cirrhosis (1). Because ICC may grow unrecognized until lesions are large enough to cause obstructing jaundice, many patients are diagnosed at a late stage and often with high-grade disease. Of those patients with more limited disease, many present with underlying hepatic dysfunction such as cirrhosis, and will not be candidates for major surgical resection (2). Ultimately, only approximately one third of patients with ICC will be candidates for resection at presentation (1,3,4). Furthermore, even in patients who are able to undergo liver resection, recurrence rates may be as high as 70% (5,6). Repeated resection after postoperative recurrence is often limited, due to the size of the liver remnant or presence of multifocal recurrence (7).
Systemic therapy offers modest benefit to patients with ICC, with median progression-free survival of 8 months and an overall survival of 11.7 months (8). Unfortunately, tolerance to chemotherapy remains a problem, with grade III/IV toxicities occurring in up to 70% of patients (8). Given all of these limitations, liver-directed therapies such as thermal ablation may play an important role in the treatment of patients with both primary and recurrent ICC.
Thermal ablation is a well-established method for the treatment of a variety of primary and metastatic tumors. It provides high rates of local tumor control and may be curative in several early-stage tumors such as lung, hepatocellular, and renal cell carcinoma (9-12). It can be performed minimally invasively, with high technical success and low rates of complications, even in patients unfit for surgical resection. Given the high rates of morbidity and mortality due to liver progression in patients with ICC, thermal ablation may serve a particularly important function in limiting intra-hepatic tumor progression and preventing liver failure (7). We present the following article in accordance with the narrative review checklist (available at http://dx.doi.org/10.21037/dmr-21-15).
Most previous reports of thermal ablation for ICC have utilized either radiofrequency (RF) or microwave (MW) technology. These technologies are similar, in that both utilize extreme temperatures in excess of 60 degrees centigrade to induce coagulative necrosis and cell death. However, these technologies differ in their method of energy delivery and tissue heating, which results in several key clinical distinctions. In RF ablation, a probe is placed within a volume of tissue and is used to generate a rapid alternating electrical current. As the current travels through the tissue, local frictional agitation generates heat around the RF probe, resulting in a rise in tissue temperature (13). The heat then propagates through thermal conduction to cause a well-defined area of tissue destruction. Because RF relies on both electrical and thermal conduction to cause tissue necrosis, variations in tissue characteristics, such as tissue water content and blood flow, can greatly impact RF ablation zones (14,15). Similarly, because of the relatively lower temperatures achieved with RF, adjacent blood vessels can cause local cooling effects that limit thermal conduction and lead to less predictable and effective ablation zones (16).
In contradistinction to RF, MW spectrum energy causes local tissue heating by means of frictional energy generated from water molecule oscillation. As a result, MW ablation does not rely on electrical conductivity and is therefore much less susceptible to local tissue characteristics. Furthermore, MW relies primarily on active tissue heating, rather than tissue conduction, to produce cellular death. This can allow for more uniform heating, higher temperatures, and larger ablation zones (17,18). Additionally, unlike RF, where multiple applicators may cause electrical interference, multiple MW probes may be combined to produce larger, confluent ablation zones (19-21). Although these factors may facilitate improvement of ablation zones, they potentially increase the risk of the procedure. Due to the increased size and temperature of the ablations performed with MW, there is a potential for increased risk of non-target ablation injuries to adjacent structures and subsequent complications.
Thermal ablation of ICC
RF ablation for ICC was first described in 2002 (6). To date, published literature consists primarily of small, retrospective studies. Table 1 summarizes fifteen case series of thermal ablation in ICC between 2005 and 2020. Several general observations can be made regarding the published data. Firstly, due to the rarity of this malignancy, most of the reports consist of relatively small populations of patients. Additionally, the majority of the reports are comprised of a mixed population of both primary ICC and recurrent ICC following prior resection, with two of the studies reporting outcomes exclusively in patients with recurrent ICC (24,27). Although this complicates the interpretation of the outcomes reported, studying recurrent cholangiocarcinoma is particularly important, as many patients will experience intraparenchymal recurrence following surgery (22,24). Finally, treatment modalities, parameters, and outcome measurements vary between studies, making comparisons between each study difficult.
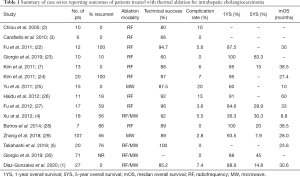
Full table
Outcomes following ablation
Most of the literature confirms the high technical success of ablation for the treatment of ICC. Assessment of technical success is typically performed by imaging evaluation with either ultrasound or CT scan at 1 month following ablation. Commonly, the ablation is deemed successful if there is complete necrosis within the treated tumor without evidence of tumoral or peri-tumoral enhancement (2). Among the case series, technical success was ≥80% in 12 series and ≥90% in 6 series.
Without treatment, median survival for ICC is 3.9 months (4). An estimate of median overall survival of among patients receiving non-surgical palliative treatments, excluding ablation, such as chemotherapy, radiation, and transarterial chemoembolization is 12.9 months (5). Twelve of the studies of thermal ablation for ICC report survival data. The 1-year overall survival in these reports range from 36–100%, with 10 of the studies reporting a >85% 1-year survival. Five-year survival was reported in 8 of the studies and ranged from 8–83%, with 6 of the studies reporting 5-year survival of <31%. The median overall survival after thermal ablation of ICC among the 11 studies reporting was 30 months, with a range 8.8–60 months. These data suggest that there can be a meaningful prolongation of survival for select patients with ICC with the addition of thermal ablation.
Complications
Complication rates following thermal ablation of ICC occur in a minority of patients, with a reported range of 5–10%. Most risks and complications of thermal ablation for ICC are similar to those of thermal ablation for other liver tumors. These included hemorrhage, infection, vascular injury or thrombosis, and non-target ablation injury to adjacent organs or structures. Commonly, patients may also experience mild symptoms of fatigue, myalgias, pain, and low-grade fever, which are generally referred to as “post-ablation syndrome.” This is typically self-limiting and treated with analgesics and anti-inflammatory medications. However, several rare complications require special attention when discussing thermal ablation of ICC. Due to the intimate relationship between the tumor and adjacent bile ducts, biliary stricture, biloma, and bile leak may occur following thermal ablation. Special care must be taken to ensure the ablation zone does not encompass any major central biliary structures. Additionally, while infections are a potential risk of any thermal ablation, patients with ICC are potentially at increased risk of infectious complications. Given the higher rate of biliary obstruction in this population, many patients will have undergone prior biliary interventions such as ERCP or biliary stenting. These procedures allow bacterial colonization of the biliary tree and are known to increase the risk of hepatic infection and abscess formation (31). Therefore, any patients with this history require extended prophylactic antibiotics to mitigate this complication.
Special considerations
Tumor size must be considered when planning thermal ablation of ICC. Historically, based primarily on data from hepatocellular carcinoma, ablation has generally been limited to patients with tumors ≤3 cm. While technical advances have improved the ability to treat larger tumors, most series of thermal ablation for ICC described diminishing technical success with larger tumors (7,20,23,24). For example, Chiou et al. reported 100% efficacy for tumors <3 cm, with decreasing efficacy in tumors 3–5 cm (2). However, it should be noted that the majority of the studies analyzed the use of RF ablation, which is known to be limited in its ability to create large ablation zones. Therefore, it is possible that the use of MW ablation, and particularly multi-probe ablation, may improve upon these results (5). Two previous studies attempted to directly compared RF to MW ablation (5,30). Giorgio et al. performed a multicenter retrospective review of 71 patients with 98 primary and recurrent ICC lesions. They demonstrated improved disease-free and overall survival in patients treated with MW ablation compared to RF ablation. Additionally, this benefit persisted in patients with tumors up to 4 cm in size (30). In contrast, Takahashi et al. found no difference between RF and MW ablation in a study of 20 patients with 50 ICC lesions. However, the mean size of lesions in this study was 1.8 cm and 88% of patients were treated with RF, which may limit the study’s ability to identify a difference between the modalities (5).
Several of the unique features of ICC may present challenges for thermal ablation. Compared with metastatic disease and primary hepatocellular carcinoma, ICC frequently has ill-defined borders and commonly demonstrates infiltrative growth on imaging. Pathologically this correlates with irregular or “rolled” tumor borders and frequently peritumoral satellites (27). These features may lessen the ability to achieve an adequate ablation that encompasses all tumor, and may increase the risk of post-ablation recurrence (4). Due to the infiltrative nature of ICC, it has been proposed that a larger ablation zone may be necessary to ensure complete tumor eradication, and an ablation margin of ≥1 cm may be needed (27). Furthermore, the approximation of the tumor to other intraperitoneal structures, such as the colon and duodenum, can further add complexity to the treatment. Previous reports have shown that, compared to tumors deep within the parenchyma, superficial ICC lesions are associated with earlier local tumor progression after ablation. This is likely due to the inability to safely achieve an adequate ablation margin (5). While these features may increase the technical challenges of performing ablation of ICC, several methods may be used to mitigate them. As stated previously, multi-probe MW ablation can achieve large ablation zones in a single session, often in excess of 5 cm, and can help ensure an adequate margin. Furthermore, for very large tumors, pre-ablative arterial embolization can work synergistically with ablation to treat the tumor, decrease heat dispersion, and help increase the volume of ablation (3). Finally, for tumors near the liver surface, numerous techniques may be employed to help minimize the risk of adjacent organ injury. These include air or hydro-dissection, balloon protection, and open or laparoscopic assistance.
Despite the high initial technical success of thermal ablation for ICC, metastatic tumor recurrence remains common, with a median time to recurrence of 10.1 months (1). Increased risk of recurrence may be due to several factors, though the evaluation of these factors is limited by the retrospective nature of the available data. Predictors of poor outcome include lymphovascular invasion, periductal infiltrating disease, elevated CA 19-9, lymph node metastasis, and poorly differentiated histology (27). Therefore, these factors should be evaluated in all patients being considered for ablative therapy.
Given the underlying liver dysfunction in many patients with ICC, there should be careful consideration of hepatic reserve when evaluating potential candidates for treatment. Based on the extensive available literature in hepatocellular carcinoma, most practitioners consider patients with mild to moderate liver dysfunction to be acceptable candidates for thermal ablation. Furthermore, Diaz-Gonzalez et al. examined ablation of ICC exclusively among patients with cirrhosis and found no specific safety concerns (1).
Finally, there remains controversy regarding which patients with recurrent cholangiocarcinoma may be best served by repeat resection versus ablation. Repeat hepatectomy can be technically challenging, but median survival after repeat hepatectomy has been reported to be as high as 20 months (32). To answer this question, Zhang et al. retrospectively compared 109 patients with recurrent ICC who had undergone either repeated hepatic resection or thermal ablation. They found no difference in overall survival among those patients with tumors less than 3 cm. Additionally, major complications were significantly higher in patients who underwent repeat hepatic resection (33).
Conclusions
ICC is a challenging cancer with poor prognosis. Many patients are ineligible for curative intent surgical resection, yet most patients who go without any treatment will not survive beyond 6 months. Thermal ablation is an effective treatment for select patients with limited primary or recurrent disease. Additionally, thermal ablation has low rates of complications and may be performed safely in patients who are poor candidates for other treatments. Patients with limited, small, and well-circumscribed tumors likely benefit most from this treatment. Broader adoption of contemporary MW ablation devices may further improve outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Yi-Jen Chen) for the series “Locoregional and systemic treatment in intrahepatic cholangiocarcinoma” published in Digestive Medicine Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the narrative review checklist. Available at http://dx.doi.org/10.21037/dmr-21-15
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-21-15). The series “Locoregional and systemic treatment in intrahepatic cholangiocarcinoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Diaz-Gonzalez A, Vilana R, Bianchi L, et al. Thermal Ablation for Intrahepatic Cholangiocarcinoma in Cirrhosis: Safety and Efficacy in Non-Surgical Patients. J Vasc Interv Radiol 2020;31:710-9. [Crossref] [PubMed]
- Chiou YY, Hwang JI, Chou YH, et al. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci 2005;21:304-9. [Crossref] [PubMed]
- Carrafiello G, Lagana D, Cotta E, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol 2010;33:835-9. [Crossref] [PubMed]
- Xu HX, Wang Y, Lu MD, et al. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol 2012;85:1078-84. [Crossref] [PubMed]
- Takahashi EA, Kinsman KA, Schmit GD, et al. Thermal ablation of intrahepatic cholangiocarcinoma: Safety, efficacy, and factors affecting local tumor progression. Abdom Radiol (NY) 2018;43:3487-92. [Crossref] [PubMed]
- Han K, Ko HK, Kim KW, et al. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol 2015;26:943-8. [Crossref] [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9 [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159-66. [Crossref] [PubMed]
- Healey TT, Dupuy DE. Radiofrequency ablation: a safe and effective treatment in nonoperative patients with early-stage lung cancer. Cancer J 2011;17:33-7. [Crossref] [PubMed]
- Psutka SP, Feldman AS, McDougal WS, et al. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 2013;63:486-92. [Crossref] [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [Crossref] [PubMed]
- Ahmed M, Brace CL, Lee FT Jr, et al. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-69. [Crossref] [PubMed]
- Bhardwaj N, Strickland AD, Ahmad F, et al. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology 2009;41:168-72. [Crossref] [PubMed]
- Lu DS, Raman SS, Vodopich DJ, et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol 2002;178:47-51. [Crossref] [PubMed]
- Eckmann MS, Martinez MA, Lindauer S, et al. Radiofrequency ablation near the bone-muscle interface alters soft tissue lesion dimensions. Reg Anesth Pain Med 2015;40:270-5. [Crossref] [PubMed]
- Aubry S, Dubut J, Nueffer JP, et al. Prospective 1-year follow-up pilot study of CT-guided microwave ablation in the treatment of bone and soft-tissue malignant tumours. Eur Radiol 2017;27:1477-85. [Crossref] [PubMed]
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135-43. [Crossref] [PubMed]
- Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol 2013;36:505-11. [Crossref] [PubMed]
- Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol 2008;19:1087-92. [Crossref] [PubMed]
- Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol 2014;83:552-8. [Crossref] [PubMed]
- Fu Y, Yang W, Wu W, et al. Radiofrequency ablation for postoperative recurrences of intrahepatic cholangiocarcinoma. Chin J Cancer Res 2011;23:295-300. [Crossref] [PubMed]
- Giorgio A, Calisti G, DE, Stefano G, et al. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single centre experience. Anticancer Res 2011;31:4575-80. [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol 2011;80:e221-5. [Crossref] [PubMed]
- Yu MA, Liang P, Yu XL, et al. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol 2011;80:548-52. [Crossref] [PubMed]
- Haidu M, Dobrozemsky G, Schullian P, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol 2012;35:1074-82. [Crossref] [PubMed]
- Fu Y, Yang W, Wu W, et al. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol 2012;23:642-9. [Crossref] [PubMed]
- Butros SR, Shenoy-Bhangle A, Mueller PR, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcome. Clin Imaging 2014;38:490-4. [Crossref] [PubMed]
- Zhang K, Yu J, Yu X, et al. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma. Int J Hyperthermia 2018;34:292-7. [Crossref] [PubMed]
- Giorgio A, Gatti P, Montesarchio L, et al. Intrahepatic Cholangiocarcinoma and Thermal Ablation: Long-term Results of An Italian Retrospective Multicenter Study. J Clin Transl Hepatol 2019;7:287-92. [Crossref] [PubMed]
- Shibata T, Yamamoto Y, Yamamoto N, et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: incidence and risk factors. J Vasc Interv Radiol 2003;14:1535-42. [Crossref] [PubMed]
- Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery 2008;143:366-74. [Crossref] [PubMed]
- Zhang SJ, Hu P, Wang N, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:3596-602. [Crossref] [PubMed]
Cite this article as: Thornblade LW, Kessler J. Thermal ablation of intrahepatic cholangiocarcinoma: a narrative review. Dig Med Res 2021;4:11.


