
Stage III colon cancer: is neoadjuvant chemotherapy ready for prime time?—A narrative review of neoadjuvant chemotherapy for colon cancer
Introduction
Stage three colon cancer remains a disease with a high risk of relapse despite current approaches to adjuvant therapy. In the most recent 2010–2016 Surveillance, Epidemiology and End Results (SEER) database cohort, patients diagnosed with regional spread of colorectal cancer to lymph nodes had a relative survival of 71.8% compared to healthy peers (1). Even in fit clinical trial populations, 5-year overall survival (OS) with combination adjuvant therapy has been measured at 73–84%, varying by trial (2,3). Five-year disease-free survival (DFS) in the same trials was 66–70%. This high relapse rate represents significant opportunity for improvement.
Worldwide, diagnosis of new cases of colorectal cancer reached more than 1.8 million cases in 2018, representing 10.2% of all cancer diagnoses (4). An incremental improvement in relapse rates would therefore result in a large number of lives saved and a reduction in costs associated with treatment of recurrent disease.
For many cancers, neoadjuvant systemic therapy (systemic therapy given before surgery) has been shown to improve outcomes compared with upfront surgery and later adjuvant chemotherapy. Neoadjuvant chemotherapy is widely used for gastric, oesophageal, bladder and breast cancers (5-8). In colon cancer, a small number of randomised clinical trials have shown promising results of neoadjuvant therapy (9,10). This paper will discuss the current treatment landscape for stage three colon cancer, the rationale and emerging evidence for neoadjuvant therapy in this disease and the ongoing trials which seek to provide more data on which to base clinical decisions. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-159).
Methods
This narrative review was based on a literature search of English language articles and abstracts included in the PubMed, EMBASE and Medline databases. Given the broad scope of the article and inclusion of current standard of care and evidence in other tumour types, a broad range of search terms were used. Regarding published data for neoadjuvant therapy in colon cancer, the search terms “neoadjuvant”, “preoperative”, “perioperative”, “chemotherapy”, “colon cancer”, “colorectal cancer”, were used. Articles were excluded if solely patients with rectal cancer were included. Due to a relatively limited amount of data in this area, trials and observational data were considered for inclusion based on relevance to the topic and included if they specifically addressed neoadjuvant chemotherapy for colon cancer, regardless of phase or study design. For currently running clinical trials, the clinicaltrials.gov database was searched using the keyword “neoadjuvant” with the filter “Colon Cancer”.
Current standard of care for stage III colon cancer
The current standard of care for stage III colon cancer is immediate resection followed by adjuvant chemotherapy. Adjuvant chemotherapy with oxaliplatin and fluoropyrimidine is the current standard of care for resected stage III colon cancer. Initially, several trials established the benefit of 5-FU and folinic acid in an adjuvant setting, the largest of these trials showing an 18% improvement in 3-year DFS [see Table 1 (11-14)]. The benefit of adding oxaliplatin was first suggested with the MOSAIC trial, showing significantly improved 5-year DFS with the addition of oxaliplatin to 5-FU infusion alone (HR 0.80; 95% CI, 0.68 to 0.93; P=0.003) (15). This was further confirmed in the NSABP C-07 trial which assessed bolus weekly 5-FU and oxaliplatin, although toxicity was more prominent than seen in the infusional 5FU regimen used in MOSAIC (16). Similarly, the XELOXA trial found that combination adjuvant therapy with capecitabine and oxaliplatin was superior to 5FU alone (17). 5FU and capecitabine have shown similar efficacy as single agents, although there has been no direct comparison in oxaliplatin based regimens in the adjuvant setting (19).
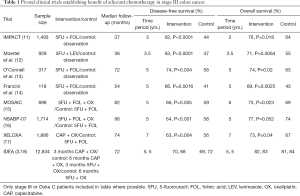
Full table
The IDEA collaboration compared three and six months of adjuvant therapy with combination fluoropyrimidine and oxaliplatin (3,18). Overall, non-inferiority of three months of therapy, as compared to six months, was not established. However, in low-risk patients (T1-3N1) when capecitabine was used with oxaliplatin, three months was non-inferior to six months. When 5FU was used or in high risk patients, six months was found to be superior to three months (18). In those patients where oxaliplatin is contraindicated, single agent fluoropyrimidine chemotherapy may be used, but there is evidence that tumours deficient in mismatch repair (MMR) are resistant to this regimen (20).
Regarding elderly patients, a pooled analysis of 3,351 trial patients including data from 506 patients over 70 concluded that adjuvant chemotherapy was equally beneficial for the older patients (21). This analysis was undertaken prior to the publication of trials regarding use of oxaliplatin in the adjuvant setting. There is conflicting evidence regarding oxaliplatin-based regimens in patients over the age of 70, with some trials showing reduced or no benefit in this age group (16,22-24). It should be noted that the proportion of patients aged over 70 included in these trials is relatively low and that analyses were post-hoc subgroup analyses, not specifically designed to answer the question of benefit in the elderly, therefore caution should be applied when extrapolating from this data.
The scientific rationale for neoadjuvant therapy
Whilst adjuvant chemotherapy is currently the standard of care for Stage III colon cancers, mounting evidence from other tumour types, including breast, bladder, gastric, gastro-oesophageal and rectal cancers suggests a neoadjuvant approach is more effective (7,8,25-37) (see Table 2). The benefits of neoadjuvant chemotherapy relate to its ability to induce tumour regression and downstaging (38), treatment of micrometastatic disease and improved adherence with and higher completion rates of systemic treatment.
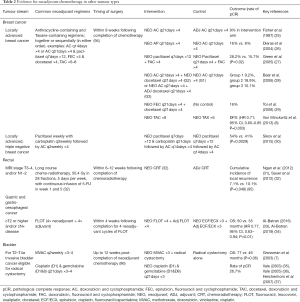
Full table
In all tumour types this field continues to evolve. Due to short median follow-up to date, for many trials the reported endpoints focus on the rate of pathological complete response (pCR). Although meaningful differences in DFS or OS may be yet to be realised, there is observational evidence that in those who achieve pCR, there are higher rates of DFS and OS (26,28).
Differences in biology between early stage colon cancer compared with metastatic disease, and the implications for design of neoadjuvant therapies
The biology of colon cancer is different between early colorectal cancer and metastatic colorectal cancer. This is observed pathologically in the difference in prevalence of MMR deficiency between different stages of colorectal cancer, and clinically in a number of adjuvant studies which demonstrate that active agents utilised in the metastatic setting may not be effective in the adjuvant setting.
MMR deficiency is found in patients with Lynch syndrome and in up to twenty percent of patients with sporadic colon cancer. The prevalence of deficient MMR is higher for early colorectal cancer: 21% in stage II and 14% in stage III (39) compared to an estimated 3.5% in metastatic colorectal cancer (40). MMR deficiency is closely correlated with microsatellite instability (MSI), and carries a more favourable prognosis in early disease, but also predicts poor response to fluoropyrimidine therapy (41). Right sided tumours are associated with higher rates of MSI (42). A retrospective study has shown that adjuvant chemotherapy with oxaliplatin and fluoropyrimidine improves disease free survival in patients with stage III deficient MMR colorectal cancer, which suggests chemosensitivity to oxaliplatin (43).
A number of adjuvant studies have shown disappointing results where active agents that are efficacious in metastatic colorectal cancer have not been shown to be beneficial in the adjuvant setting. These include irinotecan, bevacizumab and cetuximab. Several trials including the phase III randomised controlled study conducted by the Hellenic Cooperative Oncology Study Group revealed that irinotecan (a topoisomerase 1 inhibitor) in addition to 5-fluororuracil in the adjuvant setting for stage II and III colorectal cancer did not improve disease free survival or OS but was associated with increased toxicities (44). In an open label randomised phase III trial, QUASAR 2, bevacizumab (a monoclonal antibody targeting the vascular endothelial growth factor) which is effective in combination with chemotherapy in metastatic colorectal cancer, was not shown to improve outcome in stage II and III colorectal cancer when combined with capecitabine (45). Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, an agent effective in RAS wild type metastatic colon cancer in combination with other chemotherapy has not been shown to be effective when used in combination with FOLFOX in resected stage III RAS and BRAF wild type colon cancer in the European PETACC8 trial (46).
Carefully selecting agents is therefore important in the neoadjuvant setting for early disease. Due to the higher prevalence of deficient MMR tumours in the early setting, it is essential to test for this and take it into account when devising neoadjuvant treatments. Currently, surveillance as opposed to adjuvant chemotherapy is suggested in patients with high risk stage II deficient MMR colorectal cancer. For patients with high risk stage II colorectal cancer with deficient MMR, proceeding with surgery instead of utilising neoadjuvant chemotherapy will likely remain the standard of care. Furthermore, given that combination of oxaliplatin with fluoropyrimidine improved disease free survival compared to single agent fluoropyrimidine in the adjuvant setting for stage III colorectal cancer with deficient MMR; if neoadjuvant therapy was considered in this group, an oxaliplatin based regimen would be strongly recommended. Agents that have not been shown to be effective in the adjuvant setting such as irinotecan, bevacizumab and cetuximab may also be less likely to be effective in the neoadjuvant setting for early stage colorectal cancer, and if included in trials should be carefully stratified for so that their effect can be accurately assessed.
Difference between approach in colon and rectal cancer and how this came about
Surgery is the primary treatment for early stages of both colon and rectal cancer. However, unlike early stage colon cancer, management of early stage rectal cancer involves consideration of concurrent neoadjuvant chemotherapy and radiotherapy in clinical T3/T4 tumours and/or in tumours with node involvement (47). Following resection, adjuvant chemotherapy may be considered in patients with locally advanced rectal cancer (T3/T4 or node positive) unless they have received four months of neoadjuvant chemotherapy prior to surgery (48). Neoadjuvant chemoradiotherapy for locally advanced rectal cancer became standard practice following a phase III randomised controlled trial conducted by the German Rectal Study group comparing administration of concurrent chemoradiotherapy preoperatively versus during the postoperative period for locally advanced rectal cancer (49). The study revealed a lower local recurrence rate, as well as downstaging of the tumour and enabling of sphincter preservation surgery in the preoperative treatment group.
There are technical reasons that neoadjuvant chemoradiation is difficult to use in colon cancer, related to bowel mobility and therefore toxicity to overlying small bowel. Chemotherapy given alone was previously viewed with caution due to the limited response rate. These two factors delayed uptake of neoadjuvant therapy for colon cancer prior to the advent of combination chemotherapy regimens. Oxaliplatin-based chemotherapy has been shown to have a significantly increased overall response rate (50.7% vs. 22.3% in the original first-line metastatic trial) compared with 5-FU/leucovorin alone (50). This has allowed the development of combination neoadjuvant therapy regimens which have adequate disease control rates that progression is now less of a concern in the neoadjuvant setting. We will discuss the emerging data that neoadjuvant chemotherapy for colon cancer is a promising novel approach to management of this disease.
Radiologic staging of colon cancer
Identification of patients for enrolment in neoadjuvant therapy trials relies on radiologic staging of the primary tumour. In practice, the most widely available imaging modality is computed tomography (CT) scanning, which is already standard of care in newly diagnosed colon cancer to detect distant metastatic disease.
CT scanning has several limitations. It is most accurate in diagnosis of T stage. The pilot section of the FOxTROT trial (discussed later) published the accuracy of radiologic staging, finding a sensitivity of 95% and specificity of 50% for T staging (differentiating between T4 or high risk T3 (>5 mm invasion beyond muscularis propria, and lower T stages) (51). The same study found only 68% sensitivity and 42% specificity for detection of nodal metastases. Similar results were seen in a German series of 210 patients, again demonstrating higher sensitivity (94.9%) than specificity (55%) for high risk tumours (T3/4 or N+) (52). Sensitivity for T stage alone was not reported, but accuracy (correct diagnosis) of T 3–4 tumours was 91% while accuracy to diagnose positive lymph nodes was only 74%.
Specific criteria for lymph node assessment have been suggested to optimise correct diagnosis, with a combination of both irregular border, and/or internal heterogeneity being shown to increase sensitivity to 85% and specificity to 75% in a retrospective cohort of 119 patients (53).
It has been hoped that additional staging methods might optimise diagnosis but these have shown small improvements at best, at both a financial and time cost to the patient and the health system. PET scanning, when carefully optimised, showed a sensitivity of 53% but an excellent specificity of 90% for positive lymph nodes (54). A small study using MRI for staging showed moderately higher sensitivity and importantly specificity for T stage when compared with the same patients being assessed by CT (55), similar higher specificity was also seen in another small cohort (56).
It is likely that optimal staging will require a combination of imaging modalities to optimise both sensitivity and specificity, with the role of MRI and PET seeming to be most useful in increased specificity (i.e., reducing over-staging and hence over-treatment). This is an area with room for significant further optimisation and a need for larger clinical trials. Pending further data regarding optimal lymph node staging, trials to date have included patients primarily on the basis of high risk T stage (T3 with >5 mm invasion beyond muscularis propria, or T4) rather than N staging, with the result that a large number of Stage II patients have been included in trials.
Nonrandomised data
Previous concerns regarding the feasibility of neoadjuvant therapy for colon cancer have now been allayed in multiple phase II studies demonstrating the safety of this approach (9,38,57-60). Response rates even with short courses (2 cycles of CAPOX) have been very encouraging [68% radiological response (58), 42–51% of patients converted from “high risk” (qualifying for adjuvant chemotherapy) to low risk (60)]. Longer regimens of 4–6 cycles of 5-fluorouracil/folinic acid/oxaliplatin (FOLFOX) or CAPOX have also been shown to be safe, without evidence of distant progression during the pre-operative period, however without markedly increased response rates than seen with shorter courses (9,38). It is difficult to compare trials as endpoints differ from pathological grading of response, to requirement for adjuvant chemotherapy, to clinical outcomes such as R0 resection and DFS.
Apart from CAPOX and FOLFOX, other oxaliplatin based regimens have been evaluated in this setting. S-1 plus oxaliplatin (SOX) given as neoadjuvant and adjuvant chemotherapy has also been shown to be efficacious in Stage III colon cancer in a small phase II study, demonstrating 10.3% of patients with a complete pathological response, and 41.4% showing tumour regression (61). The additional benefit of this regimen is a low incidence of hand-foot syndrome.
Triplet neoadjuvant chemotherapy with FOLFOXIRI has also been assessed (57), with a trend to improvement in tumour shrinkage rate with each subsequent chemotherapy cycle, with maximal response after the third cycle. Triplet therapy was also associated with higher rates of toxicity, with more than half (56.5%) of patients experiencing grade 3 or 4 toxicities, most commonly cytopenias and gastrointestinal side effects (57). Two of the 23 patients in the FOLFOXIRI study had progressive disease during the pre-operative period, however this study included only cT4N2 tumours, higher risk patients than those included in the doublet chemotherapy trials discussed above. 91% of patients in this trial had a numeric reduction in tumour volume and 20/23 had some degree of downstaging (reduced T or N stage), including 1 pCR.
Neoadjuvant chemotherapy did not delay surgery in any of the studies and no increase in perioperative complications was noted (57,58,60). Neoadjuvant chemotherapy is associated with tumour regression and significantly less tumour positive lymph nodes (38,59). Overall, neoadjuvant chemotherapy appears to demonstrate at least moderate regression of the tumour at surgery, which in some cases results in downstaging and sparing patients of adjuvant therapy (38,58,59).
The encouraging results from these phase II trials have resulted in the instigation of a number of phase III trials designed to detect differences in DFS and OS, which will be discussed in detail below.
Randomised data
There are to date only two completed randomised studies evaluating neoadjuvant chemotherapy in colon cancer.
PRODIGE 22 was a phase II study with a primary endpoint of degree of pathological response. Patients with high risk T3 tumours (≥5 mm extramural invasion), T4 tumours or N2 nodal status (regardless of T stage) were randomised to either neoadjuvant FOLFOX4 ×4 cycles, followed by 8 cycles postoperative chemotherapy, or to standard of care (immediate surgery and adjuvant chemotherapy) (62). RAS-wild type tumours were randomised to receive chemotherapy alone vs. chemotherapy plus cetuximab, however at interim analysis the cetuximab arm was ceased due to lack of benefit. The trial failed to meet its primary endpoint (10% or more of patients with major pathological response), with only 8% of patients achieving this result. However, the intervention arm had lower pathological stage, lower risk pathological factors, and evidence of moderate pathological response at the time of surgery. This study was not designed to show a difference in DFS or OS. Three year follow up showed equivalent OS and DFS between arms (63).
The only large phase III trial addressing neoadjuvant chemotherapy for colon cancer, the FOxTROT trial, recruited patients with at least T3 disease on preoperative staging and randomised them to neoadjuvant chemotherapy +/− panitumumab, followed by adjuvant chemotherapy, or to immediate surgery followed by adjuvant chemotherapy (64).Both radiologic node- negative and node-positive patients were eligible (65). Updated results reported at the American Society of Clinical Oncology (ASCO) 2020 meeting showed a numeric reduction in 2-year failure rate (15.6% vs. 19.5%, RR 0.76, P=0.07) (66). Pathological stage was reduced in the intervention arm (66). pCR occurred in only 4% of patients. The results of the panitumumab arm are not yet published. Exploratory analysis discussed at ASCO 2020 suggested MMR deficient (d-MMR) tumours benefited less from neoadjuvant chemotherapy, with 7% of dMMR and 23% of pMMR tumours demonstrating at least moderate histological tumour regression, and with only pMMR tumours showing a significant reduction in risk of relapse at 2 years (66). Of note, follow up for this trial is ongoing and final results (including long term OS) are not yet available.
Discussion
Currently, there is insufficient evidence to recommend widespread uptake of neoadjuvant chemotherapy for colon cancer, however the results of randomised trials remain immature and further data is awaited. There is only one large phase III trial completed to date (FOxTROT, discussed in previous section) however the final results and formal publication are awaited. We discuss below the evident and the potential barriers to further development of this treatment modality.
The absolute benefit of neoadjuvant therapy in colon cancer
The absolute benefit of neoadjuvant chemotherapy in colon cancer compared to standard of care therapy appears small from the limited data available to date. The FOxTROT trial showed an absolute improvement in 2-year failure rate of 4% which was not statistically significant (10), although it is hoped that outcomes between the treatment and control arm will continue to diverge with ongoing follow-up. However, a major challenge in optimising the benefit from this treatment modality will be optimal patient selection.
In the FOxTROT trial, 48% of participants in the control arm were node-negative at surgery, and it is likely that a similarly high proportion of the intervention arm had stage II disease (67). This raises the question of possible over-treatment, as the benefit of adjuvant chemotherapy in stage II disease is uncertain and at most, small (68,69). In the PRODIGE 22 study, 33% of the control group patients were “overstaged”; included on imaging evidence of high risk T3 or positive lymph node status, but found to have low risk stage 2 disease at surgery (70).
We know from data regarding adjuvant chemotherapy that the major survival benefit from adjuvant chemotherapy is seen in stage III disease. The IMPACT-1 pooled analysis of 1,526 patients treated in trials of 5-FU and folinic acid showed a marked survival benefit in Dukes C (analogous to stage III disease) with a hazard ratio of 0.70 (95% CI, 0.53–0.92), but a non-significant 9% relative risk reduction (HR 0.91, 95% CI, 0.63–1.34) in Dukes B disease (analogous to stage II disease) (71). The addition of oxaliplatin was also shown to have negligible benefit in stage II disease (72). In clinical practice, adjuvant chemotherapy for higher risk stage II patients is still considered, given their higher risk of relapse, although the benefit has not been conclusively proven (73).
The inclusion criteria in randomised trials to date have been pragmatic, with inclusion of higher risk stage II as well as stage III patients based on CT staging (9,65). The major reason for this, as discussed earlier, is that CT staging is more accurate for T stage than for N stage and it is therefore difficult to confidently include only patients with stage III disease in a radiologically defined cohort.
It is therefore likely that current staging methods, which have been shown to have low specificity, may not be able to adequately separate out the preoperative patients who would benefit most from the neoadjuvant approach. This in turn means that it is likely the absolute benefit of neoadjuvant therapy will remain small in trial populations including large numbers of stage II patients.
Challenges in implementation of neoadjuvant chemotherapy in colon cancer
Patient selection
The risk of overtreatment should be carefully considered prior to using neoadjuvant therapy in colon cancer, due to the inherent inaccuracies in radiologic tumour staging. Exploratory data suggests there may be a role for MRI or PET in refinement of staging, however these techniques have not been included in randomised trials and data is limited to small case series (54,55); it is hoped that in future more refinement of imaging techniques may better select patients for treatment.
For patients with borderline fitness or other concerns regarding tolerance of chemotherapy, awaiting pathological staging and judicious use of adjuvant chemotherapy will likely remain the gold standard until significantly more accurate preoperative staging is available. Of note, a previous British population- based study of reasons adjuvant chemotherapy was not given to patients with stage III colon cancer found that in elderly patients (over 70 years) in particular, comorbidities precluded adjuvant chemotherapy in many patients (43% did not receive chemotherapy, of these, 60% were not offered it on the basis of comorbidities) (74).An Australian population-based study found that patients over 70 receiving oxaliplatin chemotherapy were more likely to be admitted to hospital than younger patients on doublet chemotherapy, while there was no difference in hospitalisations in patients receiving fluoropyrimidine alone (75). Both studies however showed that the patients over 70 able to receive oxaliplatin had a survival benefit similar to that seen in their younger peers (74,75). These data suggest that particular caution should be utilised when developing this treatment for the elderly, with inclusion of older patients in trials wherever possible. While the optimal neoadjuvant treatment remains oxaliplatin-based chemotherapy it is likely a significant proportion of elderly patients will continue to be unsuitable for this treatment.
Comorbidities play a role in tolerance of chemotherapy, although data are limited. A particular concern with oxaliplatin is the development of peripheral neuropathy. A systemic review conducted in 2017 suggested that various other factors increasing risk of neuropathy (diabetes mellitus, alcohol use) may increase the risk of chemotherapy-associated neuropathy, although data were conflicting between studies (76). Generally, patients with serious comorbidities are excluded from trials, limiting the extrapolation of clinical trial data to these populations. The FOxTROT trial, which had pragmatic exclusion criteria allowing for investigator judgement in most cases, still mandated a creatinine clearance of >50 mL/min, and relatively normal hepatic function, both of which parameters will have excluded a proportion of real-world colon cancer patients (65). Oxaliplatin is excreted renally, which limits use in patients with kidney dysfunction without dose adjustment, and excludes patients with severe kidney dysfunction (77). Chemotherapy is potentially toxic particularly in the elderly and combination scores using information gathered in geriatric assessment as well as routine biochemistry have been developed to predict this (78). Comorbidities need to be cautiously assessed in patients being considered for chemotherapy, and may sway the clinician toward immediate (potentially curative) surgery, rather than risking adverse effects with chemotherapy.
Addressing fear of preoperative disease progression
There is also a concern that some patients with operable disease may not respond to neoadjuvant chemotherapy, and may progress during neoadjuvant treatment, with increase in pathological stage or even development of inoperable disease. In the FOxTROT clinical trial, the mismatch repair deficient (d-MMR) subgroup showed a low rate of response (evidence of moderate or better pathological response in 7% vs. in 23% of the proficient MMR tumours), and no benefit was observed in DFS (66); suggesting that d-MMR tumours do not benefit from neoadjuvant chemotherapy and should proceed with upfront surgery. In the trial population as a whole, there was a reassuringly lower rate of incomplete surgical resection in the neoadjuvant treatment group compared with the control group (5% vs 10%, P=0.001), meaning that significant disease progression during neoadjuvant chemotherapy is unlikely (10). Similarly in the PRODIGE 22 trial, R0 resection rates were similar in both arms, again implying that significant progression while on chemotherapy was unlikely (70).
Health systems
At this stage, there is insufficient evidence for widespread adoption of neoadjuvant chemotherapy in colon cancer. However, should future data show a significant benefit of this approach, the traditional multidisciplinary approach to treatment will also require adjustment, with earlier referral to medical oncologists to facilitate pre-operative treatment. Data regarding the uptake of neoadjuvant chemotherapy for gastric cancer, where it is considered standard-of-care, shows that uptake varies by centre with higher uptake in academic centres or integrated cancer services (79). Similarly, while neoadjuvant chemotherapy has been recommended for muscle invasive bladder cancer for many years, uptake remains low in many centres, with a slow increase in use and ongoing disparity between academic centres and lower volume hospitals (80,81). A targeted multidisciplinary clinic specifically for bladder cancer has been shown to be closely associated with increasing uptake of neoadjuvant chemotherapy in one health service (82). In order to successfully implement neoadjuvant chemotherapy for colon cancer, it will be essential to invest in highly collaborative multidisciplinary teams and integrated cancer services.
Future directions
For neoadjuvant chemotherapy to become standard of care for colon cancer, an acceptable balance between toxicity and benefit will need to be struck. This could happen either in the form of treatments with reduced toxicity, or in better patient selection for the currently available, relatively toxic combination chemotherapy regimens.
There is substantial interest in immunotherapy for colon cancer. To date, immunotherapy has mainly been of benefit for patients with dMMR tumours (83,84). The NICHE study used a short course of ipilimumab and nivolumab pre operatively in patients with both pMMR and dMMR colorectal cancers, finding that all dMMR tumours responded (20/20, 12/20 with pCR) (85). Patients with pMMR tumours were randomised to either immunotherapy alone or with the addition of celecoxib. Interestingly, 4/15 patients with pMMR tumours had significant pathological responses, a finding which was associated with CD8+PD1+ T cell infiltration into the tumour (85). Further research is needed to better understand which patients with pMMR tumours might benefit from this approach. Immunotherapy is an attractive option as has a favourable safety profile compared with combination chemotherapy, as well as the ability to induce response in dMMR tumours, which have not been shown to benefit from neoadjuvant chemotherapy.
Further data is awaited regarding the benefit of currently available chemotherapy regimens in the neoadjuvant setting. The FOxTROT trial, discussed above, is yet to release final results (10). In addition to this trial there are a number of other phase III trials around the world examining the use of FOLFOX or CAPOX as neoadjuvant therapy, of which results are yet to be published (86-88). Of note, these trials have similar inclusion criteria to the FOxTROT and PRODIGE trials, and will have similar limitations regarding the use of preoperative CT staging.
Another avenue to optimise patient selection is the use of specific targetable mutations or biomarkers to identify patients who may benefit from specific regimens. An ongoing trial is examining the role of neoadjuvant Foxy-5, a targeted agent aiming to reduce tumour cell migration and metastasis in Wnt-low colon cancers (89). In future, further research into biomarkers which predict response or lack thereof to certain treatments will help further refine the use of neoadjuvant therapy. The tumour microenvironment plays an important role in tumour behaviour and emerging data shows that assessment of the tumour microenvironment in a predictive model can predict whether or not a patient will benefit from adjuvant chemotherapy for early stage colon cancer (90). It has also been shown recently that tumour-infiltrating macrophages play a part in Stage II colon cancer responsiveness to adjuvant chemotherapy, and the pattern of macrophage subtypes can predict benefit (91). These insights and future insights from ongoing investigation in this area may help to target neoadjuvant treatments to those patients who will benefit most.
Another area of interest is whether patients with an excellent response to preoperative therapy can have rationalisation or omission of postoperative therapy. This is a theoretical area at present as the rate of complete response with currently available regimens is low [4% in FOxTROT (10)], however it is hoped that better tailoring of treatment to patients most likely to respond may make this a problem to assess in clinical trials.
Novel immunotherapy approaches, including tumour vaccines and cell infusions, are also being examined, with a view to increasing efficacy of neoadjuvant treatments without compromising safety (92,93).
Despite the significant need for better patient selection for neoadjuvant treatment in this disease, there are no currently registered clinical trials specifically addressing the question of pre-operative staging, and this is a direction which will benefit from further research.
Conclusions
Despite the limitations in currently available data, neoadjuvant chemotherapy for colon cancer remains interesting as a way to optimize chemotherapy delivery and outcomes amongst colon cancer patients. If patient selection could be optimized, it would be a low cost way of repurposing currently available treatments for maximal benefit. The major challenge in trial design and proof of concept is adequate staging, with significant numbers of stage II patients included in published trials, including low risk stage II patients who are not known to benefit from adjuvant chemotherapy. Better treatments are also awaited- for the dMMR population, there is exciting early evidence of excellent activity of neoadjuvant immunotherapy; however for the majority of colon cancer patients the available systemic treatments have not changed significantly since the benefit of oxaliplatin was demonstrated more than 10 years ago. Until such time as preoperative staging is able to accurately identify stage III patients, who have a known significant benefit from currently available chemotherapy regimens in the adjuvant setting, it is unlikely that neoadjuvant chemotherapy will become standard of care. Further into the future, it is hoped that broadly active and less toxic therapies will be developed which may broaden the indications for neoadjuvant and adjuvant therapies to include both less fit patients, and also patients with lower stage disease who do not derive benefit from current treatments.
Acknowledgments
Acknowledgement to Prof Hans Prenen, University Hospital Antwerp, who was involved in developing the concept and design of this article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Colorectal Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-159
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-159). The series “Colorectal Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. ES served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Digestive Medicine Research from Sep 2019 to Sep 2021. Dr. AR reports grants from AMGEN, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Institute. SEER Cancer Stat Facts: Colorectal Cancer. Bethesda. 2020. Available online: https://seer.cancer.gov/statfacts/html/colorect.html. Accessed 3 November 2020.
- André T, de Gramont A, Vernerey D, et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol 2015;33:4176-87. [Crossref] [PubMed]
- Sobrero AF, Andre T, Meyerhardt JA, et al. Overall survival (OS) and long-term disease-free survival (DFS) of three versus six months of adjuvant (adj) oxaliplatin and fluoropyrimidine-based therapy for patients (pts) with stage III colon cancer (CC): Final results from the IDEA (International Duration Evaluation of Adj chemotherapy) collaboration. J Clin Oncol 2020;38:4004. [Crossref]
- The Global Cancer Observatory. Colorectal Cancer. In: GLOBOCAN. 2019. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 2013;31:3623-30. [Crossref] [PubMed]
- Karoui M, Rullier A, Piessen G, et al. Perioperative FOLFOX 4 Versus FOLFOX 4 Plus Cetuximab Versus Immediate Surgery for High-Risk Stage II and III Colon Cancers: A Phase II Multicenter Randomized Controlled Trial (PRODIGE 22). Ann Surg 2020;271:637-45. [Crossref] [PubMed]
- Seymour MT, Morton D. FOxTROT: an international randomised controlled trial in 1052 patients (pts) evaluating neoadjuvant chemotherapy (NAC) for colon cancer. J Clin Oncol 2019;37:350. [Crossref]
- . Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345:939-44. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8. [Crossref] [PubMed]
- O'Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997;15:246-50. [Crossref] [PubMed]
- Francini G, Petrioli R, Lorenzini L, et al. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology 1994;106:899-906. [Crossref] [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74. [Crossref] [PubMed]
- Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J Clin Oncol 2015;33:3733-40. [Crossref] [PubMed]
- Grothey A, Sobrero AF, Shields AF, et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med 2018;378:1177-88. [Crossref] [PubMed]
- Twelves CJ. Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial: overview of efficacy, safety, and cost-effectiveness. Clin Colorectal Cancer 2006;6:278-87. [Crossref] [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [Crossref] [PubMed]
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7. [Crossref] [PubMed]
- Haller DG, O'Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24. [Crossref] [PubMed]
- Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353-60. [Crossref] [PubMed]
- Meyers BM, Cosby R, Quereshy F, et al. Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review. Clin Oncol (R Coll Radiol) 2017;29:459-65. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas EP, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- Diéras V, Fumoleau P, Romieu G, et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol 2004;22:4958-65. [Crossref] [PubMed]
- Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 2005;23:5983-92. [Crossref] [PubMed]
- Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24:2019-27. [Crossref] [PubMed]
- Toi M, Nakamura S, Kuroi K, et al. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat 2008;110:531-9. [Crossref] [PubMed]
- Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015;33:13-21. [Crossref] [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697-708. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Vale C. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927-34. [Crossref] [PubMed]
- Vale CL. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. European Urology 2005;48:202-5. [Crossref] [PubMed]
- Herchenhorn D, Dienstmann R, Peixoto FA, et al. Phase II Trial of Neoadjuvant Gemcitabine and Cisplatin in Patients with Resectable Bladder Carcinoma. Int Braz J Urol 2007;33:630-8. [Crossref] [PubMed]
- Arredondo J, Baixauli J, Pastor C, et al. Mid-term oncologic outcome of a novel approach for locally advanced colon cancer with neoadjuvant chemotherapy and surgery. Clin Transl Oncol 2017;19:379-85. [Crossref] [PubMed]
- Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153-62. [Crossref] [PubMed]
- Smith CG, Fisher D, Claes B, et al. Somatic profiling of the epidermal growth factor receptor pathway in tumors from patients with advanced colorectal cancer treated with chemotherapy ± cetuximab. Clin Cancer Res 2013;19:4104-13. [Crossref] [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J Clin Oncol 2005;23:609-18. [Crossref] [PubMed]
- Baran B, Mert Ozupek N, Yerli Tetik N, et al. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res 2018;11:264-73. [Crossref] [PubMed]
- Tougeron D, Mouillet G, Trouilloud I, et al. Efficacy of Adjuvant Chemotherapy in Colon Cancer With Microsatellite Instability: A Large Multicenter AGEO Study. J Natl Cancer Inst 2016;108. [Crossref] [PubMed]
- Papadimitriou CA, Papakostas P, Karina M, et al. A randomized phase III trial of adjuvant chemotherapy with irinotecan, leucovorin and fluorouracil versus leucovorin and fluorouracil for stage II and III colon cancer: a Hellenic Cooperative Oncology Group study. BMC Med 2011;9:10. [Crossref] [PubMed]
- Kerr RS, Love S, Segelov E, et al. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): an open-label, randomised phase 3 trial. Lancet Oncol 2016;17:1543-57. [Crossref] [PubMed]
- Taieb J, Balogoun R, Le Malicot K, et al. Adjuvant FOLFOX +/- cetuximab in full RAS and BRAF wildtype stage III colon cancer patients. Ann Oncol 2017;28:824-30. [Crossref] [PubMed]
- Costas-Chavarri A, Nandakumar G, Temin S, et al. Treatment of Patients With Early-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. J Glob Oncol 2019;5:1-19. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Rectal Cancer Version 6.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed 12 July 2020.
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and Fluorouracil With or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J Clin Oncol 2000;18:2938-47. [Crossref] [PubMed]
- Dighe S, Swift I, Magill L, et al. Accuracy of radiological staging in identifying high-risk colon cancer patients suitable for neoadjuvant chemotherapy: a multicentre experience. Colorectal Dis 2012;14:438-44. [Crossref] [PubMed]
- Wiegering A, Kunz M, Hussein M, et al. Diagnostic value of preoperative CT scan to stratify colon cancer for neoadjuvant therapy. Int J Colorectal Dis 2015;30:1067-73. [Crossref] [PubMed]
- Rollvén E, Holm T, Glimelius B, et al. Potentials of high resolution magnetic resonance imaging versus computed tomography for preoperative local staging of colon cancer. Acta Radiologica 2013;54:722-30. [Crossref] [PubMed]
- Tsunoda Y, Ito M, Fujii H, et al. Preoperative Diagnosis of Lymph Node Metastases of Colorectal Cancer by FDG-PET/CT. Jpn J Clin Oncol 2008;38:347-53. [Crossref] [PubMed]
- Park SY, Cho SH, Lee MA, et al. Diagnostic performance of MRI- versus MDCT-categorized T3cd/T4 for identifying high-risk stage II or stage III colon cancers: a pilot study. Abdom Radiol (NY) 2019;44:1675-85. [Crossref] [PubMed]
- Nerad E, Lambregts DM, Kersten EL, et al. MRI for Local Staging of Colon Cancer: Can MRI Become the Optimal Staging Modality for Patients With Colon Cancer? Dis Colon Rectum 2017;60:385-92. [Crossref] [PubMed]
- Zhou H, Song Y, Jiang J, et al. A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer. Chin J Cancer Res 2016;28:598-605. [Crossref] [PubMed]
- Liu F, Yang L, Wu Y, et al. CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res 2016;28:589-97. [Crossref] [PubMed]
- de Gooyer JM, Verstegen MG. Neoadjuvant Chemotherapy for Locally Advanced T4 Colon Cancer: A Nationwide Propensity-Score Matched Cohort Analysis. Dig Surg 2020;37:292-301. [Crossref] [PubMed]
- Jakobsen A, Andersen F, Fischer A, et al. Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol 2015;54:1747-53. [Crossref] [PubMed]
- Aisu N, Yoshida Y, Komono A, et al. Phase 2 study of perioperative chemotherapy with SOX and surgery for stage III colorectal cancer (SOS3 study). Sci Rep 2019;9:16568. [Crossref] [PubMed]
- Karoui M, Rullier A, Luciani A, et al. Neoadjuvant FOLFOX 4 versus FOLFOX 4 with Cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a multicentre randomised controlled phase II trial--the PRODIGE 22--ECKINOXE trial. BMC Cancer 2015;15:511. [Crossref] [PubMed]
- Karoui M, Gallois C, Piessen G, et al. Does neoadjuvant FOLFOX chemotherapy improve the oncological prognosis of high-risk stage II and III colon cancers ? Three years’ follow-up results of the Prodige 22 phase II randomized multicenter trial. J Clin Oncol 2020;38:4110. [Crossref]
- FOxTROT Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152-60. [Crossref] [PubMed]
- FoxTROT Collaborative Group. FOxTROT protocol: A randomised trial assessing whether preoperative chemotherapy and/or an anti-EGFR monoclonal antibody improve outcome in high-risk operable colon cancer. 2012. Available online: https://www.birmingham.ac.uk/Documents/college-mds/trials/bctu/foxtrot/FOxTROTProtocolv60090712.pdf. Accessed 30 December 2020.
- Seligmann JF. FOxTROT: neoadjuvant FOLFOX chemotherapy with or without panitumumab (Pan) for patients (pts) with locally advanced colon cancer (CC). J Clin Oncol 2020;38:4013. [Crossref]
- Seymour MT, Morton D. Investigators obotIFT. FOxTROT: an international randomised controlled trial in 1052 patients (pts) evaluating neoadjuvant chemotherapy (NAC) for colon cancer. J Clin Oncol 2019;37:3504. [Crossref]
- O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381-8. [Crossref] [PubMed]
- Benson AB, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology Recommendations on Adjuvant Chemotherapy for Stage II Colon Cancer. J Clin Oncol 2004;22:3408-19. [Crossref] [PubMed]
- Karoui M, Rullier A, Piessen G, et al. Perioperative FOLFOX 4 Versus FOLFOX 4 Plus Cetuximab Versus Immediate Surgery for High-Risk Stage II and III Colon Cancers: A Phase II Multicenter Randomized Controlled Trial (PRODIGE 22). Ann Surg 2020;271:637-45. [Crossref] [PubMed]
- Labianca R, Marsoni S, Pancera G, et al. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 1995;345:939. [Crossref] [PubMed]
- Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74. [Crossref] [PubMed]
- Marsoni S. Efficacy of adjuvant fluorouracil and leucovorin in stage B2 and C colon cancer. International Multicenter Pooled Analysis of Colon Cancer Trials Investigators. Semin Oncol 2001;28:14-9. [Crossref] [PubMed]
- Ko JJ, Kennecke HF, Lim HJ, et al. Reasons for underuse of adjuvant chemotherapy in elderly patients with stage III colon cancer. Clin Colorectal Cancer 2016;15:179-85. [Crossref] [PubMed]
- Brungs D, Aghmesheh M, de Souza P, et al. Safety and Efficacy of Oxaliplatin Doublet Adjuvant Chemotherapy in Elderly Patients With Stage III Colon Cancer. Clin Colorectal Cancer 2018;17:e549-55. [Crossref] [PubMed]
- Pulvers JN, Marx G. Factors associated with the development and severity of oxaliplatin-induced peripheral neuropathy: a systematic review. Asia Pac J Clin Oncol 2017;13:345-55. [Crossref] [PubMed]
- Graham MA, Lockwood GF, Greenslade D, et al. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res 2000;6:1205-18. [PubMed]
- Retornaz F, Guillem O, Rousseau F, et al. Predicting Chemotherapy Toxicity and Death in Older Adults with Colon Cancer: Results of MOST Study. Oncologist 2020;25:e85-e93. [Crossref] [PubMed]
- Liu N, Xu Y, Rahnemai-Azar AA, et al. National Underutilization of Neoadjuvant Chemotherapy for Gastric Cancer. J Gastrointest Surg 2020;24:949-58. [Crossref] [PubMed]
- Krabbe LM, Westerman ME, Margulis V, et al. Changing trends in utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer. Can J Urol 2015;22:7865-75. [PubMed]
- Keegan KA, Zaid HB, Patel SG, et al. Increasing Utilization of Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer in the United States. Curr Urol Rep 2014;15:394. [Crossref] [PubMed]
- Nayan M, Bhindi B, Yu JL, et al. The initiation of a multidisciplinary bladder cancer clinic and the uptake of neoadjuvant chemotherapy: A time-series analysis. Can Urol Assoc J 2016;10:25-30. [Crossref] [PubMed]
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. [Crossref] [PubMed]
- Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 2018;36:773-9. [Crossref] [PubMed]
- Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566-76. [Crossref] [PubMed]
- Kyungpook National University Hospital. Neoadjuvant FOLFOX Chemotherapy for Patients With Locally Advanced Colon Cancer NCT03426904. Available online: https://ClinicalTrials.gov/show/NCT03426904. Accessed 15 December 2020.
- Vejle Hospital. Neoadjuvant Chemotherapy Versus Standard Treatment in Patients With Locally Advanced Colon Cancer NCT03970694. Available online: https://ClinicalTrials.gov/show/NCT01918527. Accessed 15 December 2020.
- Universidad de Leon. Analysis of the Effectiveness of Neoadjuvant Chemotherapy in the Treatment of Colon Cancer Locally Advanced (ELECLA) NCT04188158. Available online: https://ClinicalTrials.gov/show/NCT04188158. Accessed 15 December 2020.
- WntResearch AB. Foxy-5 as Neo-Adjuvant Therapy in Subjects With Wnt-5a Low Colon Cancer. Available online: https://ClinicalTrials.gov/show/NCT03883802. Accessed 20 December 2020.
- Zhou R, Zeng D, Zhang J, et al. A robust panel based on tumour microenvironment genes for prognostic prediction and tailoring therapies in stage I–III colon cancer. EBioMedicine 2019;42:420-30. [Crossref] [PubMed]
- Feng Q, Chang W, Mao Y, et al. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin Cancer Res 2019;25:3896-907. [Crossref] [PubMed]
- George E. Peoples. Trial of PalloV-CC in Colon Cancer. Available online: https://ClinicalTrials.gov/show/NCT03827967. Accessed 20 Dec 2020.
- Celyad Oncology SA. Dose Escalation and Dose Expansion Phase I Study to Assess the Safety and Clinical Activity of Multiple Doses of NKR-2 Administered Concurrently With FOLFOX in Colorectal Cancer With Potentially Resectable Liver Metastases (SHRINK) NCT03310008. 2017. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03310008. Accessed 15 Dec 2020.
Cite this article as: Body A, Latham S, Kong JB, Raghunath A, Segelov E. Stage III colon cancer: is neoadjuvant chemotherapy ready for prime time?—A narrative review of neoadjuvant chemotherapy for colon cancer. Dig Med Res 2021;4:16.


