
Gastrectomy for gastroparesis: when and how
Background
Gastroparesis is defined as a chronic gastric motility disorder with delayed gastric emptying in the absence of mechanical obstruction. Symptoms typically consist of nausea, vomiting, bloating, early satiety, postprandial fullness, and epigastric abdominal pain (1). The disease can be debilitating for patients, leading to nutritional deficiencies, weight loss, electrolyte disturbances, dehydration, and a decreased quality of life (2). The three most commonly identified etiologies of gastroparesis are diabetic, idiopathic, and postsurgical. Regardless of etiology, histologic examination has demonstrated a loss of the interstitial cells of Cajal underlying the disease (3).
Over the past several years, there has been a dramatic increase in the prevalence of gastroparesis among the general population in the United States (US). Recent literature estimates the current prevalence of gastroparesis at 4% of the US adult population, and the diagnosis is widely perceived to be on the rise (4,5). This has translated into a significant healthcare and economic burden. Multiple studies have demonstrated sizeable increases in gastroparesis related hospital admissions and emergency department visits (5-7). One study showed a 300% increase in gastroparesis-related hospital admissions over a 16-year period, as well as a substantial increase in hospital charges related to the disease (5). Another reported that over a 10-year period, gastroparesis accounted for $3,500 million in hospital charges and 911,963 hospital days (6). The disease has proven exceptionally difficult to manage for many patients, with relapsing symptoms and medical complications.
First line treatment for gastroparesis involves lifestyle and dietary modifications, and in the case of diabetic gastroparesis, typically includes glycemic control (8). However, this is frequently insufficient and further medical and surgical treatment modalities are often required. Medical therapies largely revolve around prokinetic and antiemetic medications, and have not considerably changed over the past few decades. The only medication reviewed and approved by the U.S. Food and Drug Administration (FDA) for the treatment of gastroparesis is metoclopramide, which is approved for four to twelve weeks of usage and carries a black box warning for tardive dyskinesia (8,9). Many other medical therapies focus on symptom control rather than addressing the underlying pathophysiology. Medical management can be limited by medication side effects or cost, and fails to be efficacious for some patients (8-10). When patients are unable to tolerate the medications or fail to improve, they are considered medically refractory and more invasive surgical options must be explored. While a variety of options exist, there is continued debate regarding the surgical treatment modalities, especially around the use of gastrectomy for the treatment of refractory gastroparesis.
In this article, we aim to review the surgical options for refractory gastroparesis, with a specific focus on the most invasive option: gastrectomy. First, we discuss the diagnosis and workup of gastroparesis. We then present a treatment algorithm for the surgical management of the disease and review the various surgical treatment options. Finally, we delve into the current literature exploring gastrectomy for refractory gastroparesis in an effort to answer when and how this modality should be utilized.
Diagnosis and preoperative workup
The gold standard for the diagnosis of gastroparesis is objective evidence of delayed gastric emptying on scintigraphy in the absence of mechanical obstruction, with associated symptomatology (8). The symptoms for gastroparesis can be quantified using the gastroparesis cardinal symptom index (GCSI), a tool developed and validated by Revicki et al. to assess the severity of symptoms associated with gastroparesis (11). There are 3 subscores within the rubric: nausea and vomiting, fullness and early satiety, and bloating (11). All patients should be evaluated with the GCSI preoperatively and postoperatively to track severity of symptoms and response to treatment.
A formal 4-hour solid phase gastric emptying scintigraphy study should be obtained for all patients. Gastric retention of >10% of the radiolabeled food bolus after 4 hours is considered abnormal (8). As the percentage of gastric retention increases at 4 hours, the severity of the disease also increases. Another option for objective evidence for diagnosis is a wireless motility capsule study, which measures the transit time of the entire gut. This study can help distinguish patients with isolated gastroparesis from patients with concomitant intestinal dysmotility, which can ultimately help with prognosis (8,9).
Additionally, all patients should undergo upper endoscopy prior to any surgical intervention. Upper endoscopy allows the surgeon to note any anatomical disturbances including a hiatal hernia, recurrence of a hernia after a prior fundoplication, mechanical obstruction such as a peptic stricture, or a large food bezoar, all of which may change surgical management. Especially in patients with postsurgical gastroparesis or a prior fundoplication, a CT scan of the chest, abdomen, and pelvis should be considered to delineate anatomy.
Treatment algorithm
While a formal management algorithm for the surgical treatment of refractory gastroparesis is not established, there is a general consensus that gastrectomy is at the end of the line after stomach-preserving measures have been attempted. One recent study by Arthur et al. described how using a tailored approach to gastroparesis can improve symptoms (12). Figure 1 outlines our surgical treatment algorithm. After workup and diagnosis of medically refractory gastroparesis is complete, our first line therapy is almost always endoscopic per-oral pyloromyotomy (POP) along the lesser curvature of the stomach. If this fails to improve symptoms, our second line therapy is typically repeat POP along the great curvature, laparoscopic pyloroplasty (LP), or gastric electrical stimulator (GES) with or without pyloroplasty, with the caveat that GES is not approved by the US FDA for postsurgical gastroparesis. If stomach-preserving interventions fail, we turn to gastrectomy with a Roux-en-Y gastrojejunostomy as the third line and final surgical treatment option.
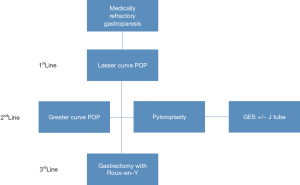
Zihni et al. recently proposed a similar algorithm for refractory gastroparesis. In their algorithm, the authors note that they first assess the nutritional status of the patient. If the patient is considered to have severe nutritional impairment, jejunal feeding access is offered to optimize nutritional status prior to any further surgical intervention. Their first line surgical therapy has traditionally been LP, however this has been variable with the development of POP. If first line intervention is insufficient, the authors then offer GES implantation. For patients who remain refractory despite these interventions, a laparoscopic subtotal gastrectomy is offered as the final step in the algorithm (13).
While the algorithm presented by Zihni et al. is similar to ours, we typically start with POP due to our experience in the treatment modality as well as the promising clinical data published about this novel surgical option. We also offer repeat pyloric intervention prior to moving down the algorithm to gastrectomy. Given the extreme step of performing a gastrectomy for a benign disease process, we promote a cautious approach for the surgical management of refractory gastroparesis.
Surgical options
Several surgical options exist for the management of refractory gastroparesis. The stomach-preserving options include GES, LP, endoscopic POP, or enteral feeding tubes. Surgical resection options include sleeve gastrectomy and subtotal or total gastrectomy.
Gastric electrical stimulation
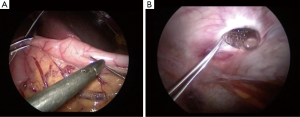
The implantation of a GES device (Enterra Therapy System, Medtronic, Inc.) received a humanitarian device exemption by the US FDA in 2000 for the treatment of refractory diabetic or idiopathic gastroparesis. The procedure is depicted in Figure 2. The device can be implanted open or laparoscopically, with two electrical leads inserted into the anterior wall of the stomach near the greater curvature 9 and 10 centimeters proximal to the pylorus in the muscular layer of the stomach wall. The pulse generator is then placed in a subcutaneous pocket under the skin (14). Multiple studies have looked at the efficacy of GES, and results are generally positive. An early double blind crossover study demonstrated a significant reduction in weekly vomiting episodes and 4-hour gastric retention. The study also measured an improved quality of life, a decrease in hospital days over the year following the procedure, and a decrease in supplementary enteral or parenteral nutritional support needs (15). Many subsequent studies have confirmed these results, with one systematic review by Zoll et al. reporting an improvement of nausea in 45.8%, vomiting in 49.8%, and epigastric abdominal pain in 40.6% of patients (14-17). However, empirical data has not demonstrated a therapeutic effect beyond placebo (15). The most common complication from the device is infection, generally of the subcutaneous pocket where the pulse generator is implanted, which typically requires device removal. Other reported complications include bowel obstruction, perforation of the stomach, and electrode dislodgement or migration (14,16).
Pyloroplasty
The goal of pyloroplasty in the treatment of refractory gastroparesis is to augment gastric emptying by preserving pyloric patency while increasing the cross-sectional area of the gastric outlet (13,18). Pyloroplasty is most commonly done laparoscopically in a Heineke-Mikulicz fashion, extending a longitudinal incision along the pylorus to the proximal duodenum, which is then closed transversely. LP has been fairly well studied for the treatment of refractory gastroparesis. Hibbard et al. reported on 28 patients who underwent LP at a single institution and demonstrated a significant reduction in prokinetic use postoperatively (89% to 14%, P≤0.0001), normalized gastric emptying time in 71% of patients, and symptoms were significantly improved after 1 month (19). A larger series by Shada et al. studied 177 patients who underwent LP for gastroparesis. The authors reported no intraoperative complications or conversions to an open procedure. Overall morbidity rate was 6.8% with 4 patients returning to the operating room and 2 confirmed leaks. There was normalization of gastric emptying time in 77% of patients. Subsequent surgical interventions for gastroparesis were required in 10.7% of patients, including GES implantation, feeding jejunostomy or gastrostomy tube, or subtotal gastrectomy (20). Overall, LP has been deemed a safe and effective first line surgical treatment option for refractory gastroparesis.
Endoscopic per-oral pyloromyotomy (G-POEM)
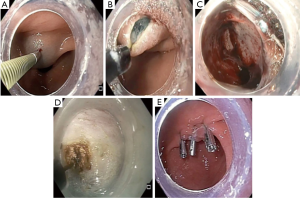
More recently, an innovative endoscopic option known as POP or G-POEM has been developed and shown promise in early literature. The steps of a POP procedure are shown in Figure 3. A mucosotomy is made after creating a mucosal bleb along the lesser curvature of the stomach. A submucosal tunnel is developed and the pylorus is identified and divided, completing the myotomy. The initial mucosotomy is then closed, typically with endoscopic clips. This can also be carried out along the greater curvature of the stomach if the patient has had prior intervention along the lesser curve pylorus. Khashab et al. reported a multicenter non-randomized study on the efficacy and safety of POP compared to medical therapy. The procedure was completed successfully in 100% of patients, with 6.7% experiencing an adverse event. At a median follow up of 5.5 months, 86% of patients had complete symptomatic response. For patients with repeat gastric emptying scans available, 47% had normalized and 35% had improved gastric emptying (21). Multiple additional studies have demonstrated therapeutic benefit from POP (22-25).
One recent study by Landrenau et al. compared outcomes of LP with POP. In a propensity matched cohort study, the authors compared 30 patients who underwent LP with 30 patients who underwent POP for refractory gastroparesis. Etiology of gastroparesis was distributed between idiopathic, postsurgical, and diabetic in both groups. Patients who underwent LP had a longer postoperative length of stay, operative time, and higher estimated blood loss, all statistically significant results. The LP group also had more complications, including surgical site infection, pneumonia, and unplanned admission to the intensive care unit, however these results were not statistically significant. Both procedures showed significant improvement in GCSI and objective gastric emptying. The authors concluded that while the procedures have comparative functional outcomes, POP has less perioperative morbidity and is therefore a safe and effective treatment for refractory gastroparesis (18).
Gastric or jejunal feeding tubes
Enteral feeding access can help to improve symptoms in refractory gastroparesis or be used to optimize nutritional status prior to other surgical interventions. A gastrostomy tube can be easily placed endoscopically and used for venting to help decrease fullness, distention, and vomiting symptoms. Gastrostomy tubes are not recommended in gastroparesis for feeding, as this may exacerbate symptoms. Jejunostomy tube placement is the preferred route for enteral nutrition, however cannot be used to vent a distended stomach. The jejunostomy tube effectively bypasses the stomach allowing adequate caloric intake, however it is common to have complications related to jejunostomy tubes (14,15).
Sleeve gastrectomy
There has been growing interest in the use of sleeve gastrectomy as a possible treatment option for refractory gastroparesis. While empirical data is sparse, there have been multiple anecdotal reports of success in improving symptoms. The largest study to date is from Lee et al., who examined 19 patients who underwent primary sleeve gastrectomy for refractory gastroparesis. They noted a relatively low morbidity rate of 11%, with average gastrointestinal quality of life scores improving from 78 preoperatively to 114 postoperatively (normal 125). Of note, body mass index did not significantly change after greater than 1 year follow up. There were 3 patients who had a recurrence of their symptoms and required subsequent formal gastrectomy greater than 1 year after their initial operation (26). While this study was small, it does demonstrate promise that laparoscopic sleeve gastrectomy may play a role in the treatment of refractory gastroparesis in the future. At this time, sleeve gastrectomy remains an experimental treatment modality and more data is needed prior to offering this as a potential first line treatment.
Gastrectomy
The end of our proposed surgical treatment algorithm and the definitive surgical option for refractory gastroparesis is subtotal or total gastrectomy. This avenue should be pursued cautiously in patients, and only after failing to maintain sustainable results with less invasive surgical treatment modalities.
Operative technique
When approaching the use of gastrectomy for refractory gastroparesis both laparoscopic and robotic minimally invasive techniques are reasonable depending on the individual surgeon’s training, experience, and resources. The approach described herein is a minimally invasive subtotal gastrectomy with Roux-en-Y gastrojejunostomy.
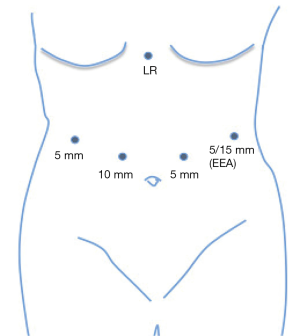
The patient is placed in the supine position with a foot-board in place and arms are secured abducted at 90 degrees for laparoscopic cases, or tucked at patient’s side for robotic cases. An orogastric tube is placed to empty the stomach prior to resection. Five 10 mm ports are placed in a gentle “U” shape across the abdomen. Our typical trocar placement is illustrated in Figure 4. If able, we use a 5 mm camera. The left upper quadrant port can be upsized from 5 to 15 mm if a circular anastomosis using an EEA stapler for an esophagojejunostomy is planned. Figure 5 depicts the basic steps of our subtotal gastrectomy. We start with mobilization of the greater curvature of the stomach using a curved tip bipolar device, which allows for controlled hemostasis. This dissection can be completed close to the gastric wall, similar to the mobilization performed during a sleeve gastrectomy. We then choose the distal resection site on the duodenum, typically just distal to the pylorus. After careful dissection under the duodenum, we use a laparoscopic or robotic linear stapler to transect the duodenum and gastroepiploic bundle. Unlike performing a gastrectomy for malignant disease, it is not necessary when performing a gastrectomy for benign disease to dissect down to the junction of the gastroepiploic vein and superior mesenteric vein, making it reasonable to take the artery and vein en bloc. We next choose the site of our our proximal resection. In patients with prior foregut surgery, we prefer to leave a small pouch or remnant stomach, preserving the left gastric artery. Our initial stapler fire is along the descending branch of the left gastric artery, and we typically complete this with staple line reinforcement to control the artery. The initial stapler fire is followed by 60 mm stapler fires horizontally on the stomach and two staple fires vertically on the stomach to create the gastric pouch. We do not routinely use staple line reinforcement on these fires. It is important to clear the debris at the staple line crotch to avoid the stapler misfiring. We then proceed to the jejunojejunostomy, measuring the biliopancreatic limb to 50 cm and the alimentary limb to 75 cm. Using a 60 mm vascular load endoGIA stapler, we create the jejunojejunostomy in a side-to-side fashion. The common enterotomy is closed in a running fashion using absorbable suture and the mesenteric defect is closed using a running-locking permanent suture. The gastrojejunostomy is most often done in an antecolic fashion. We favor a hand sewn technique for the gastrojejunal anastomosis, using 3-0 absorbable barbed suture, aligning the anastomosis site on the jejunum to the horizontal staple line on the gastric remnant. When making the gastrotomy, it is helpful to perform an endoscopy with insufflation to provide traction and countertraction and to assist with calibration of anastomosis. We then make a similarly sized jejunotomy using hook electrocautery. The posterior side of the anastomosis is sewn in two-layers in a running fashion using 3-0 absorbable barbed sutures. We routinely only complete a single-layer on the anterior side of the anastomosis; however, if there is a leak on intraoperative endoscopy we will oversew the anterior suture line with 2-0 braided absorbable suture using a seromuscular imbrication technique. An alternative approach is to perform the anastomosis with a stapled technique using either a circular 25 mm EEA stapler or a linear stapler to the desired anastomotic diameter (usually 2
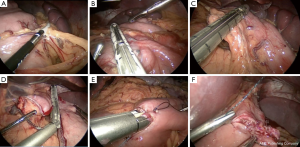
Surgical outcomes
Performing a gastrectomy for medically refractory gastroparesis has been most well-studied among postsurgical gastroparesis patients. Early research in postsurgical gastroparesis, most commonly after surgical intervention for peptic ulcer disease, was promising with multiple studies demonstrating efficacy (27-29). Eckhauser et al. studied 81 patients who underwent near total gastrectomy with Roux-en-Y reconstruction for postsurgical gastroparesis. There were no mortalities or anastomotic complications. A total of 52 patients were available for follow up interviews. With an average follow up of 56 months, 80% of patients reported long term symptom relief (27).
More recently, Bhayani et al. published a thoughtful approach to offering gastrectomy for refractory gastroparesis among various etiologies. The authors studied 35 patients with refractory gastroparesis who underwent laparoscopic total or near-total gastrectomy, 34% with diabetic, 23% with idiopathic, and 43% with postsurgical gastroparesis. With a median follow up of 6 months, 69% of patients had improved or resolved nausea (P=0.002), chronic abdominal pain resolved in 70% (P=0.3), belching resolved in 79% (P=0.03), and bloating resolved in 89% of patients (P=0.0005). The study had no mortalities, but did have a 17% leak rate, all treated with surgical reintervention. The authors concluded that while morbidity is high in this complex patient population, at a high volume center patients can be managed well with an improvement in symptoms (30).
Results of this minimally invasive approach have been published. Landrenau et al. examined the outcomes of formal gastrectomy compared to leaving the stomach in situ with a Roux-en-Y reconstruction for the treatment of gastroparesis (31). Specifically, 26 patients underwent gastric pouch creation with a Roux-en-Y gastrojejunostomy with the stomach left in situ while 27 patients underwent formal gastrectomy with Roux-en-Y reconstruction. The stomach in situ group had shorter operative times (155 vs. 223 min, P<0.001), lower estimated blood loss (24 vs. 130 mL, P<0.001), and a shorter length of stay (4.0 vs. 7.2 days, P=0.003). Leaving the stomach in situ also had a lower 30-day complication rate (7.7% vs. 44.4%, P=0.001). However, patients with the stomach in situ were more likely to require subsequent surgical intervention (23.1% vs. 3.7%, P=0.04), one of which was a remnant gastrectomy. Both procedures improved symptoms to a similar degree based on GCSI. While leaving the stomach in situ had advantages, we concluded that a formal gastrectomy may be the more definitive operation in the management of refractory gastroparesis (31).
Comparing outcomes between surgical modalities
While the majority of literature examines the efficacy of gastrectomy for the treatment of refractory gastroparesis, there has been some inquiry, largely retrospective data, comparing gastrectomy with other minimally invasive options. Zehetner et al. compared the outcomes of 103 patients with diabetic, idiopathic, and postsurgical gastroparesis who underwent GES placement or laparoscopic gastrectomy. Overall, 72 patients had a GES placed and 31 underwent gastrectomy. Morbidity was higher in the gastrectomy group (23% vs. 8.3%, P=0.06), however this was not a statistically significant result. The 30-day mortality rate was similar between the two groups (2.7% GES vs. 3% gastrectomy, P=1.00). Notably, 13 patients (18%) in the GES group were transitioned to a subtotal gastrectomy due to persistent symptoms. In the GES group, 67% of patients rated symptoms improved, compared to 87% in the gastrectomy group (P=0.02). All patients who transitioned from GES to gastrectomy reported an improvement in their symptoms (32). Sun et al. similarly compared GES (N=20) with Roux-en-Y gastrojejunostomy (N=7) in morbidly obese patients with refractory gastroparesis. In the GES group, 90% had initial symptom improvement, with 55% maintaining symptom improvement at last follow up (average 23 months). In the Roux-en-Y gastrojejunostomy group, all patients had initial symptom improvement with 71% reporting long term improvement at last follow up. There were 4 patients that required conversion from GES to gastrojejunostomy, all of whom reported subsequent symptom improvement (33).
One systematic review by Zoll et al. has compared the three major surgical interventions for refractory gastroparesis: GES, pyloric intervention (including both pyloroplasty and pyloromyotomy), and gastrectomy. Overall, pyloric intervention had the largest percentage of patients reporting improvement at 81.6% compared to GES (69.8%) and gastrectomy (67.3%). When analyzing specific symptoms, pyloric intervention improved nausea better than GES (P<0.05) and both pyloric intervention and gastrectomy improved vomiting compared to GES (P<0.05). In subgroup analyses, pyloroplasty and pyloromyotomy had similar results, as did partial and completion gastrectomy (17).
Conclusions
Gastroparesis is a complex disorder to manage. Ultimately, a gastrectomy may be necessary to control symptoms and improve patients’ quality of life. However, this definitive operation should only be considered after exhausting all organ-sparing options. While there is no “one size fits all” treatment modality for gastroparesis, our recommendation for a definitive operation for refractory gastroparesis is a subtotal gastrectomy with Roux-en-Y gastrojejunostomy. However, this should ideally be performed in a high volume center with the experience necessary to manage the high rate of postoperative complications. A reasonable alternative is to perform a Roux-en-Y gastrojejunostomy leaving the stomach in situ, understanding the possible need for completion gastrectomy in the future. Another reasonable alternative may be a sleeve gastrectomy, however there is little empirical evidence to support this surgical option. At this time, we can only recommend it be pursued as treatment for refractory gastroparesis within an approved research protocol with an aim to publish long term data.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alfredo Daniel Guerron) for the series “Advanced Laparoscopic Gastric Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-124). The series “Advanced Laparoscopic Gastric Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of Gastroparesis. Am J Gastroenterol 2013;108:18-37. [Crossref] [PubMed]
- Hejazi RA, McCallum RW. Treatment of Refractory Gastroparesis: Gastric and Jejunal Tubes, Botox, Gastric Electrical Stimulation, and Surgery. Gastrointest Endosc Clin N Am 2009;19:73-82. [Crossref] [PubMed]
- Hirsch W, Nee J, Ballou S, et al. Emergency Department Burden of Gastroparesis in the United States, 2006 to 2013. J Clin Gastroenterol 2019;53:109-13. [Crossref] [PubMed]
- Jung HK, Choung RS, Locke GR, et al. The Incidence, Prevalence, and Outcomes of Patients With Gastroparesis in Olmsted County, Minnesota, From 1996 to 2006. Gastroenterology 2009;136:1225-33. [Crossref] [PubMed]
- Wadhwa V, Mehta D, Jobanputra Y, et al. Healthcare utilization and costs associated with gastroparesis. WJG 2017;23:4428. [Crossref] [PubMed]
- Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol 2008;103:313-22. [Crossref] [PubMed]
- Grover M, Bernard CE, Pasricha PJ, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium: Clinical-histological associations in gastroparesis. Neurogastroenterol Motil 2012;24:531-9. [Crossref] [PubMed]
- Stein B, Everhart KK, Lacy BE. Gastroparesis: A Review of Current Diagnosis and Treatment Options. J Clin Gastroenterol 2015;49:550-8. [Crossref] [PubMed]
- Navas CM, Patel NK, Lacy BE. Gastroparesis: Medical and Therapeutic Advances. Dig Dis Sci 2017;62:2231-40. [Crossref] [PubMed]
- Grover M, Farrugia G. Stanghellini vs. Gastroparesis: a turning point in understanding and treatment. Gut 2019;68:2238-50. [Crossref] [PubMed]
- Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): Development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res 2004;13:833-44. [Crossref] [PubMed]
- Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc 2018;32:977-82. [Crossref] [PubMed]
- Zihni AM, Dunst CM, Swanström LL. Surgical Management for Gastroparesis. Gastrointest Endosc Clin N Am 2019;29:85-95. [Crossref] [PubMed]
- Hejazi RA, McCallum RW. Treatment of Refractory Gastroparesis: Gastric and Jejunal Tubes, Botox, Gastric Electrical Stimulation, and Surgery. Gastrointest Endosc Clin N Am 2009;19:73-82. [Crossref] [PubMed]
- Borrazzo EC. Surgical Management of Gastroparesis: Gastrostomy/Jejunostomy Tubes, Gastrectomy, Pyloroplasty, Gastric Electrical Stimulation. J Gastrointest Surg 2013;17:1559-61. [Crossref] [PubMed]
- Abell TL, Bernstein V-RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. The American Motility Society Task Force on Gastroparesis (members in alphabetical order). Neurogastroenterol Motil 2006;18:263-83. [Crossref] [PubMed]
- Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: a systematic review. J Surg Res 2018;231:263-9. [Crossref] [PubMed]
- Landreneau JP, Strong AT, El-Hayek K, et al. Laparoscopic pyloroplasty versus endoscopic per-oral pyloromyotomy for the treatment of gastroparesis. Surg Endosc 2019;33:773-81. [Crossref] [PubMed]
- Hibbard ML, Dunst CM, Swanström LL. Laparoscopic and Endoscopic Pyloroplasty for Gastroparesis Results in Sustained Symptom Improvement. J Gastrointest Surg 2011;15:1513-9. [Crossref] [PubMed]
- Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc 2016;30:1326-32. [Crossref] [PubMed]
- Khashab MA, Ngamruengphong S, Carr-Locke D, et al. Gastric per-oral endoscopic myotomy for refractory gastroparesis: results from the first multicenter study on endoscopic pyloromyotomy (with video). Gastrointest Endosc 2017;85:123-8. [Crossref] [PubMed]
- Strong AT, Rodriguez J, Kroh M, et al. Safety and Feasibility of Per-Oral Pyloromyotomy as Augmentative Therapy after Prior Gastric Electrical Stimulation for Gastroparesis. J Am Coll Surg 2019;229:589-95. [Crossref] [PubMed]
- Strong AT, Landreneau JP, Cline M, et al. Per-Oral Pyloromyotomy (POP) for Medically Refractory Post-Surgical Gastroparesis. J Gastrointest Surg 2019;23:1095-103. [Crossref] [PubMed]
- Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis: Endoscopic pyloromyotomy in gastroparesis. J Gastroenterol Hepatol 2018;33:1829-33. [Crossref] [PubMed]
- Rodriguez JH, Haskins IN, Strong AT, et al. Per oral endoscopic pyloromyotomy for refractory gastroparesis: initial results from a single institution. Surg Endosc 2017;31:5381-8. [Crossref] [PubMed]
- Lee AM, Fuchs KH, Varga G, et al. Sleeve gastrectomy for treatment of delayed gastric emptying—indications, technique, and results. Langenbecks Arch Surg 2020;405:107-16. [Crossref] [PubMed]
- Eckhauser FE, Conrad M, Knol J, et al. Safety and long-term durability of completion gastrectomy in 81 patients with postsurgical gastroparesis syndrome. Am Surg 1998;64:711-6. [PubMed]
- McCallum RW, Polepalle SC, Schirmer B. Completion gastrectomy for refractory gastroparesis following surgery for peptic ulcer disease: Long-term follow-up with subjective and objective parameters. Digest Dis Sci 1991;36:1556-61. [Crossref] [PubMed]
- Speicher JE, Thirlby RC, Burggraaf J, et al. Results of Completion Gastrectomies in 44 Patients with Postsurgical Gastric Atony. J Gastrointest Surg 2009;13:874-80. [Crossref] [PubMed]
- Bhayani NH, Sharata AM, Dunst CM, et al. End of the Road for a Dysfunctional End Organ: Laparoscopic Gastrectomy for Refractory Gastroparesis. J Gastrointest Surg 2015;19:411-7. [Crossref] [PubMed]
- Landreneau JP, Strong AT, El-Hayek K, et al. Gastrectomy versus stomach left in situ with Roux-en-Y reconstruction for the treatment of gastroparesis. Surg Endosc 2020;34:1847-55. [Crossref] [PubMed]
- Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc 2013;27:61-6. [Crossref] [PubMed]
- Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc 2015;29:2683-9. [Crossref] [PubMed]
Cite this article as: Thelen AE, El-Hayek K. Gastrectomy for gastroparesis: when and how. Dig Med Res 2021;4:13.


