
Narrative review of hepatocellular carcinoma: from molecular bases to therapeutic approach
Introduction
To date, hepatocellular carcinoma (HCC) is considered a serious public health threat in most parts of the world comprising a broad spectrum of liver diseases with important clinical and histological differences within them, and therefore, variable prognosis depending on the characteristics of each patient. Although great technological and scientific advances have been done in recent decades, HCC still represents the 17th most prevalent cause of cancer in the world, and the third-leading cause of cancer mortality (1).
Understanding and educating our population about the most important causes that have been associated with the development of HCC, as well as discussing and implementing a series of specific strategies that have been shown to be effective in the places where they have been carried out successfully, could represent a window of opportunity for low-cost primary prevention that associates substantial benefits in all national and international health systems that are willing to fulfill these objectives. In the long run, this could reduce the exposure to environmental and occupational risk factors associated with the development of chronic liver disease (CLD) representing a decrease in the surveillance costs of HCC.
Similarly, the medical field is getting each time even closer towards an ideal, more precise, and personalized medicine through the discovery and understanding of several genetic and epigenetic mechanisms involved in different diseases. In the area of oncological hepatology, great advances have also been seen in this regard. So, it would be essential to review what have been the most important advances, and the clinical relevance of these findings in the medical practice.
For this purpose, the objective of this review is to discuss the main scientific advances in the molecular biology of HCC, concisely discussing its clinical significance, the new horizons to consider, the risk factors associated with HCC development, the new advances in the therapeutic approach of this cancer, and the primary prevention measurements that have most demonstrated effectiveness in reducing the clinical spectrum of CLDs and their consequent evolution to HCC.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-116).
Epidemiology aspects
According to data from the International Agency for Research on Cancer (IARC), only in 2018 there was an incidence of 841,080 new cases of liver cancer in the world and a mortality of 781,631 deaths predominantly in male gender (1). Moreover, HCC is twice as common in men as in women (male-to-female ratio exceeding 2.5 for incidence and mortality) (2,3).
In the past, HCC was seen almost as an exclusive condition of East Asian and Sub-Saharan African population (4). However, despite the fact that these groups continue to have high incidences of liver cancer (17.7 cases per 100,000 inhabitants in Eastern Asia) (1), the global epidemic of overweight and obesity, as well as the high consumption of alcoholic beverages have conditioned a noticeable increase in the prevalence of CLD, and a transition in the main etiologies of liver cirrhosis and HCC worldwide (5). Globally, hepatitis B virus (HBV) remains as the leading cause of cirrhosis and HCC due to the lack of universal vaccination in most low-income and lower-middle income countries (6). Interestingly, recent evidence points to the fact that alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) and its severe form, nonalcoholic steatohepatitis (NASH) are becoming important causes of cirrhosis in Asia, potentially exceeding HBV in years to come (7,8). In upper-middle income and high-income Western countries where they have rigorous vaccination programs against HBV, things change a little. In the United States, hepatis C virus (HCV) is the leading cause of liver cirrhosis and liver transplantation (9,10), while NAFLD has become the most rapidly growing cause of liver morbidity and mortality in North America (9,11) and together with ALD representing the two main CLDs in age-standardized death rate and age-standardized years of life lost in the last decade (12). This reveals the need for more aggressive and effective preventive strategies against these health problems that are becoming more frequent in the daily life of our society.
In addition, there is genetic susceptibility among population groups with certain diseases that put them at risk to develop HCC. For instance, a study reported a significant increase in the prevalence of cirrhosis among first-degree relatives of patients with cirrhosis. Meanwhile, another study found familial clustering and potential maternal linkage for insulin resistance for NAFLD and cirrhosis (13). Moreover, mutations in the genes for hemochromatosis (HFE), alpha 1-antitrypsin deficiency (SERPINA1), glycogen storage diseases (G6PC, SLC37A4), porphyria (HMBS, UROD), tyrosinemia (FAH) and Wilson’s disease (ATP7B) increase susceptibility to HCC while genome-wide association studies (GWAS) in Asians with HBV and HCV support the evidence of polymorphism related with HCC, in this case was several loci located in HLA region (14)
Regarding the incidence rate of HCC, it is impressive to observe how from 1992 to 2017 the incidence rate has practically doubled (4.5 to 8.7 new cases per 100,000 inhabitants) (15). If we add to the equation, the lack of current criteria for the surveillance of patients at risk for the development of HCC, as well as the poor pharmacological response of current treatments in advanced clinical stages, the discouraging prognosis of HCC in the near future are widely exposed.
On the other hand, the economic burden that HCC demands is not a small thing to consider. The diagnosis and treatment of this disease imply an enormous expense in both medical and economic resources, with variable costs between the different studies reported. In Canada, the 5-year net cost of care of a patient with HCC was reported at $77,509 ($60,410–$94,607) representing a higher expense of this condition compared to other cancers (12,16). In the United Stated, the direct costs per patient per year (PPPY) ranged from $29,354 to $58,529 with median overall costs of up to $176,456 per patient (17), while in Taiwan, the direct costs of HCC were a little lower with an estimated cost PPPY of $ 25,716 (18).
Molecular pathogenesis
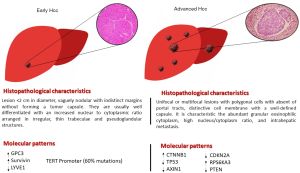
HCC is the result of a series of disturbances at the cellular and molecular level, derived from a chronic liver insult that produces significant damage to liver cells, and completely deregulates cells proliferation mechanisms (19). This in turn begins to trigger the formation of a vaguely nodular lesion with increasingly known histological and molecular patterns, which as the liver insult progresses, begins to spread throughout all segments of the liver architecture until the development of a clinically-advanced cancer (Figure 1) (19,20). HCC is a very heterogeneous tumor. Recent basic research findings have suggested an important role for liver progenitor cells in the development of HCC (21,22). On one side, progenitor cells per se could give a rise to the development of neoplastic cells, and in a similar way, mature hepatocytes could undergo dedifferentiation into progenitor cells and also give a rise to neoplastic cells (23). Although these pathways are still unclear, it has been suggested that liver cancers with stem cell patterns are more aggressive and have a worse prognosis than those without these characteristics (24,25). Irrespective of the involvement or not of progenitor cells, it is clear that dysregulations between liver cells and their microenvironment is a crucial step for the development of neoplastic lesions (26). Approximately, 90% of liver tumors develop under chronic inflammation conditions (23). In this sense, this could explain why immune-related therapies are each time more used as a therapeutic strategy for HCC.
Within this complex relationship between liver cells and their microenvironment, extracellular vesicles (EVs) are worth mentioning their increasingly evident role in cancer immunoregulation. EVs are vital communication pathways that possess cells with the rest of their environment in physiological and pathological situations (27). In HCC, EVs have been associated with local spread through the release of oncogenic micro-RNAs like -584, -517c and -378 (28). Furthermore, EVs could decrease the pharmacological response to immunological therapies with immune checkpoint inhibitors by promoting chemoresistance of neoplastic cells (29). Unfortunately, the great findings made in the basic sciences of HCC have not yet been successfully transferred to clinical practice. The molecular alterations most frequently associated with the development and progression of HCC continue to represent a challenge for its implementation as prognostic biomarkers, and as effective therapeutic targets.
Genomic events
TERT promoter
The most frequent and important mutation identified in patients since early stages of HCC is in the TERT promoter, seen in almost 60% of patients with this condition (30-32). Some studies have also identified this mutation in dysplastic nodules (31). On the other hand, in patients with overt HCC, other gene mutations have been discovered as important biologic modulators of this tumor. HCV infection (and absence of HBV) is associated with mutations in this gene (33-35).
Wnt/ β-catenin pathway
The Wnt/β-catenin pathway regulates stem cells pluripotency and cell fate decision during development. In HCC, CTNNB1-gene mutation has been found on over 40% of liver tumors (36,37). Its upregulation is believed to favor the proliferation of tumor cells in advanced HCC stages (38). Mutations in this gene are associated with young age and moderately/poorly differentiated HCV-related HCC (34,35,39). Otherwise, AXIN1 serves as a negative regulator of the Wnt/β-catenin pathway and its downregulation in HCC specimens has been found in almost 20% of cases (36,38).
TP53 pathway
Mutations in TP53 signaling are more associated with HBV infection and absence of HCV (35). On the other hand, TP53 mutation is also related to diet since certain toxins present in a variety of food, such as aflatoxins are involvement in the development of HCC due to epoxidation and DNA adduction (40). These mycotoxins are produced by Aspergillus fungi and contaminate grains and cereals, especially in endemic areas of HBV like Southeast Asia. In fact, there is a kind of synergism between HBV infection and aflatoxins exposure (41,42). Moreover, chronic dietary exposure to aflatoxin B1 is capable to induce TP53 mutation at codon 249 of that gene by adduction of AGG to AGT, Arginine to Serine (R249S mutation) (43).
TP53 signaling downregulation is a characteristic commonly found in most cancers and in HCC is not the exception. In liver tumors, TP53 mutation is associated with worse prognosis and poor clinical outcomes due to a downregulation of immune response (44). Also, the TP53 signaling is an essential modulator of angiogenesis thought the regulation of the vascular endothelial growth factor (VEGF-A) (38). Mutations in TP53 signaling are more associated with HBV infection and absence of HCV (35).
Other pathways
The CDKN2A, also called P16, is another key tumor suppressor gene that suppresses the activity of the oncogenes CDK4 and CDK6, and its mutation is seen in a variable proportion of patients with overt HCC of both HBV and HCV etiologies (45). Likewise, the ribosomal protein S6 kinase (RPS6KA3) plays a crucial regulation in the MAPK/ERK and mTOR signaling. Mutations in this gene are associated with an altered cell growth and differentiation (38). Finally, the PTEN is a tumor suppressor gene involved in antagonizing the PI3K-AKT-mTOR pathway. Its downregulation is found in half of patients with HCC (46).
Metabolic pathways
Metabolic dysfunction plays an important role in the development of HCC, this is important due to the higher prevalence of metabolic syndrome or just metabolic diseases alone, such as diabetes. The case of patients with NAFLD/NASH is particularly interesting because they present patatin-like phospholipase-3 (PNPLA3) I148M sequence variant that is an independent risk factor for HCC. The proinflammatory state of NAFLD/NASH that derived from adipose tissue generate the perfect conditions to develop a primary lesion and DNA, hence, mutations in cell lines (47).
On the other hand, the effects of adipose tissue even in the absence of NAFLD is still important; it seems trough different models of liver metabolic inflammation that intrahepatic CD4 T cells population may be depleted by lipid-mediated mitochondrial dysfunction (48). Regarding to this matter, patient with obesity exhibit a leaky gut that combined with dysbiosis, the translocation of microbiota-associated molecular patterns (MAMPs) and metabolites from gut to the liver induce activation of hepatic stellate cells (HSCs) to form fibrosis and therefore HCC development by inducing a senescence-associated secretory phenotype (SASP) in HSCs (48,49). Another important mechanism related to obesity is lipotoxicity, where lipotoxic free acids and cytokines like chemokine C-C motif ligand (CCL) from adipose tissue activate Kupfer cells (KCs) and consequently HSCs leading to an abnormal production of extracellular matrix (50).
Finally, patients with T2DM suffer from severe forms of metabolic dysfunction because insulin resistance and hyperinsulinemia increase the formation of free fatty acid which leads to activation of adipose tissue and accumulation of reactive oxygen species (ROS) within it. This causes impairment of signaling cascade such as PI-3 kinase, adiponectin, IL, TNF-α, and even more generation of ROS. Finally, those process activate NF-kB and, STATs signaling pathway and HSCs leading to hepatocarcinogenesis (51).
Epigenetic events
Epigenetics is currently an expansive field of study. Micro-RNAs are small non-coding RNA molecules that are involved in RNA silencing and post-transcriptional regulation of gene expression. Their study in cancer has clarified our knowledge in this field and has helped us to develop novel biomarkers and selective drugs specific for each microRNAs profile (26,52). However, we are barely touching the surface of this amazing field of epigenetics, so there is still much work to be done. In HCC, micro-RNA-122 is probably the most studied molecule in this regard. Micro-RNA-122 is a liver-specific micro-RNA that has a key function in the Wnt/β-catenin pathway and its deregulation is believed to confers resistance to pharmacological treatment in patients with HCC (53). Moreover, there are new types of endogenous functional non-coding RNAs (ncRNAs) that have been demonstrated to play important roles in the development of HC. Thus, long non-coding RNAs (lncRNAs) induce liver carcinogenesis by regulating crucial molecular pathways such as the Wnt-Beta Catenin and the STAT3 (51). In addition, circular RNAs (circRNAs) such as circRHOT1 in HCC patients predicted a poor prognosis (54). Pathway analysis suggested that putative target genes of these essential circRNA are involved in HCC-associated signaling pathways, such as Wnt, transforming growth factor beta (TGFβ), and Ras (55).
The studies of both molecules have shown promising results as tools in HCC prognosis. Due to their secretion into body fluids, lncRNAs were proposed also as non-invasive biomarkers in patients with HCC (51).
Unfortunately, the heterogenicity of the few studies carried out, and the inability to differentiate between chronic liver injury and HCC make that it is still not possible to take advantage of the full potential of this biomarker (56,57). Other micro-RNAs studied in HCC are micro-RNA-200a (58), micro-RNA-214 (59), micro-RNA-155 (60), micro-RNA-221 (61), and micro-RNA-21 (62).
Immunological events
During the last decade, a concept known as tumor microenvironment (TME) has become one of the most important models in explaining the progression of cancer. The complex ecosystem of HCC incorporates not only tumor cells, but also nonmalignant cells, vessels, lymphoid organs or lymph nodes, nerves, intercellular components, and metabolites located within the tumor. In fact, about of 30% of HCCs have immune activation. The interplay between all those cells allows to active pathways involved in tumor growth, survival and even metastasis (3,63).
TME in all cancers are mainly composed by immune cells besides cancer cells. In HCC, TME is mainly infiltrated by lymphocytes; each type of t cells, such as regulatory T cell (Treg) and cytotoxic T cells, play a role in tumor invasion and metastasis; even recurrence after resection (48,64).
Moreover, Treg who accumulate mainly in tumoral liver tissues, suppress immune activity against the tumor by inhibition of CD8 T cells perforins and granzymes activity. In addition, CD8 T cells can be inhibited by other immune cells such KCs by phagocytic and cytokine secretion activities, especially by releasing IL-10 and TGFβ (20). In addition to the latter mechanism, KCs suppress CD8 T cells activity by producing programmed death-ligand 1 (PDL-1), which at the same time inhibit cytotoxic T lymphocyte antigen 4 (CTL-4) favoring HCC malignant progression (48,65,66) Finally, when KCs are activate by IL-1 and TNF-α, the produce osteoponin, a protein involved in angiogenesis, fibrogenesis and carcinogenesis (66,67).
Other macrophages populations besides KCs also play a major role in HCC. Tumor cells can attract and activate macrophages through a variety of growth factor and interleukins; thus, they suffer a transformation into activated M2 macrophages. Increased content of these macrophages associates with angiogenesis and metastasis. Further, it is known that tumor growing means a lack of architecture in its structure, this also apply to TME. An abnormal structure and disorganized growth promote hypoxia leading to activation of M2 macrophages with a phenotype for immunosuppression (48,66).
Therefore, an imbalance among immune cells in HCC TME not only induce to tumoral growing but also survival due to inhibition of immune response and by increasing levels in TGF-β and angiogenic factors, providing subtracts for cancer cells.
Risk factors
As it was stated before, HCC is almost two times more frequent in males than females. The latter, among other factors, may be related with sex hormones imbalance. As an example, in 2001 a case control study in Greece reported that men with HCC had higher levels of estradiol and lower levels of testosterone when compared with controls (68). In addition to the previous study, in 2016 another research found that total testosterone levels were significantly decreased in subjects with HCC but there was no relation between HCC and other sex hormones such as progesterone. In fact, they conclude that there was no strong relation between sex hormones and HCC (69). Furthermore, there is controversial evidence in those kind of studies since there is wide contrasting evidence between them. Yet, it is important to notice that sex hormones play a role in HCC carcinogenesis despite the lack of clear relation with a specific hormone. Besides, in vitro studies have shown that dihydrotestosterone (DHT) and testosterone enhance the growth and proliferation of hepatic tumor cell line by the increasing DHT receptors on tumoral cells and surrounding liver tissue (70). On other hand, estrogen receptor, progesterone receptor and androgen receptor which are also involved in cell growth and proliferation are detected in a variable proportion of HCC. Moreover, estrogen induction of microsomally activated catechols by aryl hydrocarbon hydroxylase and estrogen 2-/4-hydroxylase causes excess of free radicals and unrepaired DNA adducts and strand breaks, that produce a mutagenic and irreversible DNA damage (71).
In contrast, there are modifiable risk factors also related with gender. In this matter, metabolic factors like NAFLD/NASH, are the most important because they are becoming an increasingly common cause of HCC worldwide (3). Fox example, type II diabetes mellitus (T2DM) is more common in males than females, obesity is more prevalent in females than males while males are overall more likely than females to have NAFLD/NASH (72).
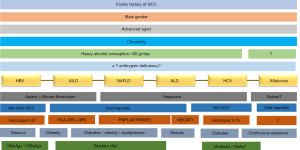
Regarding to ethnicity, Hispanic and Asian race are the two most important ethnic groups associated with HCC development due to higher prevalence viral hepatitis and susceptibility to aggressive metabolic diseases, especially for Hispanics (73-75). The latter explains that undoubtedly, the most important risk factor for HCC development is to count with a preexisting CLD since either metabolic diseases, viral infection or gender may confer a liver lesion that can be progress from steatosis or chronic inflammation to fibrosis and cirrhosis (Figure 2) (19). Cirrhotic patients evolve faster to HCC at a rate of 1% to 4% per year after cirrhosis is established (74,76). The most frequent CLD etiologies that evolve to HCC at a 5-year period are hemochromatosis (21%), HCV cirrhosis (17%), and HBV cirrhosis (10–15%) (74).
HBV
HBV is the leading cause of cirrhosis and HCC worldwide representing at least 50% of all cases of HCC (77). According to the WHO, 257 million people were living with chronic HBV infection in 2015 (78). The main risk factors for the development of HCC in HBV-infected livers are family history of HCC, demographic factors (male gender, advanced age, Asian or African ethnicity), hypertension (79), environmental factors (heavy alcohol consumption, tobacco smoking, exposure to aflatoxins) (80), and viral factors [higher levels of HBV DNA, positivity of HB surface antigen and HB e antigen, chronicity of infection, co-infection with HCV, hepatitis D virus (HDV) and/or human immunodeficiency virus (HIV)] (80). In Asian population, genotype C is more associated with cirrhosis and HCC development, while in East Europe and North America, genotype D is the most frequent in this regard (81). Also, genotype D is associated with HCC in young HBV-infected people without cirrhosis (82).
Hepatitis C virus
HCV is the most frequent etiology of cirrhosis in the Unites States (9). WHO estimates that globally there are 71 million people with chronic HCV infection (78). Active HCV infection has an estimated increased risk for HCC development of 15–20 folds (74). Risk factors for progression to HCC include family history of HCC, demographic factors (male gender, advanced age, Hispanic ethnicity), hypertension, diabetes, obesity, heavy alcohol consumption, and viral factors (genotypes 3 and 1b, chronicity of infection, co-infection with HBV and/or HIV) (83-85). There is still some uncertainty as to whether or not new direct-acting antiviral (DAAs) agents against HCV may be associated with the development of de novo HCC, and with the risk of tumor recurrence. The studies conducted in this regard have shown conflicting results (86-89). A recent meta-analysis supports the position of no association between DAAs and HCC development and recurrence (90).
NAFLD
NAFLD is the hepatic manifestation of metabolic syndrome (91). With the increasing epidemic of overweight/obesity as well as other metabolic diseases like T2DM and hypertension, it is believed that NAFLD will represent the most frequent CLD, and therefore will be the leading cause of cirrhosis and HCC in the near future (92,93). Risk factors for HCC development are family history of HCC, demographic factors (male gender, advanced age, Hispanic ethnicity), obesity, T2DM, hypertension, dyslipidemia, gut dysbiosis, high-fat, high-cholesterol, high-fructose diet (Western diet) and genetic modifiers (PNPLA3 and TM6SF2) (94,95).
Alcohol
Heavy alcohol drinking is an important risk factor for HCC, either by causing direct liver damage by the toxic metabolites derived from ethanol, or by synergizing with viral infections or another CLD, especially when the consumption is above 60 g/day (20,96,97). Compared with non-drinking persons, the pooled relative risk for HCC development in heavy drinking persons (>3 drinks per day) was 1.16 (95% CI, 1.01–1.34) (98).
Autoimmune liver diseases (AILD)
AILD are uncommon causes of HCC. A recent systematic review and meta-analysis found that the pooled incidence rate for HCC in patients with autoimmune hepatitis (AIH) was 3.06 per 1,000 patient-years (P=0.002) and 10.07 per 1,000 patient-years (P=0.015) in AIH cirrhosis (99). The overall recurrence of HCC in AIH is estimated in 5.1% to 6.2% (100). Asian ethnicity, advanced age, male gender, and high alcohol consumption were found to be associated with HCC development, while concomitant primary biliary cholangitis (PBC) was associated with lower HCC risk (83).
In PBC, the incidence of HCC in a Chinese cohort was 4.13% and was considerably higher in men. The significant risk factors found were body mass index ≥25 (P=0.045) and history of alcohol intake (P=0.040) (101).
Aflatoxins
Aflatoxins are metabolites derived from the fungi Aspergillus flavus and Aspergillus parasiticus. They are poisonous carcinogens and mutagens found in soil, decaying vegetation, hay, and grains in tropical and subtropical climates in sub-Saharan Africa, Eastern Asia, and South America. Aflatoxin B1 exposure has been related with an increased risk of HCC acting synergistically with HBV (102). A meta-analysis comprising 5 studies found an increased in the risk of cirrhosis associated with aflatoxins exposure with an adjusted pooled odds ratio of 2.5 (P=0.429) (103). Interestingly, specific mutations in TP53 and in ADGRB1 gene in HCC associated with aflatoxin exposure (104).
Surveillance
HCC surveillance continues to represent a challenge for many health professionals due to the lack of evidence in the adequate selection of patients for their study, the high cost of some imaging studies, and the lack of adequate biomarkers with high diagnostic sensitivity and specificity that are cost-effective for its standardization. To address that problem there are important efforts to identify some of the populations that would benefit from HCC screening to improve surveillance. According to current guidelines of the AASLD, the patient at the highest risk for HCC are Asian male and female with VBH infection, all carriers with VHB or VHC with cirrhosis and/or family history with HCC, genetic hemochromatosis with cirrhosis and patients with alpha-1 antitrypsin deficiency and cirrhosis (105). There is also a recommendation to perform abdominal ultrasound (US) with or without serum alpha-fetoprotein (AFP) every 6 months in cirrhotic Child-Pugh stage A and B patients, and non-cirrhotic HBV patients with moderate to severe risk for HCC. All patients on the waiting list for liver transplantation (including cirrhotic Child-Pugh C) should be screened for HCC (106-108).
Although abdominal US is a cost/effective tool for HCC detection, it has suboptimal performance in men, overweight subjects, Child-Pugh B cirrhosis and ALD cirrhosis, while it was inadequate in Child-Pugh C cirrhosis and NASH cirrhosis (109). In this regard, contrast-enhanced US (CEUS) could be a more reliable study to perform in these patients as it has shown in a recent meta-analysis of 53 studies a pooled sensitivity and specificity to detect HCC of 0.85 (95% CI: 0.84–0.86) and 0.91 (95% CI: 0.90–0.92), respectively. A pooled positive and negative likelihood ratio of 6.28 (95% CI: 4.49–8.77) and 0.16 (95% CI: 0.12–0.22), respectively, a pooled diagnostic odds ratio of 55.01 (95% CI: 35.25–83.47), and an area under the curve of 0.9432 (110). However, reliably studies to assess its utility in patients with suboptimal characteristics for US screening are still lacking. Serological marker AFP has only demonstrated to be reliable when it was combined with US (111). Other biomarkers like des-gamma-carboxyprothrombin, lens culinaris agglutinin-reactive fraction of AFP, glypican-3, etc. still need further evidence to be considered (112). Circulating-free DNA of HCC in plasma is a novel tool for diagnosis and probably for surveillance that could be used in the near future (113).
Diagnosis
All patients under US surveillance in whom a lesion suggestive of malignancy ≥1 cm is found, should be immediately submitted to multiphasic contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) for diagnostic confirmation (106–108). If the lesion is <1 cm it should be monitored closely every 3–6 months for 1–2 years (114). If the lesion remains or disappears, routine surveillance should be done. If the lesion grows or a new lesion develops, CT or MRI must be performed.
The Liver Imaging Reporting and Data System (LI-RADS) emerged as an easy-to-interpret classification system for reporting standardization of images obtained by CT and MRI of patients with suspected HCC diagnosis (114). A systematic review supports LI-RADS as an excellent tool for HCC diagnosis presenting a high discriminatory degree against other liver malignancies (115). In cirrhotic patients with an US liver lesion ≥1 cm submitted to CT or MRI where a solid ≥2 cm lesion with arterial phase non-rim hyperenhancement with late wash-out is found, HCC is confirmed, and no liver biopsy should be done. However, when the lesion does not satisfactorily achieve this criteria, individualized decisions should be done, and liver biopsy could be performed. CEUS could be performed as a second-line option when CT or MRI was inconclusive, and as a prior step before liver biopsy (106-108).
Staging
Since almost all patients with HCC have a pre-existing CLD that determines the clinical condition of the patient depending on the years of evolution and the associated complications, the TNM scale did not adequately satisfy the staging and prognosis of patients with HCC. As a result of this need, different scales began to emerge that not only evaluated the size and tumor invasion, but also evaluated the patient’s liver function and quality of life.
Since the Okuda score proposed in 1985, different staging systems have emerged such as Cancer of the Liver Italian Program (CLIP), Tokyo score, bilirubin-albumin-AFPL3-AFP-DCP (BALAD), Advanced Liver Cancer Prognostic System (ALPCS), Hong Kong Liver Cancer (HKLC), Italian Liver Cancer Study (ITA-LI.CA), etc. (116) Most of these classifications have the disadvantage of having only been validated in their own regions of origin, and only for certain etiologies of HCC. Although there is not an universally fully accepted classification, the Barcelona Clinic Liver Cancer (BCLC) established in 1999 was the first system to recommend evidence-based clinical and surgical treatment for each stage (117). Currently, the BCLC classification is the recommended staging system by the European Association For the Study of The Liver and the American Association for the Study of Liver Diseases for its external validation in multiple clinical trials, and for being a dynamic classification in constant evolution that offers evidence-based first-line and second-line treatment strategies for each class of HCC (106,107).
Treatment
Therapeutic management of HCC is a complex subject to discuss. Ideally, each case should be individualized and evaluated by a multidisciplinary group that includes hepatologists, oncologists, surgeons, and radiologists with experience in this type of tumor. However, the resources available to each institution, the experience of the hospital center, and the patient’s decision must be considered in all cases. Staging systems can help us evaluate which options are the most viable for each patient. In this context there are two important scales, the BCLC staging criteria and Eastern Cooperative Oncology Group (ECOG) Scale of Performance Status have become an important tool in the decision of treatment modality. The second one is a five-point scale on which higher numbers reflect greater disability where stage 0 (BCLC stages 0-B) may be treated with ablation, resection, transplantation, or chemoembolization while patients in stages 1–2 (BCLC stage C) require systemic therapies with sorafenib among other. Finally, those in stages beyond 2 (BCLC stage D) are non-transplantable and benefit from supportive care (3,118,119).
Resection
Resection is considered the therapeutic management of choice in those patients with a solitary tumor ≤3 cm with preserved liver function, and normal portal pressure ≤10 mmHg (BCLC stage 0 or A). Resection even showed greater overall survival in BCLC stage B patients ideal for resection compared to any locoregional treatment, whereas in BCLC stage B not ideal for resection, it was superior to embolization but not to ablation (120). Regarding the type of approach, laparoscopic liver resection has shown a superiority over open liver resection as it is significantly associated with fewer transoperative complications, better successful achievement of R0 resection, wider resection margin, minor days of hospitalization, and lower morbidity and 30-day mortality rates with no differences in tumor recurrence and overall survival (121). Unfortunately, tumor recurrence with resection is high with an estimated 70% recurrence rate at a 5-year period (122).
Ablation
Image-guided percutaneous radiofrequency ablation is considered a good option in the management of early-stage HCC that are not candidate for surgery. Ethanol injection is considered another modality of ablation but has no shown worst results vs. radiofrequency (123). A meta-analysis showed that radiofrequency ablation has the maximum benefit in terms of overall survival and recurrence-free survival (compared with resection) in Child-Pugh A stage, single-nodule tumor <2 cm and AFP <20 ng/mL (124). Other ablation techniques in current study are new-generation microwave ablation and irreversible electroporation. Prospective confident studies that evaluate these techniques are still lacking.
Transarterial embolization therapies
Transarterial chemoembolization (TACE) is the first-line treatment for HCC patients BCLC stage B or as down staging therapy in potential patients for LT. TACE consists on the joint administration of chemotherapeutic agents (doxorubicin or cisplatin and lipiodol), and the subsequent embolization of the arteries that supply blood to the tumor causing an entrapment of cytotoxic agents confined to the neoplastic cells, ischemia and reduction in tumor burden. Transarterial embolization is a similar modality in which the introduction of chemotherapeutic agents is avoided. Both were demonstrated to be superior in overall survival compared with tamoxifen (125) and symptomatic treatment (126) in unresectable HCC patients, however, there is still a lack of consensus about which transarterial modality is the most effective and secure for patients. Although there is still no clear evidence suggesting superiority of TACE vs. TAE (127,128), most authors preferer TACE as the best option for intermediate stage HCC, and it has also been suggested in advanced stage HCC (BCLC stage C) by achieving a longer median overall survival compared with sorafenib (9.2 vs. 7.4 months respectively) (129), but it was found to be less cost effective (130).
In recent years, the introduction of TACE with drug eluting beads (DEB-TACE) was presented as a better modality that would deliver chemotherapeutic agents (mainly doxorubicin) with lower adverse effects. A meta-analysis found that DEB-TACE had higher overall survival and was safer than conventional TACE suggesting that DEB-TACE could be the most adequate option to consider in intermediate stage HCC (131).
Systemic therapies
Nowadays, BCLC staging criteria is one of the most useful system to determinate therapeutic strategies of HCC. Patients in stage C, which means an advance stage with extrahepatic spread o portal invasion but preserved liver functions, are the ones who received systemic therapy. In this case, there are 5 main drugs: sorafenib and lenvatinib in the first line and regorafenib, cabozantinib and ramucirumab in the second line (3,119).
Before the development of sorafenib in 2008, there were no effective systemic treatments that prolonged overall survival in patients with advanced stage HCC. Sorafenib is a multiple kinase inhibitor that acts on different signaling pathways especially on the Raf/MEK/ERK pathway and also acts as an anti-angiogenic agent by inhibiting VEGF-2, platelet-derived growth factor, and c-KIT receptor. Sorafenib achieved a longer median overall survival vs. placebo (10.7 vs. 7.9 months) in the European-American trial (SHARP) (132) and a median overall survival of 6.5 vs. 4.2 months in the placebo arm in the Asian Pacific trial (133). Therefore, sorafenib is considered the first-line treatment in advanced stage HCC or intermediate stage HCC that failed locoregional therapies.
Other treatments, such as linifanib (134), brivanib (135), erlotinib (136), and sunitinib (137), subsequently emerged with the intention of emulating or surpassing the results obtained by sorafenib as first-line treatments, however, none of them showed significant results until the arrival of lenvatinib, another multiple kinase inhibitor, which demonstrated a median overall survival not significantly different vs. sorafenib (13.6 vs. 12.3 months respectively) with similar adverse effects reported in both groups in a non-inferiority clinical trial (138). Therefore, in 2018, lenvatinib obtained by FDA approval as first-line treatment in conjunction with sorafenib for HCC.
For the second-line treatment, regorafenib was tested in HCC patients at Child-Pugh stage A that tolerated sorafenib but still progressed despite treatment. Regorafenib demonstrated an overall survival time of 10.6 vs. 7.8 months in the placebo arm (139) and was approved by the FDA as second-line treatment in 2014. Cabozantinib is another second-line approved treatment that showed a median overall survival time of 10.2 vs. 8.0 months in placebo group in previously treated HCC patients who presented progression (140).
Immune-based therapy is an increasingly notorious reality in the treatment of patients with cancer. Ramucirumab is a VEGF-2 immune checkpoint inhibitor that in a randomized double-blind multicenter phase 3 clinical trial failed to improve overall survival vs. placebo in patients with advanced HCC who failed first-line treatment, however, later it was shown to improve overall survival in patients with advanced HCC and serum AFP levels ≥400 ng/mL (141), being approved as a second-line treatment in HCC patients with AFP ≥400 ng/mL. The programmed cell death-1 (PD-1) immune checkpoint inhibitor nivolumab showed an objective response rate of 15% in the dose-escalation phase and 20% in the dose-expansion phase leading to its approval by the FDA as a second-line treatment (142). The use of nivolumab as first-line treatment compared to sorafenib is currently being evaluated in a randomized multi-center phase III study (NCT02576509) (143). Pembrolizumab is another PD-1 inhibitor that recently failed in its phase III clinical trial by no achieving significant outcomes in median overall survival time vs. placebo [13.9 vs. 10.6 months respectively (P=0.0238)] (144). Recently, the results of the phase 3 clinical trial of atezolizumab plus bevacizumab vs. sorafenib in unresectable HCC patients who had not previously received systemic treatment were recently published. Remarkably, overall survival at one year was 67.2% with atezolizumab-bevacizumab and 54.6% with sorafenib, with a median progression-free survival time of 6.8 and 4.3 months, respectively (145). These findings suggest that atezolizumab-bevacizumab could be considered in coming years as the first-line treatment in unresectable HCC patients.
Prevention strategies and future directions
As we have seen, despite the great advances in diagnosis and in the different therapeutic modalities that exist for the management of HCC, future projections indicate a growing wave of new cases of HCC, which translates into an increase in mortality from this type of cancer. For this reason, reinforcing the strategies that have been shown to be effective in reducing the number of factors associated with this disease is of vital importance.
Reducing the incidence of viral hepatitis by 90% and mortality by 65% by 2030 is an astonishing goal that the WHO has set as part of the Global Health Sector Strategy on Viral Hepatitis 2016–2021 (146). Within this strategy, it is proposed to make a universal coverage of the HBV vaccine, continuing medical education in the populations at greatest risk though a sustainable and financeable program specific for each region where it is applied. Regarding ALD, although alcohol abstinence is the main goal to be achieved in patients with harmful alcohol consumption, in clinical practice it is difficult to achieve this goal, as it represents a radical change in the lifestyle of patients leading to a poor rate of success in many cases. Recent studies have demonstrated that by achieving a reduction of one or two levels in the WHO drinking risk levels is enough to improve physical health and quality of life of patients, and predict a lower likelihood of liver disease (147-150). Therefore, if we motivate patients to achieve a slight and gradual reduction in their alcohol consumption until they reach to an acceptable point of consumption, could represent a more effective strategy that allows reducing the burden of harmful alcohol consumption and all their direct and indirect consequences. Finally, since NAFLD is becoming the all-leading CLD worldwide, better strategies to combat metabolic diseases should be done. Recently, we discuss the political, economic and social strategies they were carrying out in Europe against NAFLD and how they could be extrapolated to a global context (151). Basically, limitation of fast food and sweetened beverages advertising, nutrition labelling, implementation of lifestyle changes and nutritional education programs in schools are the most important things to implement, and hopefully this could reduce the metabolic diseases burden that we are currently experiencing today.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Digestive Medicine Research for the series “Current Status and Future Expectations in the Management of Gastrointestinal Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-116
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-116). The series “Current Status and Future Expectations in the Management of Gastrointestinal Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. NMS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer. Liver Cancer 2020. Available online: http://gco.iarc.fr/ [last accessed on 24 December 2020].
- Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol 2020;72:250-61. [Crossref] [PubMed]
- Villanueva A. Hepatocelular carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Wallace MC, Preen D, Jeffrey GP, et al. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol 2015;9:765-79. [Crossref] [PubMed]
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019;70:151-71. [Crossref] [PubMed]
- Sayiner M, Golabi P, Younossi ZM. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig Dis Sci 2019;64:910-7. [Crossref] [PubMed]
- Singh SP, Panigrahi S, Mishra D, et al. Alcohol-associated liver disease, not hepatitis B, is the major cause of cirrhosis in Asia. J Hepatol 2019;70:1031-2. [Crossref] [PubMed]
- Shah AS, Amarapurkar DN. Natural History of Cirrhosis of Liver after First Decompensation: A Prospective Study in India. J Clin Exp Hepatol 2018;8:50-7. [Crossref] [PubMed]
- Paik JM, Golabi P, Younossi Y, et al. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020;72:1605-16. [Crossref] [PubMed]
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547-55. [Crossref] [PubMed]
- Méndez-Sánchez N, Zamarripa-Dorsey F, Panduro A, et al. Current trends of liver cirrhosis in Mexico: Similitudes and differences with other world regions. World J Clin Cases 2018;6:922-30. [Crossref] [PubMed]
- Paik JM, Golabi P, Biswas R, et al. Nonalcoholic Fatty Liver Disease and Alcoholic Liver Disease are Major Drivers of Liver Mortality in the United States. Hepatol Commun 2020;4:890-903. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogénesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021;73:4-13. [Crossref] [PubMed]
- National Cancer Institute. Cancer of the Liver and Intrahepatic Bile Duct - Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/livibd.html [last accessed on 20 July 2020].
- Thein H-H, Isaranuwatchai W, Campitelli MA, et al. Health care costs associated with hepatocellular carcinoma: a population-based study. Hepatology 2013;58:1375-84. [Crossref] [PubMed]
- Kohn CG, Singh P, Korytowsky B, et al. Humanistic and economic burden of hepatocellular carcinoma: systematic literature review. Am J Manag Care 2019;25:SP61-73. [PubMed]
- Nguang SH, Wu CK, Liang CM, et al. Treatment and Cost of Hepatocellular Carcinoma: A Population-Based Cohort Study in Taiwan. Int J Environ Res Public Health 2018;15:2655. [Crossref] [PubMed]
- Motola-Kuba D, Zamora-Valdés D, Uribe M, et al. Hepatocellular carcinoma. An overview. Ann Hepatol 2006;5:16-24. [Crossref] [PubMed]
- Méndez-Sánchez N, Valencia-Rodriguez A, Vera-Barajas A, et al. The mechanism of dysbiosis in alcoholic liver disease leading to liver cancer. Hepatoma Res 2020;6:5. [PubMed]
- Chen Y, Wong PP, Sjeklocha L, et al. Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology 2012;55:563-74. [Crossref] [PubMed]
- Tarlow BD, Pelz C, Naugler WE, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 2014;15:605-18. [Crossref] [PubMed]
- Sia D, Villanueva A, Friedman SL, et al. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017;152:745-61. [Crossref] [PubMed]
- Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med 2006;12:410-6. [Crossref] [PubMed]
- Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 2006;25:3818-22. [Crossref] [PubMed]
- Méndez-Sánchez N, Valencia-Rodríguez A, Coronel-Castillo C, et al. The cellular pathways of liver fibrosis in non-alcoholic steatohepatitis. Ann Transl Med 2020;8:400. [Crossref] [PubMed]
- Yang J, Li C, Zhang L, et al. Extracellular Vesicles as Carriers of Non-coding RNAs in Liver Diseases. Front Pharmacol 2018;9:415. [Crossref] [PubMed]
- Kogure T, Lin WL, Yan IK, et al. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 2011;54:1237-48. [Crossref] [PubMed]
- Lv LH, Wan YL, Lin Y, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012;287:15874-85. [Crossref] [PubMed]
- Nault JC, Villanueva A. Intratumor molecular and phenotypic diversity in hepatocellular carcinoma. Clin Cancer Res 2015;21:1786-8. [Crossref] [PubMed]
- Nault JC, Calderaro J, Di Tommaso L, et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 2014;60:1983-92. [Crossref] [PubMed]
- Kwa WT, Effendi K, Yamazaki K, et al. Telomerase reverse transcriptase (TERT) promoter mutation correlated with intratumoral heterogeneity in hepatocellular carcinoma. Pathol Int 2020;70:624-32. [PubMed]
- Chen YL, Jeng YM, Chang CN, et al. TERT promoter mutation in resectable hepatocellular carcinomas: a strong association with hepatitis C infection and absence of hepatitis B infection. Int J Surg 2014;12:659-65. [Crossref] [PubMed]
- Pezzuto F, Izzo F, Buonaguro L, et al. Tumor specific mutations in TERT promoter and CTNNB1 gene in hepatitis B and hepatitis C related hepatocellular carcinoma. Oncotarget 2016;7:54253-62. [Crossref] [PubMed]
- Kawai-Kitahata F, Asahina Y, Tanaka S, et al. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J Gastroenterol 2016;51:473-86. [Crossref] [PubMed]
- Takai A, Dang HT, Wang XW. Identification of drivers from cancer genome diversity in hepatocellular carcinoma. Int J Mol Sci 2014;15:11142-60. [Crossref] [PubMed]
- Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet 2012;44:694-8. [Crossref] [PubMed]
- Khemlina G, Ikeda S, Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer 2017;16:149. [Crossref] [PubMed]
- Tornesello ML, Buonaguro L, Tatangelo F, et al. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics 2013;102:74-83. [Crossref] [PubMed]
- Rushing BR, Selim MI. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol 2019;124:81-100. [Crossref] [PubMed]
- McCullough AK, Lloyd RS. Mechanisms underlying aflatoxin-associated mutagenesis - Implications in carcinogenesis. DNA Repair (Amst) 2019;77:76-86. [Crossref] [PubMed]
- Ferreira RG, Cardoso MV, de Souza Furtado KM, et al. Epigenetic alterations caused by aflatoxin b1: a public health risk in the induction of hepatocellular carcinoma. Transl Res 2019;204:51-71. [Crossref] [PubMed]
- Villar S, Ortiz-Cuaran S, Abedi-Ardekani B, et al. Aflatoxin-Induced TP53 R249S Mutation in HepatoCellular Carcinoma in Thailand: Association with Tumors Developing in the Absence of Liver Cirrhosis. PLoS One 2012;7:e37707 [Crossref] [PubMed]
- Long J, Wang A, Bai Y, et al. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine 2019;42:363-74. [Crossref] [PubMed]
- Zhou Y, Wang XB, Qiu XP, et al. CDKN2A promoter methylation and hepatocellular carcinoma risk: A meta-analysis. Clin Res Hepatol Gastroenterol 2018;42:529-41. [Crossref] [PubMed]
- Subbiah IM, Falchook GS, Kaseb AO, et al. Exploring response signals and targets in aggressive unresectable hepatocellular carcinoma: an analysis of targeted therapy phase 1 trials. Oncotarget 2015;6:28453-62. [Crossref] [PubMed]
- Kanda T, Goto T, Hirotsu Y, et al. Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma. Int J Mol Sci 2020;21:1525. [Crossref] [PubMed]
- Hou J, Zhang H, Sun B, et al. The immunobiology of hepatocellular carcinoma in humans and mice: Basic concepts and therapeutic implications. J Hepatol 2020;72:167-82. [Crossref] [PubMed]
- Schwabe RF, Greten TF. Gut microbiome in HCC - Mechanisms, diagnosis and therapy. J Hepatol 2020;72:230-8. [Crossref] [PubMed]
- Mendez-Sanchez N, Cruz-Ramon VC, Ramirez-Perez OL, et al. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int J Mol Sci 2018;19:2034. [Crossref] [PubMed]
- Gramantieri L, Baglioni M, Fornari F, et al. LncRNAs as novel players in hepatocellular carcinoma recurrence. Oncotarget 2018;9:35085-99. [Crossref] [PubMed]
- Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatr Res 2007;61:24R-29R. [Crossref] [PubMed]
- Fu X, Calin GA. miR-122 and hepatocellular carcinoma: from molecular biology to therapeutics. EBioMedicine 2018;37:17-8. [Crossref] [PubMed]
- Wang L, Long H, Zheng Q, et al. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol Cancer 2019;18:119. [Crossref] [PubMed]
- Qiu L, Wang T, Ge Q, et al. Circular RNA Signature in Hepatocellular Carcinoma. J Cancer 2019;10:3361-72. [Crossref] [PubMed]
- Klingenberg M, Matsuda A, Diederichs S, et al. Non-coding RNA in hepatocellular carcinoma: Mechanisms, biomarkers and therapeutic targets. J Hepatol 2017;67:603-18. [Crossref] [PubMed]
- Xu J, Wu C, Che X, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 2011;50:136-42. [Crossref] [PubMed]
- Xiao F, Zhang W, Zhou L, et al. microRNA-200a is an independent prognostic factor of hepatocellular carcinoma and induces cell cycle arrest by targeting CDK6. Oncol Rep 2013;30:2203-10. [Crossref] [PubMed]
- Wang J, Li J, Wang X, et al. Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun 2013;439:47-53. [Crossref] [PubMed]
- Han ZB, Chen HY, Fan JW, et al. Up-regulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantation. J Cancer Res Clin Oncol 2012;138:153-61. [Crossref] [PubMed]
- Li J, Wang Y, Yu W, et al. Expression of serum miR-221 in human hepatocellular carcinoma and its prognostic significance. Biochem Biophys Res Commun 2011;406:70-3. [Crossref] [PubMed]
- Tomimaru Y, Eguchi H, Nagano H, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol 2012;56:167-75. [Crossref] [PubMed]
- Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther 2020;5:166. [Crossref] [PubMed]
- Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol 2015;7:2080-90. [Crossref] [PubMed]
- Polidoro MA, Mikulak J, Cazzetta V, et al. Tumor microenvironments in primary liver tumors: A challenging role of natural killer cells. World J Gastroenterol 2020;26:4900-18. [Crossref] [PubMed]
- Novikova MV, Khromova NV, Kopnin PB. Components of the Hepatocellular Carcinoma Microenvironment and Their Role in Tumor Progression. Biochemistry (Mosc) 2017;82:861-73. [Crossref] [PubMed]
- Wen Y, Jeong S, Xia Q. Role of Osteopontin in Liver Diseases. Int J Biol Sci 2016;12:1121-8. [Crossref] [PubMed]
- Kuper H, Mantzoros C, Lagiou P, et al. Estrogens, testosterone and sex hormone binding globulin in relation to liver cancer in men. Oncology 2001;60:355-60. [Crossref] [PubMed]
- El Mahdy Korah T, Abd Elfatah Badr E, Abd Elfatah Badr E, et al. Relation between sex hormones and hepatocellular carcinoma. Andrologia 2016;48:948-55. [Crossref] [PubMed]
- De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol 2002;193:59-63. [Crossref] [PubMed]
- Di Maio M, Daniele B, Pignata S, et al. Is human hepatocellular carcinoma a hormone-responsive tumor? World J Gastroenterol 2008;14:1682-9. [Crossref] [PubMed]
- Wu EM, Wong LL, Hernandez BY, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res 2018;4:66. [Crossref] [PubMed]
- Pomenti S, Gandle C, Abu Sbeih H, et al. Hepatocellular Carcinoma in Hispanic Patients: Trends and Outcomes in a Large United States Cohort. Hepatol Commun 2020;4:1708-16. [Crossref] [PubMed]
- Kim HS, El-Serag HB. The Epidemiology of Hepatocellular Carcinoma in the USA. Curr Gastroenterol Rep 2019;21:17. [Crossref] [PubMed]
- El-Serag HB, Lau M, Eschbach K, et al. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med 2007;167:1983-9. [Crossref] [PubMed]
- Bartolomeo N, Trerotoli P, Serio G. Progression of liver cirrhosis to HCC: an application of hidden Markov model. BMC Med Res Methodol 2011;11:38. [Crossref] [PubMed]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030-44. [Crossref] [PubMed]
- World Health Organization. Global hepatitis report, 2017. Available online: https://www.who.int/publications-detail-redirect/global-hepatitis-report-2017 [last accessed on 22 July 2020].
- Xiang X, You XM, Zhong JH, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol 2017;67:885-6. [Crossref] [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-1273.e1. [Crossref] [PubMed]
- Kao JH, Chen PJ, Lai MY, et al. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology 2003;124:327-34. [Crossref] [PubMed]
- Ni YH, Chang MH, Wang KJ, et al. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology 2004;127:1733-8. [Crossref] [PubMed]
- Mittal S, El-Serag HB. Epidemiology of HCC: Consider the Population. J Clin Gastroenterol 2013;47:S2-6. [Crossref] [PubMed]
- Raimondi S, Bruno S, Mondelli MU, et al. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol 2009;50:1142-54. [Crossref] [PubMed]
- Kanwal F, Kramer JR, Ilyas J, et al. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology 2014;60:98-105. [Crossref] [PubMed]
- Tahata Y, Sakamori R, Urabe A, et al. Clinical outcomes of direct-acting antiviral treatments for patients with hepatitis C after hepatocellular carcinoma are equivalent to interferon treatment. Hepatol Res 2020;50:1118-27. [Crossref] [PubMed]
- Singal AG, Rich NE, Mehta N, et al. Direct-Acting Antiviral Therapy Not Associated with Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019;156:1683-1692.e1. [Crossref] [PubMed]
- Mashiba T, Joko K, Kurosaki M, et al. Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS One 2018;13:e0194704 [Crossref] [PubMed]
- Chan PPY, Levy MT, Shackel N, et al. Hepatocellular carcinoma incidence post direct-acting antivirals in hepatitis C-related advanced fibrosis/cirrhosis patients in Australia. Hepatobiliary Pancreat Dis Int 2020;19:541-6. [Crossref] [PubMed]
- Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67:1204-12. [Crossref] [PubMed]
- Almeda-Valdes P, Aguilar-Olivos N, Uribe M, et al. Common features of the metabolic syndrome and nonalcoholic fatty liver disease. Rev Recent Clin Trials 2014;9:148-58. [Crossref] [PubMed]
- Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896-904. [Crossref] [PubMed]
- Méndez-Sánchez N, Villa AR, Chávez-Tapia NC, et al. Trends in liver disease prevalence in Mexico from 2005 to 2050 through mortality data. Ann Hepatol 2005;4:52-5. [Crossref] [PubMed]
- Benhammou JN, Lin J, Hussain SK, et al. Emerging risk factors for nonalcoholic fatty liver disease associated hepatocellular carcinoma. Hepatoma Res 2020;6:35. [PubMed]
- Anstee QM, Reeves HL, Kotsiliti E, et al. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 2019;16:411-28. [Crossref] [PubMed]
- Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323-31. [Crossref] [PubMed]
- Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol 2005;3:1150-9. [Crossref] [PubMed]
- Turati F, Galeone C, Rota M, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol 2014;25:1526-35. [Crossref] [PubMed]
- Tansel A, Katz LH, El-Serag HB, et al. Incidence and Determinants of Hepatocellular Carcinoma in Autoimmune Hepatitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2017;15:1207-1217.e4. [Crossref] [PubMed]
- Valean S, Acalovschi M, Dumitrascu DL, et al. Hepatocellular carcinoma in patients with autoimmune hepatitis - a systematic review of the literature published between 1989-2016. Med Pharm Rep 2019;92:99-105. [Crossref] [PubMed]
- Zhang XX, Wang LF, Jin L, et al. Primary biliary cirrhosis-associated hepatocellular carcinoma in Chinese patients: incidence and risk factors. World J Gastroenterol 2015;21:3554-63. [Crossref] [PubMed]
- Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis 2013;22:305-10. [PubMed]
- Mekuria AN, Routledge MN, Gong YY, et al. Aflatoxins as a risk factor for liver cirrhosis: a systematic review and meta-analysis. BMC Pharmacol Toxicol 2020;21:39. [Crossref] [PubMed]
- Zhang W, He H, Zang M, et al. Genetic Features of Aflatoxin-Associated Hepatocellular Carcinoma. Gastroenterology 2017;153:249-262.e2. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- European Association for The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169-77. [Crossref] [PubMed]
- Zhang J, Yu Y, Li Y, et al. Diagnostic value of contrast-enhanced ultrasound in hepatocellular carcinoma: a meta-analysis with evidence from 1998 to 2016. Oncotarget 2017;8:75418-26. [Crossref] [PubMed]
- Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706-1718.e1. [Crossref] [PubMed]
- Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019;157:54-64. [Crossref] [PubMed]
- Labgaa I, Villacorta-Martin C, D’Avola D, et al. A pilot study of ultra-deep targeted sequencing of plasma DNA identifies driver mutations in hepatocellular carcinoma. Oncogene 2018;37:3740-52. [Crossref] [PubMed]
- Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056-65. [Crossref] [PubMed]
- Tang A, Bashir MR, Corwin MT, et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018;286:29-48. [Crossref] [PubMed]
- Karademir S. Staging of hepatocellular carcinoma. Hepatoma Res 2018;4:58. [Crossref]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Hsu CY, Lee YH, Hsia CY, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 2013;57:112-9. [Crossref] [PubMed]
- Doycheva I, Thuluvath PJ. Systemic Therapy for Advanced Hepatocellular Carcinoma: An Update of a Rapidly Evolving Field. J Clin Exp Hepatol 2019;9:588-96. [Crossref] [PubMed]
- Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015;62:440-51. [Crossref] [PubMed]
- Sotiropoulos GC, Prodromidou A, Kostakis ID, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg 2017;69:291-311. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of Hepatocellular Cancer After Resection: Patterns, Treatments, and Prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Shiina S, Sato K, Tateishi R, et al. Percutaneous Ablation for Hepatocellular Carcinoma: Comparison of Various Ablation Techniques and Surgery. Can J Gastroenterol Hepatol 2018;2018:4756147 [Crossref] [PubMed]
- Casadei Gardini A, Marisi G, Canale M, et al. Radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of overall survival and recurrence-free survival. Onco Targets Ther 2018;11:6555-67. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [Crossref] [PubMed]
- Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998;27:1578-83. [Crossref] [PubMed]
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [Crossref] [PubMed]
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 2007;30:6-25. [Crossref] [PubMed]
- Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology 2012;263:590-9. [Crossref] [PubMed]
- Chen S, Peng Z, Wei M, et al. Sorafenib versus Transarterial chemoembolization for advanced-stage hepatocellular carcinoma: a cost-effectiveness analysis. BMC Cancer 2018;18:392. [Crossref] [PubMed]
- Zou JH, Zhang L, Ren ZG, et al. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis 2016;17:510-7. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol 2015;33:172-9. [Crossref] [PubMed]
- Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-24. [Crossref] [PubMed]
- Zhu AX, Rosmorduc O, Evans TRJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:559-66. [Crossref] [PubMed]
- Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013;31:4067-75. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-96. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Clinical Trials. An Investigational Immuno-therapy Study of Nivolumab Compared to Sorafenib as a First Treatment in Patients with Advanced Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02576509 [last accessed on 28 July 2020].
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193-202. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Available online: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ [last accessed on 26 July 2020].
- Witkiewitz K, Kranzler HR, Hallgren KA, et al. Drinking Risk Level Reductions Associated with Improvements in Physical Health and Quality of Life Among Individuals with Alcohol Use Disorder. Alcohol Clin Exp Res 2018;42:2453-65. [Crossref] [PubMed]
- Knox J, Wall M, Witkiewitz K, et al. Reduction in Nonabstinent WHO Drinking Risk Levels and Change in Risk for Liver Disease and Positive AUDIT-C Scores: Prospective 3-Year Follow-Up Results in the U.S. General Population. Alcohol Clin Exp Res 2018;42:2256-65. [Crossref] [PubMed]
- Witkiewitz K, Falk DE, Litten RZ, et al. Maintenance of World Health Organization Risk Drinking Level Reductions and Posttreatment Functioning Following a Large Alcohol Use Disorder Clinical Trial. Alcohol Clin Exp Res 2019;43:979-87. [Crossref] [PubMed]
- Witkiewitz K, Hallgren KA, Kranzler HR, et al. Clinical Validation of Reduced Alcohol Consumption After Treatment for Alcohol Dependence Using the World Health Organization Risk Drinking Levels. Alcohol Clin Exp Res 2017;41:179-86. [Crossref] [PubMed]
- Méndez-Sánchez N, Valencia-Rodríguez A. Caveats for the implementation of global strategies against non-alcoholic fatty liver disease. J Hepatol 2020;73:220. [Crossref] [PubMed]
Cite this article as: Méndez-Sánchez N, Valencia-Rodríguez A, Coronel-Castillo CE, Qi X. Narrative review of hepatocellular carcinoma: from molecular bases to therapeutic approach. Dig Med Res 2021;4:15.


