
Expanding living donor liver transplantation in the Western world: changing the paradigm
Introduction
Initially conceived as a deceased donor operation, liver transplantation (LT) has been necessarily restricted by the availability of donor grafts. The lack of access to donor grafts resulting from extremely low deceased donor rates remains prevalent in Asia; however, even in Western countries where organ donation is culturally accepted and even promoted, the supply of donor grafts has not proportionally increased to adequately meet increasing demand. At the end of 2019, over 12,000 patients in the United States were active on the national liver transplant waiting list, however only 8,372 deceased donor liver transplants (DDLT) were performed that year (1) leaving one-third of actively listed patients waiting >1 year for an available donor graft. Current deceased donor rates have also failed to meet the demands for LT in Canada and Europe where the widening donor organ supply-demand gap in both regions have led to an estimated 20-25% waitlist mortality over the past decade (2,3).
By expanding the donor pool, LDLT has reduced the risk of waitlist mortality and broadened patient eligibility for LT beyond the limitations of a MELD-dependent graft allocation system. In addition, the elective nature of LDLT provides a timing advantage—allowing patients to undergo surgery after preoperative medical optimization but prior to becoming critically ill from liver failure.
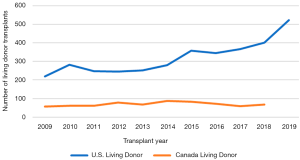
Despite these advantages and their association with improved outcomes compared to DDLT (4-8), the expansion of LDLT in Western countries has not been nearly as robust as that in Asia, comprising only 4.5% of total LT performed in the United States and 15.6% of transplants in Canada in 2019 (1,2). Despite increases in experience, trends in living donor transplant cases in the U.S. and Canada in the last decade have remained stable with time (Figures 1,2). Aggregate reported data on number of LDLTs performed by multiple national LT registries from 2010–2014 have also demonstrated similar trends across Europe ranging from as low as <1% of total LT in French transplant centers to around 8% in Germany (9). Conversely, LDLT constituted approximately 90% of LT activity between 1995-2005 in Asian transplant centers in South Korea, Taiwan, Japan, and Hong Kong becoming the predominant form of LT in this region soon after its implementation (10,11).
In light of numerous and recent single-center, multi-center, and national analyses supporting overall superior survival outcomes after LDLT compared to DDLT, the remaining major barrier to wider utilization of LDLT outside of Asia is the risk of perioperative recipient, but more importantly, donor morbidity and mortality in the setting of a technically demanding and complex procedure. As efforts in North American and Europe to further maximize deceased donor organ supply have plateaued, it increasingly becomes imperative to address these obstacles and allow for LDLT to play a much greater role in the accepted treatment of liver disease. This review will summarize the current status of LDLT in North America and identify the strategies, resources, and infrastructures necessary to change the paradigm towards a more durable and integrated application of LDLT in the West.
Current status and recipient outcomes from North American LDLT experiences
The findings from the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) established by the National Institutes of Health in 2002 were critical in shifting Western perceptions of LDLT and its role in addressing the risk of waitlist mortality. As the first prospective multi-center study evaluating LDLT donor and recipient outcomes, this consortium of nine U.S. experienced adult LDLT transplant centers demonstrated a 56% lower mortality risk in LDLT patients when compared with a matched cohort of patients waiting for DDLT (12). It additionally demonstrated a significant and sustained unadjusted 10-year survival benefit of LDLT compared to DDLT (70% vs. 64%) (13). These findings of superior survival outcomes with LDLT were consistent when compared with non-A2ALL transplant centers (14) and have since been reliably reproduced in other large single-center studies (4-8). Most recently, the largest single center LDLT experience in the United States at the University of Pittsburgh Medical Center compared outcomes of 245 adult LDLT with DDLT performed from 2009–2019 demonstrating a survival advantage at 3-year post-transplant (86% vs. 80%, P=0.03) (15). These analyses clearly illustrate how LDLT implementation within the context of the current liver allocation system intrinsically improves survival probability—even when overall graft survival rates are comparable to DDLT—through effective waitlist mortality reduction.
Significantly higher risk of biliary and vascular complications after LDLT attributed to the caliber and size difference of the bile ducts and vessels was reported by the A2ALL consortium and earlier single-center series (16). However, interestingly, the risk of hepatic artery/portal vein thrombosis and biliary stricture was not statistically different between LDLT and DDLT in the University of Pittsburgh cohort at 3-year follow-up. Although the series did re-demonstrate a higher incidence of biliary leak after LDLT compared to DDLT (11.8% vs. 7.1%, P=0.03), this occurrence was lower than the 26% reported by A2ALL (17).
The A2ALL consortium and non-A2ALL U.S. transplant centers concurrently determined that center LDLT experience and volume were essential to achieving improved outcomes. Early center experience (≤20 LDLT cases) was associated with higher risk of graft failure [HR 1.83 (1.2–2.8) P=0.005] and conversely, increased survival was demonstrated at higher case numbers (>30) (18). Given these conclusions, the lower technical complication rates reported by the University of Pittsburgh LDLT series compared to its predecessors may be attributed to the center’s exceptionally high LDLT rates (87.5 per 100-person years in 2018) and implementation of surgical modifications to the procedure, such as routine graft venoplasty for outflow reconstruction and more frequent use of hepaticojejunostomy for biliary reconstruction, to decrease recipient complications (15). These data attest to the technical demands and challenges of the procedure but also demonstrate that increased center experience can overcome differences in surgical complications between LDLT and DDLT after the learning curve has been passed.
Subgroup analyses evaluating outcomes for special and expanded criteria LT populations from multiple U.S. transplant centers have demonstrated comparable, if not improved, patient and graft survival in LDLT compared to DDLT. A2ALL follow-up studies performed within HCV-infected cohorts found similar overall 3- and 5-year patient and graft survival between LDLT and DDLT with appropriate center experience (>20 LDLT cases) (19,20). Early A2ALL post-transplant analyses on mortality and recurrence in HCC patients reported comparable overall survival but significantly higher 3-year recurrence rates (29% vs. 0%, P=0.02) in patients who underwent LDLT compared to DDLT raising concern that shorter time to transplant leads to the inclusion of HCC patients with more aggressive tumor biology who otherwise may have eventually been dropped from DDLT consideration for disease progression (21). In contrast, more recently, the University of Toronto reported their intention-to-treat analysis in a larger prospective study of 219 HCC patients listed for LDLT and 632 listed for DDLT and demonstrated a survival benefit with a 33% mortality risk reduction in the LDLT due to shorter waiting period and lower dropout rates (22). As-treated analysis of post-transplant survival outcomes after LDLT compared to DDLT were again found to be comparable but did not detect differences in HCC recurrence between the two groups, contrary to earlier reports. With increasing LDLT experience, several North American centers have begun to expand LDLT eligibility to higher-risk recipients including high MELD (>25) patients, retransplants, acute liver failure, and tumors outside of accepted DDLT criteria (15,23-25). The University of Pittsburgh demonstrated in their series similar outcomes in these populations after LDLT compared to DDLT and even improved survival in the elderly with LDLT (15).
Finally, cost and resource utilization analyses at LDLT centers have shown conflicting data on inpatient hospital costs associated with LDLT however, the overall cost benefit to the healthcare system resulting from improved recipient survival and post-transplant outcomes is clearly evident across all studies. Cost analysis of A2ALL data found higher hospitalization rates and increased inpatient costs associated with management of living donor evaluation and post-surgical care, however these differences were not significant at experienced transplant centers (26). In contrast, analyses by the University of Toronto and Pittsburgh have demonstrated that, in facilitating transplantation at an earlier stage in liver disease progression, LDLT promotes faster post-operative recovery and shorter hospital stays in transplanted cirrhotics leading to decreased hospital costs and better resource utilization (14,27).
Overall, the current literature to date—including expanded data from more recent large single-center LDLT studies such as the University of Pittsburgh and the University of Toronto, overwhelmingly supports the safety and applicability of LDLT expansion at North American centers based on recipient and graft outcomes when compared to DDLT. However, these studies have also demonstrated that quality of LDLT recipient and graft outcomes directly correlate with transplant center experience and technical expertise. These factors are even more important in the consideration of donor-related outcomes. This perceived demand for zero to near-zero patient post-LT mortality in addition to comfortability with a well-established—although limited—DDLT program is hindering the wider utilization of a proven superior option and it is imperative to pursue effective strategies to overcome this barrier.
Optimizing donor selection and outcomes in LDLT
Ensuring donor safety is the foremost priority of any live donor program which begins with careful and methodical donor evaluation, particular in the setting of LDLT given the operative risks of a major hepatic resection. All experienced LDLT transplant programs are currently equipped with standard operating live donor evaluation protocols that ensure a thorough assessment prior to organ donation. These generally involve (I) informed and complete consent to donation (II) pre-operative medical and surgical evaluation to ensure that live donors are healthy individuals with minimal, if any, comorbidities (III) imaging studies to assess for adequate graft to recipient weight ratio (GRWR) and remnant volumes, in addition to anatomic suitability, including presence of vascular and biliary structure variations (28,29). Routine pre-operative laboratory and psychological screenings required for DDLT are also performed.
While donor selection must be strict to minimize their perioperative morbidity and mortality, it is important to not evaluate donor suitability in a vacuum—rather, in the context of (I) the LDLT center experience/technical expertise and (II) recipient needs and compatibility. Older age, high BMI/obesity, and borderline GRWR, were all historically major restrictions to LDLT due to theoretical concern for risk of increased donor complications and poorer outcomes overall however, experienced North American centers are increasingly reporting on the safety and comparable outcomes using live donors from these higher-risk populations as their technical expertise and comfort improves (30-32). High BMI, for example, is no longer considered a contraindication for living donation at many U.S. LDLT centers but rather an indication for further testing, particularly to detect possible non-alcoholic fatty liver disease (NAFLD) and over 15% of live donors in the U.S. were reported to have BMI ≥30 (33).
Social and public education factors are also important considerations in expansion of the live donor organ pool and, consequently, the LDLT program size and volume in North America. The University of Toronto has harnessed strong social altruism and support in their community to integrate anonymous donation in their LDLT program and have performed 21 anonymous adult LDLTs to date (26). This center, in addition to the University of Pittsburgh, has also increasingly utilized the media and public educational programs to increase live donor organ awareness (14,26). Because of its underutilization and potential for misinformation, it is important that potential live donors be informed about the actual risks and benefits of LDLT and given full autonomy to make the best decision for them.
Optimal donor selection not only relies on having a large live donor organ pool composed of healthy individuals, but also requires consideration of the type of donor graft most fitted for a recipient’s transplant needs. Here, striking the delicate balance between minimizing surgical donor risk and maximizing recipient graft outcomes is paramount. At minimum, the donor graft volume needs to be large enough to meet the functional demands of the recipient—generally defined by GRWR >0.8—in order to avoid small-for-size syndrome. Because of this, right-lobe (RL) live donor grafts have historically been preferred to ensure adequate graft function and survival however, left-lobe (LL) grafts have been associated with fewer surgical complications. Successful utilization of LL grafts to minimize donor risk is appealing but for many years, the tradeoff between avoiding donor risk and avoiding graft insufficiency was highly debated. A recent series at UCSF comparing five-year outcomes between RL and LL grafts in LDLT demonstrated that appropriate use of LL grafts does not impact long-term patient or graft survival, however more frequently required graft portal flow modification compared to RL grafts (34). These findings justified the shift towards LL graft use to further reduce donor morbidity however, fine discretion by the surgeon in assessing the correct graft to use for each recipient is still needed, for example selecting RL grafts for high MELD cases.
Finally, the evolution of minimally invasive liver surgery and its application to LDLT for live donor hepatectomies has served, in some centers, as another potential strategy to mitigate donor surgical risk. The first laparoscopic hepatectomy for use in pediatric LDLT was documented in 2002 and since then, several single-center series—mostly in Asia where LDLT predominates—have reported the feasibility of purely laparoscopic or hybrid laparoscopically-assisted donor hepatectomies (35-38). As more LDLT centers have begun pursuing laparoscopic approaches to live donor surgery, an international multi-institutional study from both Western and Eastern centers evaluating its safety and efficacy demonstrated comparable donor outcomes to open standard donor hepatectomies (39). Despite the potential for faster post-operative recovery and added appeal to potential live donors, the steep learning curve of this procedure compounded with the experience required for standard open LDLT poses a significant hurdle to LDLT growth already hindered by concern over donor outcomes.
Optimizing the LDLT framework to improve implementation and growth
In addition to minimization of donor and recipient risk, the success and growth of LDLT in Western regions requires widespread changes to existing infrastructures and policies in order to optimize LDLT care on a systems-level basis. This first entails setting up an effective multi-disciplinary team and clinic for thorough and expeditious donor evaluation to be able to schedule surgeries in a timely manner. The LDLT program established at the University of Toronto well exemplifies this model and is headed by a dedicated liver transplant surgeon, a full-time medical director, and a full-time nursing coordinator—all supported by administrative assistants forming donor and recipient teams closely coordinating together (28). Other major LDLT programs, such as the one established at the University of Pittsburgh, are fully incorporated into the center’s overall LT program but also rely on a multi-disciplinary approach to evaluation for LDLT with involvement from specialized transplant hepatologists, nutritionists, social workers, and behavioral health professionals, in addition to surgeon input (40).
Secondly, adopting an “LDLT-first” approach during initial LT evaluation by offering LDLT as the first and best option for most liver disease with low MELD scores will contribute to tackling waitlist mortality in addition to expanding LDLT experience. Achieving this requires a vigorous outreach and education campaign on the safety and benefits of living liver donation to overcome current patient reluctance to participate as potential donors, as well as reticence to offer LDLT by referring healthcare providers. The University of Toronto utilized an outcomes data-driven education campaign and a professional commitment to a “donor safety first” approach to instill confidence in their LDLT program amongst their referral base (28). Starting in 2018, the University of Pittsburgh has invested in a primarily digital marketing campaign for LDLT as a broader outreach strategy to gain awareness of living liver donation (41). Even with optimized infrastructures in place at established transplant centers to execute a high volume of LDLTs, these outreach and education efforts are critical to shift the paradigm of liver disease management back toward LDLT in North America.
Third, centralization of LDLT to experienced high-volume centers equipped with specialized personnel and resources is necessary to accumulate the experience required to ensure superior patient outcomes. Vigorous transplant training programs must integrate training on LDLT surgical techniques and medical management, in particular, live donor and minimally invasive procedures, in order to develop LDLT expertise early on in the next generation of LT surgeons.
Conclusion
Increased utilization of LDLT in the West first requires increased acceptance of LDLT. Several published donor deaths in the United States dealt a devastating blow to the growth of LDLT in the early 2000s and only in the past 1–2 years has total LDLT volume in the U.S. recovered to levels when this procedure peaked. Since then, donor mortality—although not zero—has remained low between 0.2–0.4% and reported donor complication rates typically ranging between 20–40% (15). Despite continuing minimization of donor complications, prevailing intolerance of non-zero donor mortality and perceptions of inflated donor risk have contributed to the underutilization of LDLT. Overcoming these barriers requires, not avoidance of LDLT—a procedure with numerous advantages and a potential solution to the critical organ shortage—but rather constructing a framework that optimizes donor and recipient variables for improved outcomes and minimized risks. At the heart of it, LT as a field needs to change to optimize conditions for both DDLT and LDLT in tandem to work towards the goal of zero waitlist mortality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Drs. Giuliano Testa, Greg McKenna, and Johanna Bayer) for the series “Living Donor Liver Transplantation” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-87). The series “Living Donor Liver Transplantation” was commissioned by the editorial office without any funding or sponsorship. Dr. Tran reports grants from National Institutes of Health, grants from Burroughs Wellcome Fund, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 2004 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Department of Health and Human Serves, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA. Available online: http://optn.transplant.hrsa.gov/data/view-data-reports/national-data. May 2020.
- Canadian Institute for Health Information. Treatment of End-State Organ Failure in Canada, Canadian Organ Replacement Register, 2009-2019: Extra-renal Transplants-Data Tables. Ottawa, ON: CIHI, 2019.
- Goldaracena N, Barbas S. Living donor liver transplantation. Curr Opin Organ Transplant 2019;24:131-7. [Crossref] [PubMed]
- Brown RS, Russo BW, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med 2003;348:818-25. [Crossref] [PubMed]
- Abt PL, Mange KC, Olthoff KM, et al. Allograft survival following adult-to-adult living donor liver transplantation. Am J Transplant 2004;4:1302-7. [Crossref] [PubMed]
- Maluf DG, Stravitz RT, Cotterell AH, et al. Adult living donor versus deceased donor liver transplantation, a 6-year single center experience. Am J Transplant 2005;5:149-56. [Crossref] [PubMed]
- Pomposelli JJ, Verbesey J, Simpson MA, et al. Improved survival after live donor adult liver transplantation (LDALT) using right lobe grafts: program experience and lessons learned. Am J Transplant 2006;6:589-98. [Crossref] [PubMed]
- Shah SA, Levy GA, Greig PD, et al. Reduced mortality with right-lobe living donor compared to deceased-donor liver transplantation when analyzed from the time of listing. Am J Transplant 2007;7:998-1002. [Crossref] [PubMed]
- Nadalin S, Capobianco I, Fabrizio P, et al. Living donor liver transplantation in Europe. Hepatobiliary Surg Nutr 2016;5:159-75. [PubMed]
- Lee SG. Living-donor transplantation in adults. British Medical Bulletin 2010;94:33-48. [Crossref] [PubMed]
- Chen CL, Kabiling CS, Concejero AM. Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol 2013;10:746-51. [Crossref] [PubMed]
- Berg CL, Gillespie BW, Merion RM, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology 2007;133:1806-13. [Crossref] [PubMed]
- Olthoff KM, Smith AR, Abecassis M, et al. Defining long-term outcomes with living donor liver transplantation in North America. Ann Surg 2015;262:465-75. [Crossref] [PubMed]
- Olthoff KM, Abecassis MM, Emond JC, et al. Outcomes of adult living donor liver transplantation: comparison of the Adult-to-adult Living Donor Liver Transplantation Cohort Study and the national experience. Liver Transpl 2011;17:789-97. [Crossref] [PubMed]
- Humar A, Ganesh S, Jorgensen D, et al. Adult living donor versus deceased donor liver transplant (LDLT Versus DDLT) at a Single Center: Time to Change our Paradigm for Liver Transplant. Ann Surg 2019;270:444-51. [Crossref] [PubMed]
- Abu-Gazala S, Olthoff K. Status of Adult Living Donor Liver Transplantation in the United States: Results from the A2ALL Cohort Study. Gastroenterol Clin North Am 2018;47:297-311. [Crossref] [PubMed]
- Samstein B, Smith AR, Freise CE, et al. Complications and Their Resolution in Recipients of Deceased and Living Donor Liver Transplants: Findings from the A2ALL Cohort Study. Am J Transplant 2016;16:594-602. [Crossref] [PubMed]
- Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg 2005;242:314-23. [PubMed]
- Terrault NA, Shiffman ML, Lok AS, et al. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transpl 2007;13:122-9. [Crossref] [PubMed]
- Terrault NA, Stravitz RT, Lok AS, et al. Hepatitis C disease severity in living versus deceased donor liver transplant recipients: an extended observation study. Hepatology 2014;59:1311-9. [Crossref] [PubMed]
- Fisher RA, Kulikb LM, Freise CE, et al. Hepatocellular Carcinoma Recurrence and Death Following Living and Deceased Donor Liver Transplantation. Am J Transplant 2007;7:1601-8. [Crossref] [PubMed]
- Goldaracena N, Gorgen A, Doyle A, et al. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J Hepatol 2019;70:666-73. [Crossref] [PubMed]
- Feng S. Living donor liver transplantation in high Model for End-Stage Liver Disease score patients. Liver Transpl 2017;23:S9-S21. [Crossref] [PubMed]
- Urrunaga NH, Rachakonda VP, Magder LS, et al. Outcomes of living versus deceased donor liver transplantation for acute liver failure in the United States. Transplant Proc 2014;46:219-24. [Crossref] [PubMed]
- Mendizabal M, Silva MO. Liver transplantation in acute liver failure: A challenging scenario. World J Gastroenterol 2016;22:1523-31. [Crossref] [PubMed]
- Merion RM, Shearon TH, Berg CL, et al. Hospitalization Rates Before and After Adult-to-Adult Living Donor or Deceased Donor Liver Transplantation. Ann Surg 2010;251:542-9. [Crossref] [PubMed]
- Barbas AS, Goldaracena N, Dib MJ, et al. Early intervention with live donor liver transplantation reduces resource utilization in NASH: the Toronto experience. Transplant Direct 2017;3:e158. [Crossref] [PubMed]
- Levy GA, Selzner N, Grant DR. Fostering liver living liver donor transplantation. Curr Opin Organ Transplant 2016;21:224-30. [Crossref] [PubMed]
- Abu-Gazala S, Olthoff K. Current Status of Living Donor Liver Transplantation in the United States. Annu Rev Med 2019;70:225-38. [Crossref] [PubMed]
- Trotter JF, Wisniewski KA, Terrault NA, et al. Outcomes of donor evaluation in adult-to-adult living donor liver transplantation. Hepatology 2007;46:1476-84. [Crossref] [PubMed]
- Lo CM. Evaluating the living donor: expansion by innovation. Hepatol Int 2016;10:242-4. [Crossref] [PubMed]
- Goldaracena N, Sapisochin G, Spetzler V, et al. Live Donor Liver Transplantation With Older (≥50 Years) Versus Younger (<50 Years) Donors. Ann Surg 2016;263:979-985. [Crossref] [PubMed]
- Yamamoto K, Takada Y, Fujimoto Y, et al. Nonalcoholic steatohepatitis in donors for living donor liver transplantation. Transplantation 2007;83:257-62. [Crossref] [PubMed]
- Braun HJ, Dodge JL, Roll GR, et al. Impact of Graft Selection on Donor and Recipient Outcomes Following Living Donor Liver Transplantation. Transplantation 2016;100:1244-50. [Crossref] [PubMed]
- Baker TB, Jay CL, Ladner DP, et al. Laparoscopy-assisted and open living donor right hepatectomy: a comparative study of outcomes. Surgery 2009;146:817-23. [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Hasegawa Y, et al. Minimally invasive donor hepatectomy: evolution form hybrid to pure laparoscopic technique. Ann Surg. 2015;261:e3-e4. [Crossref] [PubMed]
- Suh KS, Yi NJ, Kim T, et al. Laparoscopy-assisted donor right hepatectomy using a hand port system preserving the middle hepatic vein branches. World J Surg 2009;33:526-33. [Crossref] [PubMed]
- Samstein B, Cerqui D, Rotellar F, et al. Totally laparoscopic full left hepatectomy for calculated small-for-size LDLT in adults: proof of concept. Am J Transplant 2013;13:2462-6. [Crossref] [PubMed]
- Soubrane O, Eguchi S, Uemoto S, et al. Minimally Invasive Donor Hepatectomy for Adult Living Donor Liver Transplantation: an International, Multi-Institutional Evaluation of Safety, Efficacy, and Early Outcomes. Ann Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- UPMC Transplant Services: Liver Transplant Information and Process. 2020. Available online: https://www.upmc.com/services/transplant/liver/process
- Park A. UPMC's campaign for living-donor liver transplants resulted in 20,000 inquiries in its 1st year — 2 marketing leaders explain how. 2019 Nov 20. Available online: https://www.beckershospitalreview.com/digital-marketing/upmc-s-campaign-for-living-donor-liver-transplants-resulted-in-20-000-inquiries-in-its-1st-year-2-marketing-leaders-explain-how.html.
Cite this article as: Tran L, Humar A. Expanding living donor liver transplantation in the Western world: changing the paradigm. Dig Med Res 2020;3:52.



