
A case of Castleman disease and literature review
Introduction
Castleman disease (CD) is also known as angiofollicular lymphadenopathy, giant lymphadenopathy and Benjamin CD. It was first reported and named by Benjamin Castleman, director of Pathology Department of Massachusetts General Hospital in 1956 (1). At present, no clear cause has been found, but it may be related to the following factors: virus infection, immune deficiency and abnormal immune regulation, abnormal cytokine secretion. All lymph nodes can be involved, most of which are in the mediastinum (2), which accounts for about 60%. The second is the retroperitoneum, neck and armpit. According to the statistics of, the incidence rate of male and female is not significantly different. Foreign data show that women are higher than men. It can be divided into two types, unicentral Castleman disease (UCD) and multicentric castle disease (MCD). The therapeutic effect of CD is closely related to clinical classification. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/dmr-20-49) (3).
Case presentation
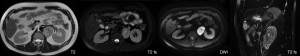
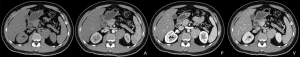
A 37-year-old male was admitted to our hospital for “abdominal mass for one month”. The patient came to our hospital for diagnosis and treatment due to occasional abdominal dull pain one month ago without any other discomfort. No positive physical signs were found in physical examination. MR showed a mass before the left psoas muscle, neurogenic tumor was considered, and the tumor is close to the upper part of the psoas muscle and the left diaphragm (Figure 1). The patient had no basic diseases, no smoking and alcohol and other bad habits. After admission, the related examination was carried out. The blood routine showed that the white blood cell count was 4.91×109/L, hemoglobin was 148 g/L, monocyte percentage was 8.5%, lymphocyte percentage was 19.6%. The liver and kidney functions were normal, AFP, CA199, CA125, CEA were normal. The CT scan showed a neurogenic tumor near the left psoas muscle could be considered. Retroperitoneal lymph nodes were enlarged (Figure 2). On January 10, 2020, the tumor was resected. During the operation, a tumor was found, ranging from the anterior upper part of the left retroperitoneal psoas major muscle, the foot of the left diaphragm to the bifurcation of the iliac artery, with a maximum of 5.1 cm × 4.3 cm × 1.5 cm, with a capsule, abundant blood vessels on the surface, unclear boundary, close to the left side of the spine and the inferior vena cava. Postoperative pathology showed that the lymph node membrane was intact, the lymphoid follicles proliferated, the germinal center atrophied, the blood vessels between the follicles obviously proliferated with hyaline degeneration of the tube wall, and the lymphoid nuclei between the local follicles slightly increased. The results of immunohistochemistry showed that CD10+, Ki-67 proliferation index increased, CD3+, CD20+, CD23+, CD10 and bcl-6 showed atrophic germinal center, BCL-2 germinal center (−), Ki-67 (germinal center about 80%+). Combined with the morphology and immunohistochemistry results, the lesions were consistent with CD, Hyaline Vascular type (Figure 3). The patient was followed-up by CT three months later, no recurrence was showed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
CD is a heterogeneous disease, the cause is not clear at present. According to recent researches, it may be closely related to the following factors: (I) virus infection: Chang (4) first found human herpesvirus-8 (HHV-8) and HIV, and analyzed the process of HHV-8 and HIV infection leading to CD in detail. Later Reddy (5) found HHV-8 and HIV plays an important role in the pathogenesis of CD. At the same time, antiviral treatment can alleviate the disease of some patients, which also supports that virus infection is related to CD. However, the evidence about virus infection is mostly found in MCD, while the report about virus infection in UCD is less. (II) Immune deficiency and abnormal immune regulation: some studies have shown that HHV-8 infection is almost 100% in the MCDs related to acquired immune deficiency syndrome, while 40% in the MCDs unrelated to acquired immune deficiency syndrome, indicating the role of immune deficiency in the disease. In addition, CD can show autoimmune hemocytopenia. RF, Coombs test and ANA (+) suggest that CD may be related to the body immune mechanism. (III) Cytokines: it has been confirmed that the level of IL-6 in serum and involved lymph nodes of patients with MCD is increased. Yoshizaki (6) treated 7 patients with MCD with human recombinant Anti-IL-6 receptor antibody, the systemic symptoms were improved. After the treatment, the number and size of lymph follicles in the involved lymph nodes were significantly reduced, indicating that lymph node follicular hyperplasia was directly related to IL-6.
According to the clinical characteristics, it can be divided into two types (7): (I) UCD: the disease manifested as a single lymph node or a single extranodal disease, including multiple lymph nodes involved in a single area (oligocentric form). (II) MCD: according to whether HHV-8 is infected or not, MCD can be further divided into HHV-8 positive MCD, which is common in HIV infected patients or immunosuppressed population, and HHV-8 negative MCD, also known as idiopathic MCD (iMCD). According to the pathological characteristics, it can be divided into three types: Hyaline Vascular type (HV-CD), Plasma Cell type (PC-CD) and mixed type (mix CD). In UCD, HV-CD is the most common type, while PC-CD is the most common type in MCD. Under the hyaline vascular type, lymphofollicular hyperplasia in lymph nodes is seen. The vascular hyperplasia in the center of the follicle is accompanied by hyaline degeneration of the blood vessel wall, the heart becomes smaller, and the follicular dendritic cells with vesicular nucleus are “burned” like, and the outer layer of small lymphocytes are arranged in concentric circles like “onion skin”; the plasma cell type is characterized by hyaline degeneration of capillaries and “onion skin” like changes in the follicles, which are not obvious, and the deposition of pink and amorphous eosinophils can be seen between the filtering bubbles, and a large number of mature plasma cells diffuse and proliferate Immunohistochemical staining showed that CD35 and CD21 were expressed in the follicular dendritic cells in the germinal center, polyclonal in the lymphocytes, and low expression in Ki-67 index.
Clinically, 47–81% of the patients with UCD, the most common pathological type is HV type (76–91%), the median age of onset is 30–40 years old, the most frequently involved mediastinal lymph nodes (60–75%), mainly manifested as single site lymphadenopathy, more than 90% of the patients have no systemic symptoms; 19–53% of the patients with MCD, PC type MCD is the most common, accounting for 77%, the onset age is later (median age 50–60 years old). Usually, it is characterized by multiple lymphadenopathy and multiple organ involvement. The patients have long-term fever, night sweat, general weakness, emaciation, hemocytopenia (such as anemia and thrombocytopenia), elevated serum IL-6, hyperimmunoglobulinemia, hepatosplenomegaly, etc. (8). In clinic, the diagnosis of UCD is mostly after operation, but the diagnosis of MCD is difficult. It is necessary to exclude autoimmune diseases, infectious diseases and tumor diseases. Pathological diagnosis is the gold standard of CD diagnosis. FNA alone or core needle biopsy is not suitable as the basis of CD initial diagnosis, and it is necessary to obtain enough samples to combine the necessary immunophenotype.
The therapeutic effect of CD is closely related to clinical classification, in which UCD is mainly treated by surgery, and the involved lymph nodes need to be removed during the operation, and the prognosis is good after the tumor is completely removed (9). For the cases that are not suitable for complete resection, comprehensive treatment such as preoperative chemotherapy, radiotherapy, interventional embolization can be performed (10), so that the tumor can be reduced and then operated again. In recent years, there are also reports that rituximab, hormone and cyclophosphamide are effective in the treatment of unresectable UCD (11). The 3-year and 5-year disease-free survival rates of patients with UCD are 90% and 81% respectively (12), and postoperative recurrence cases are mostly seen in incomplete resection and neglect of involved lymph nodes cases (13). However, there is no “standard” treatment plan for MCD before 2018. In 2017, relevant consensus and diagnostic standards were published for the first time in the world. The etiology of HHV8+ MCD is clear and there are clear and effective treatment methods (14), while the proportion of iMCD in patients with MCD is 33–58%, and the proportion of iMCD in Chinese population is higher, which can occur in any age group with unknown etiology and poor prognosis. Some studies have reported its 5-year survival rate between 55–77%, it is a hot spot and the most rapid development field in recent years. Following the proposal of the diagnosis standard of iMCD in 2017, the first international consensus on iMCD treatment was released in 2018 in view of the lack of treatment guidelines and the disorder of diagnosis and treatment, and was updated in 2019, pushing the diagnosis and treatment in this field to a new height.
To sum up, this paper reported a case of surgical treatment of localized CD, shared surgical experience, and summarized the clinical characteristics of the disease by consulting relevant literature and consensus of guidelines, so as to provide useful experience for clinicians.
Acknowledgments
Funding: This work was supported by the Science and Technology Planning Project of Guangdong Province, China (2017A020215014) and the Medical Scientific Research Foundation of Guangdong Province, China (A2017274).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/dmr-20-49
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr-20-49). YH serves as the Editor-in-Chief of Digestive Medicine Research. CZ serves as an unpaid Associate Editor-in-Chief of Digestive Medicine Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer 1956;9:822-30. [Crossref] [PubMed]
- Nadir A, Colak N, Koktener A, et al. Isolated intrapulmonary Castleman's disease: a case report, review of the literature. Ann Thorac Cardiovasc Surg 2014;20:689-91. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994;266:1865-9. [Crossref] [PubMed]
- Reddy D, Mitsuyasu R. HIV-associated multicentric Castleman disease. Curr Opin Oncol 2011;23:475-81. [Crossref] [PubMed]
- Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood 1989;74:1360-7. [Crossref] [PubMed]
- Frizzera G. Castleman's disease and related disorders. Semin Diagn Pathol 1988;5:346-64. [PubMed]
- Dispenzieri A, Armitage JO, Loe MJ, et al. The clinical spectrum of Castleman's disease. Am J Hematol 2012;87:997-1002. [Crossref] [PubMed]
- Talarico F, Negri L, Iusco D, et al. Unicentric Castleman's disease in peripancreatic tissue: case report and review of the literature. G Chir 2008;29:141-4. [PubMed]
- Bora U, Ferrat D, Omer S, et al. Role of the radiotherapy in the management of recurrent Castleman’s disease. Gulhane Tip Dergi 2014;56:54-6. [Crossref]
- Estephan FF, Elghetany MT, Berry M, et al. Complete remission with anti-CD20 therapy for unicentric, non-HIV-associated, hyaline-vascular type, Castleman's disease. Cancer Invest 2005;23:191. [Crossref] [PubMed]
- Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman's disease: a systematic review of 404 published cases. Ann Surg 2012;255:677-84. [Crossref] [PubMed]
- Bowne WB, Lewis JJ, Filippa DA, et al. The management of unicentric and multicentric Castleman's disease: a report of 16 cases and a review of the literature. Cancer 1999;85:706-17. [Crossref] [PubMed]
- Uldrick TS, Polizzotto MN, Aleman K, et al. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood 2014;124:3544-52. [Crossref] [PubMed]
Cite this article as: Jiang J, Hao T, Chen J, Yang S, Li J, Huang C, Li M, Wu W, Zhang C, He Y. A case of Castleman disease and literature review. Dig Med Res 2020;3:79.



