Effect of early oral feeding on gastrointestinal motility in patients undergoing colorectal resection: a meta-analysis of randomized clinical trials
Introduction
Colorectal cancer (CRC) has been a serious threat to human health and high mortality rate in the world (1). Surgery is currently the first-line treatment, however CRC surgery is a relatively big trauma to patients. There are still two major issues for postoperative treatment: how to improve the gastrointestinal (GI) function while improving the wound healing, and how to offer enough and efficient nutrition to patients to improve recovery from surgery. The commonly supportive treatment is nutrition feeding after the resumption of bowel sounds and first flatus or defecation. However, the time of feeding and the feeding path remains controversial. The conventional oral feeding (COF) is gradually introduced following the first bowel sounds and flatus or defecation. This strategy is mainly based on the concern that early oral feeding (EOF) may increase anastomotic leakage and prolonged postoperative ileus (2,3). However, this practice has been challenged by findings from several GI physiologic studies stating that the optimal nutritional status and maintenance of GI function significantly contribute to wound healing (4,5). These findings show that EOF will lead to postoperative ileus as a paralysis of the entire bowel with the complete absence of any functional contractile activity is misleading. Postoperative ileus is usually transient and clinically not obvious. In actuality, oral feeding within 24 hours after colorectal surgery is tolerated, and the feed is absorbed (6-9). Although EOF might be a promising one, whether early or later oral feeding benefit patients still remains unsure. Enteral nutrition includes oral and enteral tubes. Feeding through enteral tubes could lead to various complications, including aspiration pneumonia, discomfort, tube occlusion, tube malposition, and epistaxis, which may affect the recovery of postoperative GI function. Although it is a general opinion that oral feeding is superior to feeding via other paths, the benefits and adverse effects of oral feeding has not been fully evaluated. There are increasingly more reports of randomized controlled trials (RCTs) on nutritional support after CRC surgery. There are previous meta-analyses combined studies of oral feeding and transcatheter feeding or upper GI track operation. The current meta-analysis focuses on the benefits and adverse effects of EOF and COF on the recovery of GI function.
Methods
The present meta-analysis was carried out using a protocol designed according to the Cochrane Handbook recommendations; it was performed in consistency with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (10).
Data sources and search strategy
We searched PubMed, the Cochrane Database of Systematic Reviews (Cochrane Reviews), and EMBASE for appropriate RCTs published between January 1966 and October 2017. The full PubMed search strategy is presented as follows: ((early OR immediate) AND (oral OR enteral) AND (feed OR nutrition OR diet)) AND ((Colorect, rect, sigmoid, bowel, intestine, Colorectal Neoplasms, Neoplasms, Colorectal, or Neoplasm, Colorectal or Cancers, Colorectal)) AND ((Resection OR surgery OR operation OR Laparotomy OR laparoscopy or laparoscope)) AND ((Colonic Diseases OR surgery)) AND ((Gastrointestinal Motilities or Motilities, Gastrointestinal or Motility, Gastrointestinal or Intestinal Motility or Intestinal Motilities or Motilities, Intestinal or Motility, Intestinal)) AND ((randomized controlled trial or random or human)). The search strategy was designed to identify RCTs of EOF therapy compared to the COF in patients that underwent colorectal surgery. There were no language restrictions. References of retrieved studies and identified reviews were manually searched.
Study selection
Titles and abstracts of records obtained from searches were first independently examined by two well trained researchers (Z Jiang and QC Chen). Following the initial abstract assessment, the full-text of all identified studies was acquired. Eligible studies were RCTs that met the following criteria: (I) population: patients underwent elective CRC surgery; (II) experimental intervention: administration of EOF program, without tube feeding; (III) comparison intervention: conventional (traditional) oral feeding; and (IV) outcomes: primary outcomes include time to first bowel sounds, first flatus, defecation, and length of hospital stay. Secondary outcomes include nausea and vomiting and nasogastric tube reinsertion and complications (wound infection, pneumonia, anastomotic dehiscence and postoperative total complications). We defined EOF as any oral caloric intake commencing within 24 h postoperatively. COF was defined as withholding oral intake until passage of flatus or bowel movement or longer than 24 h postoperatively. To be included, a study should have reported at least one of the relevant outcome measures listed above. Studies were excluded according to the following exclusion criteria: tube feeding, emergency surgery, parenteral nutrition, use of immune-enhancing feeding products fast-track programs, including other interventions that might influence postoperative GI motility except EOF, inability to identify whether feeding was given within 24 h, or no data available for meta-analysis. Two investigators (Z Jiang and QC Chen) independently assessed studies for inclusion, and any disagreements between the two reviewers were resolved by consensus or the involvement of another author (LX Cao).
Data extraction and study characteristics
Two authors (Z Jiang and QC Chen) independently extracted data from studies and entered them into a predefined database. Any discrepancies were identified and resolved through discussion with a third author (LX Cao) if necessary. Extracted information from each eligible study included: (I) study bibliographic information including the name of the first author, year of publication, and number of patients in each group; (II) patient information including age, gender; (III) EOF detailed protocol and control interventions; and (IV) outcome measurements. The outcomes were time to first bowel sounds, flatus, defecation, length of hospital stays and all complications. If data on the postoperative outcomes were absent or incomplete, the corresponding author of the study was contacted to request for the missing data. The data extraction followed the intention-to-treat basis whenever possible.
Assessment of quality of the included studies
Two authors (Z Jiang and QC Chen) independently assessed the methodological quality of the included studies by using the Cochrane approach (11,12). Each trial was judged for low, unclear, or high risk of bias. The quality of the evidence for each outcome was evaluated with the grading of recommendations assessment, development, and evaluation (GRADE) (11-13). Disagreement was resolved through consensus and deep discussion.
Data synthesis and analysis
Treatment effects for dichotomous outcomes were measured with relative risk (RR), and for continuous outcome measures were measured with weighted mean difference (WMD). Pooled estimates were presented with 95% CI. Heterogeneity was explored by the Cochran Q statistic and characterized with I2. A fixed-effect model was used for meta-analysis in the absence of significant heterogeneity, defined as a P value >0.10 and I2 <50%. In case of significant heterogeneity, we employed the random-effects model; the exception was if few trials dominated the available evidence or if small-trial bias was statistically significant (14,15). For all other comparisons, a P value <0.05 was considered statistically significant, and all tests were two-sided. Data analysis was performed with Review Manager (RevMan, Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014).
Results
Study characteristics
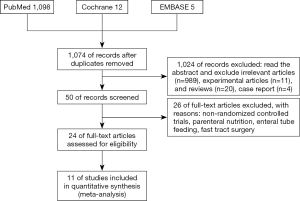
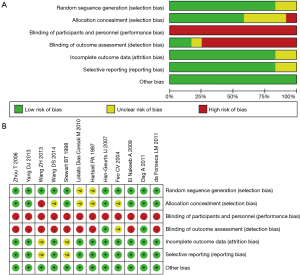
The initial search of electronic databases identified 1,074 published studies (Figure 1). After applying the study exclusion criteria, 11 RCTs (1,216 patients) (16-26) were included in the analysis; there was a total of 611 patients in the EOF group and 605 in the COF group. The main characteristics of the 11 trials are shown in Tables 1,2. The results of risk of bias are shown in Figure 2. Eight of the 11 included studies detailed the methods of randomization used (17-22,25,26), and two provided inadequate information on randomization methods (23,24). Six studies described the blinding methods used: blinding numbers and blinding of the study assessors (17,18,20,21,25,26). We categorized two studies as of unclear risk bias based on the assessment of reporting bias (19,25). All of the included studies had a high risk of performance bias because the experimental and control interventions could not be blindly implemented.
Table 1
| Author | Data | Sample size | Age | Randomization | Main outcomes | Type of surgery | |||
|---|---|---|---|---|---|---|---|---|---|
| EOF | COF | EOF | COF | ||||||
| Wang D (16) | 2014 | 43 | 45 | 60.4±6.5 | 60.3±4.9 | Random number table | Duration of postoperative fever; interval to flatus; hospital stay; medical cost; complication morbidity | LS | |
| Zhou T (17) | 2006 | 161 | 155 | 55.3±16.7 | 57.1±19.8 | Random number table | Time to first flatus; defecation; hospital stay; complication | OS | |
| da Fonseca LM (18) | 2011 | 24 | 26 | 57.4±16.3 | 51.7±13.3 | Patients were randomly assigned to groups by a computer program | Time to first flatus; defecation; hospital stay; tolerance of solid diet; complication | LS | |
| Wang ZH (19) | 2013 | 24 | 24 | 56.3±11.5 | 54.7±12.5 | Random number table | Time to first flatus; hospital stay; complication; hospital cost; complication | LS | |
| Dag A (20) | 2011 | 99 | 100 | 62±12.33 | 61±15.82 | A computer-generated list immediately after surgery by an independent computer consultant | Time to first flatus; hospital stay; complication; intestinal movements; time to defecation | LS | |
| EI Nakeeb A (21) | 2009 | 60 | 60 | 52.3±12.5 | 56.3±11.6 | Randomization was achieved using sealed envelopes | Hospital stay; complications; times to first passage of flatus; time to first stool; vomiting | OS | |
| Feo CV (22) | 2004 | 50 | 50 | 67.6±10.2 | 67.6±10.4 | Patients were assigned by means of a computerized randomization list | Bowel movement; hospital stay; nausea and vomiting; complications | OS | |
| Hartsell PA (23) | 1997 | 29 | 29 | N/A | N/A | Patients were prospectively randomized | Hospital stay; complications; nausea and vomiting | OS | |
| Lobato Dias Consoil M (24) | 2010 | 14 | 15 | 47.4±16.7 | 54.5±10.1 | Patients were randomized to either traditional care or the early fed group | hospital stay; complications; first flatus | LS & OS | |
| Stewart BT (25) | 1998 | 40 | 40 | N/A | N/A | Pre-operatively the patients were randomly assigned according to a computer number generator to one of two groups | Passed flatus; bowel sounds; hospital stay; complications; rate of vomiting | OS | |
| Han-Geurts IJ (26) | 2007 | 67 | 61 | N/A | N/A | Randomization was according to a computer- generated list with blocked sequences, and was carried out by telephone call in the operating theatre before surgery | Passed flatus; bowel sounds; hospital stay; complications; rate of vomiting; first defecation; time to reinsertion of gastric tube | OS | |
N/A, not available; LS, laparoscopy; OS, open surgery.
Table 2
| Trial | Protocol | Duration of observation |
|---|---|---|
| Wang D (16) | Water POD 1, gradually increase fluid diet to semiliquid diet; POD 2 in take TPF-FOS | POD 1 to POD 7 |
| Zhou T (17) | Water immediately, gradually to a liquid fiberless diet POD 1, and a semi-liquid fiber diet POD 3 | Hospital discharge |
| da Fonseca LM (18) | Liquid diet (approximately 500 cm3) on POD 1, and regular diet within the next 24 h, as tolerated and at their discretion | Hospital discharge (POD 7) |
| Wang ZH (19) | Water POD 1, gradually increase fluid diet to semiliquid diet; POD 2 in take TPF-FOS | Until discharge (tolerated solid diet and normal defecation) |
| Dag A (20) | Fluid diet approximately 12 h after the operation, gradually increased to a solid diet as tolerated | During hospital stay |
| EI Nakeeb A (21) | Began fluids on POD 1 and advanced to a regular diet within the next 24–48 h when tolerated | During hospital stay |
| Feo CV (22) | Drink the day after the operation, eat a soft diet the following day regardless of the passage of flatus, and then advanced to solid food as tolerated | Until on the day of discharge |
| Hartsell PA (23) | Began a full liquid diet on POD 1 | During hospital stay |
| Lobato Dias Consoil M (24) | 500 mL of restricted fluid were received on POD 1, and a free diet was received immediately if no nausea or vomiting was observed | Until hospital discharge |
| Stewart BT (25) | Free fluids from 4 h after the surgery and progressed to a solid diet from POD 1 at their own discretion | Until hospital discharge |
| Han-Geurts IJ (26) | Resumed an oral diet on any day after surgery | Until hospital discharge |
POD, postoperative day; TPF-FOS, enteral nutritional suspension.
Primary outcomes
Time to first flatus was reported in six studies incorporated after screening. Three of the 11 studies did not report the mean or SD for this outcome; authors were contacted for additional information but with none responding. Based on the reported data, time to first flatus was significantly shorter in the EOF group (WMD −0.58 days; 95% CI: −0.70 to −0.47 days; P<0.00001, from a fixed effects model), with significant heterogeneity across trials (χ2 =4.97, P=0.29, I2 =19%) compared to the COF group (Figure 3A).
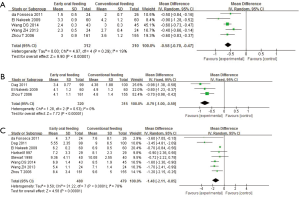
According to data from four trials, time to first defecation was reduced in the EOF group (WMD −0.79 days; 95% CI: −1.00 to −0.59 days; P<0.00001, from a fixed effects model) with heterogeneity across trials (χ2 =1.28, P=0.53, I2 =0%) compared to the COF group (Figure 3B).
Length of hospital stay was also assessed in all of the included studies. Three of the trials did not report the mean or SD for length of hospital stay; authors were contacted for additional information but did not respond. Thus, the analysis for length of hospital stay was based on eight trials. Compared with the COF group, hospital stay was significantly reduced in the EOF group (WMD −1.48 days; 95% CI: −2.11 to −0.85 days; P<0.00001, from a random effects model), with some evidence of heterogeneity between trials (χ2 =31.22, P<0.0001, I2 =78%) (Figure 3C).
Secondary outcomes
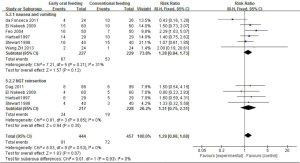
Nausea and vomiting were reported in six studies. EOF tended to be associated with a higher risk of nausea and vomiting compared to COF, although the difference was statistically non-significant (RR 1.28; 95% CI: 0.94 to 1.73; P=0.12). According to data from five studies, the difference in NGT reinsertion was not statistically significant between the EOF and COF groups (RR 1.31; 95% CI: 0.75 to 2.31; P=0.35) with no heterogeneity (χ2 =0.81, P=0.85, I2 =0%) (Figure 4).
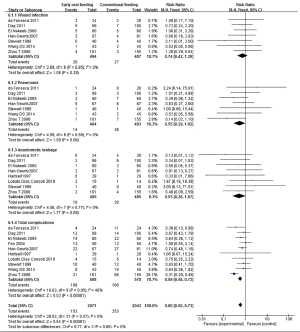
Postoperative complications were analyzed in the included studies. Wound infection was reported in 7 studies, pneumonia in 7 studies, and anastomotic dehiscence in 8 studies. There were 10 total studies reported with postoperative complications. There were no statistically significant differences between the EOF and the COF groups in the risk of wound infection, pneumonia, and anastomotic dehiscence (respectively RR 0.74; 95% CI: 0.42 to 1.29; P=0.29; RR 0.55; 95% CI: 0.29 to 1.02; P=0.06; RR 0.53; 95% CI: 0.26 to 1.07; P=0.08), with little heterogeneity between trials (respectively χ2 =2.69, P=0.85, I2 =0%; χ2 =4.89, P=0.56, I2 =0%; χ2 =4.06, P=0.77, I2 =0%). The results showed that EOF reduced the risk of total complications compared to COF (RR 0.59; 95% CI: 0.48 to 0.73; P<0.00001), with little heterogeneity between trials (χ2 =16.63, P=0.05, I2 =46%) (Figure 5).
Discussion
This meta-analysis determined the effect of EOF on several postoperative GI motility outcomes in patients undergoing elective colorectal surgery. Our primary analysis included trials with low risk of bias and that showed EOF being associated with a significant reduction in time to first flatus, defecation, length of hospital stay, and actually decreasing total postoperative complications compared with COF. There were no statistical differences in the risk of wound infection, pneumonia, anastomotic dehiscence, rate of NGT reinsertion, nausea, and vomiting. EOF is one of some important elements in fast-track surgery which can enhance recovery after colorectal surgery (27,28), and the evidence is mainly based on two meta-analyses (7,29,30). However, there is inadequate evidence. Firstly, both meta-analyses included many studies in which all or some patients had undergone other GI surgeries rather than colorectal surgery, such as upper GI surgery (31) and small bowel operations (32,33). Secondly, studies of oral feeding and tube feeding were combined in these meta-analyses. Fast-track colorectal surgery indicates early NGT removal, EOF, and early mobilization rather than early tube feeding (27,28). This is the first updated meta-analysis to evaluate GI function following EOF compared to following COF in patients undergoing colorectal surgery. The meta-analysis shown that EOF was beneficial for patients to recover and can be an available option for clinical treatment.
Almost every patient that underwent GI surgery experienced disorders of GI dysfunction. The major postoperative determinant of GI function is ileus, which is defined as a transient impairment of intestinal motility following abdominal surgery. It has a complex pathogenesis involving the surgical stress response and inhibitory neural reflexes. Postoperative ileus is thought to influence toleration of oral intake, and vice versa, but reports on possible interactions are controversial (33-35). Diminishing complications and enhancing recovery to allow adequate food intake are the main objectives. A fasting period of 3 to 4 days, or until passage of flatus or defecation after colorectal surgery, has been common practice and is often accompanied by decompression of the stomach with a nasogastric tube and intravenous administration of fluids or nutrition. These procedures have been performed habitually, though controversy still exists. However, recent research on EOF after GI surgery has demonstrated its clinical advantages, especially in the field of colorectal surgery.
The present study followed the recommendations of the PRISMA statement and was strengthened by the stringent inclusion criteria, rigorous search strategy, and avoidance of language limitation. What’s more, this meta-analysis was based on 11 RCTs, and 9 of these trials (1,078 patients) were published after 2004. With the development of the surgical and anesthetic practices that have changed over recent years, the results of the present study will be more representative than the previous meta-analyses for current colorectal surgery. The results indicate that EOF is effective and safe for recovery of GI motility after colorectal resection.
There were several limitations to the present meta-analysis. First, the time to first flatus, defecation, and length of hospital stay were presented as median (range), percentage, and time of postoperative complications in some trials which could not be included in our present meta-analysis. In addition, none of the incorporated studies used blinding method for the observers or patients. For feeding protocols, however, they could not lend themselves to double (observers and patients) or single (patients only) blinding, as both would have detected the introduction of food. Second, high statistical heterogeneity was identified in the length of hospital stay. Third, the included studies did not adequately evaluate total hospital costs and quality of life after surgery, which are very important outcomes for patients undergoing elective colorectal surgery. Finally, because only studies of EOF were included in this meta-analysis, our findings may not necessarily be generalized to patients with early postoperative tube feeding.
In conclusion, we found that EOF after elective colorectal resection is optional and safe in improving fast recovery. EOF was associated with a lower incidence of postoperative complications and a reduction in time to first flatus, defecation, and length of hospital stay. Further high-quality RCTs of EOF with long-term follow-up and quality of life are necessary to assess hospital costs and quality of life in patients undergoing elective colorectal surgery.
Acknowledgments
Funding: Department of Finance of Guangdong Province (150-9), Traditional Chinese Medicine Bureau of Guangdong Province (20181103), and the Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (YN2016QJ18).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2019.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer. Colorectal cancer estimated incidence, mortality and prevalence worldwide in 2012[EB/OL]. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Bisgaard T, Kehlet H. Early oral feeding after elective abdominal surgery – what are the issues? Nutrition 2002;18:944-8. [Crossref] [PubMed]
- Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2005;CD004929. [PubMed]
- Shoar S, Naderan M, Mahmoodzadeh H, et al. Early Oral Feeding After Surgery for Upper Gastrointestinal Malignancies: A Prospective Cohort Study. Oman Med J 2016;31:182-7. [Crossref] [PubMed]
- Osland EJ, Memon MA. Early postoperative feeding in resectional gastrointestinal surgical cancer patients. World J Gastrointest Oncol 2010;2:187-91. [Crossref] [PubMed]
- Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus ‘nil by mouth’ after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ 2001;323:773-6. [Crossref] [PubMed]
- Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev 2006;CD004080. [PubMed]
- Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J. Gastrointest Surg 2009;13:569-75. [Crossref] [PubMed]
- Osland E, Yunus RM, Khan S, et al. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta-analysis. JPEN J Parenter Enteral Nutr 2011;35:473-87. [Crossref] [PubMed]
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [Crossref] [PubMed]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available online: https://training.cochrane.org/handbook
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193-206. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Wang D, Zhong B, Zhao P, et al. A randomized control study of early oral enteral nutrition after colorectal cancer operation. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:977-80. [PubMed]
- Zhou T, Wu XT, Zhou YJ, et al. Early removing gastrointestinal decompression and early oral feeding improve patients' rehabilitation after colorectostomy. World J Gastroenterol 2006;12:2459-63. [Crossref] [PubMed]
- da Fonseca LM, Profeta da Luz MM, Lacerda-Filho A, et al. A simplified rehabilitation program for patients undergoing elective colonic surgery--randomized controlled clinical trial. Int J Colorectal Dis 2011;26:609-16. [Crossref] [PubMed]
- Wang ZH, Zhong B, Xiang JY, et al. Effect of early oral enteral nutrition on clinical outcomes after colorectal cancer surgery. Zhonghua Wei Chang Wai Ke Za Zhi 2013;16:735-8. [PubMed]
- Dag A, Colak T, Turkmenoglu O, et al. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (Sao Paulo) 2011;66:2001-5. [Crossref] [PubMed]
- El Nakeeb A, Fikry A, El Metwally T, et al. Early oral feeding in patients undergoing elective colonic anastomosis. Int J Surg 2009;7:206-9. [Crossref] [PubMed]
- Feo CV, Romanini B, Sortini D, et al. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg 2004;74:298-301. [Crossref] [PubMed]
- Hartsell PA, Frazee RC, Harrison JB, et al. Early postoperative feeding after elective colorectal surgery. Arch Surg 1997;132:518-20. [Crossref] [PubMed]
- Lobato Dias Consoli M, Maciel Fonseca L, Gomes da Silva R, et al. Early postoperative oral feeding impacts positively in patients undergoing colonic resection: results of a pilot study. Nutr Hosp 2010;25:806-9. [PubMed]
- Stewart BT, Woods RJ, Collopy BT, et al. Early feeding after elective open colorectal resections: a prospective randomized trial. Aust N Z J Surg 1998;68:125-8. [Crossref] [PubMed]
- Han-Geurts IJ, Hop WC. kok NF. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg 2007;94:555-61. [Crossref] [PubMed]
- Thornton L, Reader H, Stojkovic S, et al. Has the 'Fast-Track' referral system affected the route of presentation and/or clinical outcomes in patients with colorectal cancer? World J Surg Oncol 2016;14:158. [Crossref] [PubMed]
- Kehlet H. Fast-track colorectal surgery. Lancet 2008;371:791-3. [Crossref] [PubMed]
- Lewis SJ, Egger M, Sylvester PA, et al. Early enteral feeding versus ‘nil by mouth’ after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ 2001;323:773-6. [Crossref] [PubMed]
- Zhuang CL, Ye XZ, Zhang CJ, et al. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg 2013;30:225-32. [Crossref] [PubMed]
- Mahmoodzadeh H, Shoar S, Sirati F, et al. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surg Today 2015;45:203-8. [Crossref] [PubMed]
- Rohatiner T, Wend J, Rhodes S, et al. A prospective single-institution evaluation of current practices of early postoperative feeding after elective intestinal surgery. Am Surg 2012;78:1147-50. [PubMed]
- Kawamura YJ, Uchida H, Watanabe T, et al. Early feeding after oncological colorectal surgery in Japanese patients. J Gastroenterol 2000;35:524-7. [Crossref] [PubMed]
- Bozzetti F, Braga M, Gianotti L, et al. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet 2001;358:1487-92. [Crossref] [PubMed]
- Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg 2000;87:1480-93. [Crossref] [PubMed]
Cite this article as: Jiang Z, Chen QC, Zhang JH, Cao LX, Chen ZQ. Effect of early oral feeding on gastrointestinal motility in patients undergoing colorectal resection: a meta-analysis of randomized clinical trials. Dig Med Res 2019;2:17.