
Leptin in breast milk prevents hyperlipidemia in adult female rats born small for gestational age
Introduction
Small-for-gestational-age (SGA) infants, namely those born at a weight below the tenth percentile for gestational age, experience rapid weight gain during early childhood and have a high risk of developing obesity, cardiovascular diseases, and type 2 diabetes during adulthood (1-4). Such conditions may be caused by nutritional programming (5,6); however, the underlying mechanisms remain unclear. Obesity has been associated with premature birth and low birth weight (7-9). Breastfeeding during early life might prevent obesity in children and adults (10-12). Human milk contains hormones, growth factors, immunoglobulins, cytokines, and enzymes that support the growth and passive defenses of infants (13-15), in which leptin plays an essential role (16).
Leptin regulates body fat by inhibiting food intake and stimulating catabolic, autonomic, and neuroendocrine responses that direct nutrient stores away from fat compartments. Circulating leptin concentrations are closely correlated with body mass index (BMI) (17-19). Leptin in milk during the early stages of lactation may provide a mechanism for thermoregulation, satiation, and homeostatic endocrine and metabolic control in the neonate (20,21). Marked decreases in leptin and insulin levels in the mature milk of mothers of SGA newborns contributed to rapid weight gain in these newborns (22); however, the exact mechanisms are unclear. To explore whether breast milk leptin may prevent lipid metabolism disorder in adulthood in SGA infants, we assessed the role of leptin through subcutaneous injections of a leptin antagonist in breast-fed SGA pups and oral supplementation of leptin in formula-fed SGA pups. We subsequently evaluated the pups’ body weight (BW), BMI, and serum fat profiles at various time points after birth.
Methods
Animal model
The experimental protocol was approved by the Medical Animal Care & Welfare Committee of Shantou University Medical College (No. SUMC 2016-145). Three-month-old Sprague-Dawley rats were examined and quarantined for 1 week. Mating was confirmed by verifying the presence of spermatozoa in vaginal smears. The animals were housed in a regulated environment with a constant temperature of 25 °C, 50% humidity, and a 12:12-h light-dark cycle. Pregnant rats were divided into two groups (n=10 per group), ad-libitum-fed group and restrictedly-fed group, who were fed ad libitum or restrictedly to 30% of regular food intake throughout pregnancy respectively. After birth, the BW, nose-to-anus length, and abdominal circumference (AC) of all pups were recorded. BMI was calculated by dividing BW (g) by nose-to-anus length (cm2). Pups with a birth weight within the 95% confidence interval limits for litter and sex in the ad-libitum-fed group were designated as appropriate for gestational age (AGA) rats. Pups in restrictedly-fed group weighed less than two standard deviations (SDs) below the mean AGA weight were designated as SGA rats. Because male and female rats differ in terms of weight, BMI, and leptin level, we selected female rats as the experimental subjects.
Groups and treatments
After birth, female SGA rats were randomly assigned to the following four groups: (I) SB: breast-fed SGA rats; (II) SF: formula-fed SGA rats; (III) SBLA: breast-fed SGA rats that received leptin antagonist injections (Triple Rat Recombinant; ProSpec, Israel); and (IV) SFL: formula-fed SGA rats that received orally fed leptin (PeproTech, USA). The leptin antagonist was dissolved in 0.4% NaHCO3 at a concentration of 1 mg/mL, and a dose of 2.5 µg/g was subcutaneously injected between 16:00 and 17:00 once daily from day 1 to day 20. The dosage used in the experiment was based on our pilot data in the previous study (23). Recombinant murine leptin (1 ng/µL) was dissolved in water. From day 1 to day 20 of life, during the first 2 h of the light cycle, 20 µL of the recombinant murine leptin solution was administered orally to the pups by using a pipette and the amount was as follows: 1.0, 2.0, 3.0, 4.0, 5.0, 6.3, 7.5, 8.8, 10.0, 11.3, 15.6, 17.2, 18.8, 20.3, 21.9, 23.5, 25.0, 26.6, 39.4, and 43.8 ng, respectively (24). This leptin dosage was five times the amount of daily leptin ingested from maternal milk (25).
Breast-fed AGA pups were designated as AB group. The rats in breast-fed groups, namely AB, SB, and SBLA, were fed by the mother rats. The rats in formula-fed groups, namely SF and SFL, were separated from the mother rats and artificially fed through a thin tube. After day 20, all rats were fed rat chow. The composition of formula milk was close to that of breast milk. Standard laboratory chow comprising 6% fat, 21% protein, and 55% carbohydrates with an energy content of 3.66 kcal/g (Laboratory Animal Center of Shantou University Medical College) was provided. BW determined food consumption from day 21 to day 120 of life. No significant differences were observed among the study groups at any age (P>0.05 for all comparisons; data not shown).
Enzyme-linked immunosorbent assay (ELISA) analysis
On days 18, 30, 90, and 120 of life, the blood was drawn by puncturing the retro-orbital plexus under light ether anesthesia after overnight fasting (10 h) to assess serum leptin, total cholesterol (TC), and triglyceride (TG) levels. Blood samples were centrifuged at 2,500 ×g for 15 min at 4 °C. The entire serum was immediately frozen at −80 °C until subsequent analysis. Leptin levels were measured using a rat/mouse leptin ELISA kit (Santa Cruz, USA). TC and TG levels were measured using a rat/mouse cholesterol/TG ELISA kit (Santa Cruz, USA).
Statistical analysis
Results were analyzed by conducting analyses of variance (ANOVAs). For all analyses, the level of significance was set at P<0.05. All values are expressed herein as mean ± SD.
Results
As showed in Figure 1, compared with the breast-fed AGA rats, the breast-fed SGA rats had lower weight on days 90 and 120 (P<0.05 for all the comparisons), but similar BMI on every time point (P>0.05 for all the comparisons). SGA rats had higher leptin level on every time point (P<0.05 for all the comparisons). Referring to the fat profiles, SGA rats had lower TG level on days 18 and 30 (P<0.05 for all the comparisons), but similar TG level on days 90 and 120 (P>0.05 for all the comparisons) compared with the AGA rats. SGA rats had higher TC level on days 30, 90, but lower TC level on day 120 compared with the AGA rats (P<0.05 for all the comparisons). Although SGA rats were smaller than the AGA rats, they were prone to get lipid metabolism disorder when growing up.

As showed in Figure 2, the weight and BMI of the formula-fed SGA rats were lower than those of the breast-fed SGA rats on days 18 and 30 (P<0.05 for all the comparisons), but were similar on days 90 and 120 (P>0.05 for all the comparisons). Compared with breastfed SGA rats, formula-fed SGA rats had lower leptin level on days 18, 90, and 120 and higher TC and TG levels on day 120 (P<0.05 for all the comparisons). Hyperlipidemia was present in the adult SGA rats that were fed with formula milk during the neonatal period.
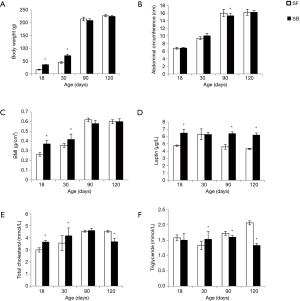
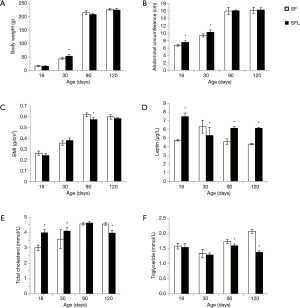
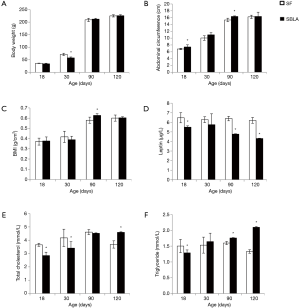
As the leptin level was low in formula-fed SGA rats, which might be related to the hyperlipidemia, we investigated the effect of leptin in breast milk by supplying leptin to formula-fed rats or injection of leptin antagonist to breast-fed rats. As leptin was added to the formula milk, the leptin-added-formula-fed rats, which were named SFL group, had higher leptin levels and lowered BMI, TC, and TG levels on days 90 and 120 compared with the formula-fed rats (P<0.05 for all comparisons; Figure 3). Otherwise, after leptin antagonist was injected, the rats in SBLA group had lower leptin level and higher BMI, TC, and TG levels compared with the breast-fed SGA rats on days 90 and 120 (P<0.05 for all comparisons; Figure 4). The observed hyperlipidemia in formula-fed rats was improved as leptin was added to the milk; otherwise, the prevalence of hyperlipidemia was increased as the effect of leptin in breast milk was inhibited.
Discussion
In this study, we developed a model of undernutrition in pregnant rats by restricting 30% of ad libitum consumption throughout gestation; this led to the delivery of SGA infants, similar to other studies (26,27). The nutritional status of the mother during pregnancy or that of the infant during the first year of life can exert long-term effects on metabolism in adulthood (28). These effects are referred to as programming. Undernourishment during development can cause an adaptive response that programs offspring to prioritize organ growth and increases the metabolic efficiency in preparation for an environment with sparse resources; this is the core theory of the thrifty phenotype hypothesis. Programming becomes detrimental when postnatal nutrition is more plentiful than prenatal nutrition; offspring exhibit rapid catch-up growth and subsequent obesity, which is a critical risk factor for noncommunicable diseases in adulthood, including metabolic syndrome and coronary heart disease (29). Epidemiological studies in humans and controlled animal studies have revealed that nutritional programming in early periods of life is a phenomenon that affects metabolic and physiological functions throughout life and can even affect the next generation (30,31). In our study, the observed BW, AC, and BMI levels in SGA female rats were lower than those in AGA rats during the neonatal period; however, the adult SGA female rats developed a high BMI and hyperlipidemia, which closely resembled clinical and metabolic abnormalities observed in humans born with a low weight.
The regulation of nutritional imprinting on hormonal and epigenetic mechanisms is complimentary. The central part, including the hypothalamic-pituitary-adrenal axis (32) and growth hormone-insulin-like growth factor axis (33), as well as peripheral tissue such as adipose tissue (34), may play a crucial role in regulation induced by nutritional programming. In these complex systems, leptin is critical, particularly in the process of lipid metabolism (35). Leptin is produced primarily in fat cells but is also produced in other organs and tissues such as the placenta and breast milk tissues. In our study, we found that within 18 days of birth, the leptin levels were higher in the breastfed SGA group than in the formula-fed SGA group, but the levels were similar in these two groups after the weaning period, thereby indicating that breast milk is the primary source of leptin during lactation. After day 18 of life, the number and volume of fat cells began to gradually increase, thereby enhancing the ability of fat cells to synthesize and secrete leptin. In this study, the peak leptin level was attained on day 90, and the levels gradually decreased after that. These results are similar to those of another study that showed that the leptin level was closely related to the amount of body fat tissue present and an increase in BW (36).
A comparison between breastfed female SGA and AGA rats revealed that there were higher TC levels, but the TG level was lower on days 30 and 90 in the SB group, indicating that female SGA rats experience hypercholesterolemia at the juvenile stage. Compared with the breastfed SGA group, formula-fed SGA rats had a lower leptin level and higher TC and TG levels. After leptin was added to the formula, the SFL group exhibited lower BMI, TC, and TG levels than the formula-fed SGA group did. Leptin deficiency was hypothesized to disrupt the lipid metabolism-regulating effect, leading to hyperlipidemia in the formula-fed female SGA group. To verify this hypothesis, the SBLA group, in which the rats were fed the leptin antagonist to inhibit the effect of leptin, was analyzed; higher BMI, TC, and TG levels were observed in this group compared with the SB group. These results indicate that breastfeeding may prevent obesity and hyperlipidemia during adulthood in SGA female rats because of the leptin in breast milk. Another study obtained a similar result and determined that peripheral leptin administration reduced BW under normal food intake (37). Leptin can influence the proliferation and differentiation of infant adipocytes and can help prevent obesity in later life.
In summary, breast milk is the primary source of leptin during lactation. Leptin plays a vital role in preventing obesity and hyperlipidemia during adulthood in SGA rats. However, the exact underlying mechanisms require further investigation.
Acknowledgments
Funding: The work was funded by the clinical scientific research fund from Wu Jieping Medical Fund, China (research number: 320.6750.14183).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Changhua Zhang and Liang Li) for the series “Nutritional Support for Digestive Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2019.08.02). The series “Nutritional Support for Digestive Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experimental protocol was approved by the Medical Animal Care & Welfare Committee of Shantou University Medical College (No. SUMC 2016-145) in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J 2015;36:551-9. [Crossref] [PubMed]
- Crume TL, Scherzinger A, Stamm E, et al. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 2014;22:608-15. [Crossref] [PubMed]
- Morrison JL, Duffield JA, Muhlhausler BS, et al. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol 2010;25:669-77. [Crossref] [PubMed]
- Skilton MR. Fetal growth and the ethnic origins of type 2 diabetes. Diabetologia 2015;58:422-4. [Crossref] [PubMed]
- Martínez JA, Cordero P, Campión J, et al. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc Nutr Soc 2012;71:276-83. [Crossref] [PubMed]
- Lane RH. Fetal programming, epigenetics, and adult onset disease. Clin Perinatol 2014;41:815-31. [Crossref] [PubMed]
- Hui LL, Lam HS, Leung GM, et al. Late prematurity and adiposity in adolescents: evidence from “Children of 1997” birth cohort. Obesity (Silver Spring) 2015;23:2309-14. [Crossref] [PubMed]
- Visentin S, Grumolato F, Nardelli GB, et al. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis 2014;237:391-9. [Crossref] [PubMed]
- Mercuro G, Bassareo PP, Flore G, et al. Prematurity and low weight at birth as new conditions predisposing to an increased cardiovascular risk. Eur J Prev Cardiol 2013;20:357-67. [Crossref] [PubMed]
- Spatz DL. Preventing obesity starts with breastfeeding. J Perinat Neonatal Nurs 2014;28:41-50. [Crossref] [PubMed]
- Oddy WH, Mori TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab 2014;64:262-70. [Crossref] [PubMed]
- Škledar MT, Milošević M. Breastfeeding and time of complementary food introduction as predictors of obesity in children. Cent Eur J Public Health 2015;23:26-31. [Crossref] [PubMed]
- Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr 2010;156:S3-7. [Crossref] [PubMed]
- Garofalo R. Cytokines in human milk. J Pediatr 2010;156:S36-40. [Crossref] [PubMed]
- Walker WA. The dynamic effects of breastfeeding on intestinal development and host defense. Adv Exp Med Biol 2004;554:155-70. [Crossref] [PubMed]
- Uçar B, Kirel B, Bör O, et al. Breast milk leptin concentrations in initial and terminal milk samples: relationships to maternal and infant plasma leptin concentrations, adiposity, serum glucose, insulin, lipid and lipoprotein levels. J Pediatr Endocrinol Metab 2000;13:149-56. [Crossref] [PubMed]
- Owecki M, Nikisch E, Miczke A, et al. Leptin, soluble leptin receptors, free leptin index, and their relationship with insulin resistance and BMI: high normal BMI is the threshold for serum leptin increase in humans. Horm Metab Res 2010;42:585-9. [Crossref] [PubMed]
- Mitsuyama S, Abe F, Kimura M, et al. Association between leptin gene expression in subcutaneous adipose tissue and circulating leptin levels in obese patients with psoriasis. Arch Dermatol Res 2015;307:539-44. [Crossref] [PubMed]
- Houde AA, Légaré C, Biron S, et al. Leptin and adiponectin DNA methylation levels in adipose tissues and blood cells are associated with BMI, waist girth and LDL-cholesterol levels in severely obese men and women. BMC Med Genet 2015;16:29-38. [Crossref] [PubMed]
- Savino F, Benetti S, Liguori SA, et al. Advances on human milk hormones and protection against obesity. Cell Mol Biol 2013;59:89-98. [PubMed]
- Savino F, Sardo A, Rossi L, et al. Mother and infant body mass index, breast milk leptin and their serum leptin values. Nutrients 2016;8:383-92. [Crossref] [PubMed]
- Nunes M, da Silva CH, Bosa VL, et al. Could a remarkable decrease in leptin and insulin levels from colostrum to mature milk contribute to early growth catch-up of SGA infants? BMC Pregnancy Childbirth 2017;17:410-6. [Crossref] [PubMed]
- Attig L, Solomon G, Ferezou J, et al. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 2008;32:1153-60. [Crossref] [PubMed]
- Sánchez J, Oliver P, Miralles O, et al. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 2005;146:2575-82. [Crossref] [PubMed]
- Picó C, Sanchez J, Oliver P, et al. Role of leptin present in maternal milk in the control of energy balance during the post-natal period. Genes Nutr 2007;2:139-41. [Crossref] [PubMed]
- Reamon-Buettner SM, Buschmann J, Lewin G. Identifying placental epigenetic alterations in an intrauterine growth restriction (IUGR) rat model induced by gestational protein deficiency. Reprod Toxicol 2014;45:117-24. [Crossref] [PubMed]
- Menendez-Castro C, Fahlbusch F, Cordasic N, et al. Early and late postnatal myocardial and vascular changes in a protein restriction rat model of intrauterine growth restriction. PLoS One 2011;6:e20369. [Crossref] [PubMed]
- Lucas A. Long-term programming effects of early nutrition–implications for the preterm infant. J Perinatol 2005;25:S2-6. [Crossref] [PubMed]
- Barker DJ, Eriksson JG, Forsén T, et al. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31:1235-9. [Crossref] [PubMed]
- Remacle C, Bieswal F, Bol V, et al. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am J Clin Nutr 2011;94:1846S-1852S. [Crossref] [PubMed]
- Fernandez-Twinn DS, Constância M, Ozanne SE. Intergenerational epigenetic inheritance in models of developmental programming of adult disease. Semin Cell Dev Biol 2015;43:85-95. [Crossref] [PubMed]
- Hollanders JJ, Heijboer AC, van der Voorn B, et al. Nutritional programming by glucocorticoids in breast milk: targets, mechanisms and possible implications. Best Pract Res Clin Endocrinol Metab 2017;31:397-408. [Crossref] [PubMed]
- Holt RI. Fetal programming of the growth hormone-insulin-like growth factor axis. Trends Endocrinol Metab 2002;13:392-7. [Crossref] [PubMed]
- Symonds ME, Pope M, Sharkey D, et al. Adipose tissue and fetal programming. Diabetologia 2012;55:1597-606. [Crossref] [PubMed]
- Doneray H, Orbak Z, Yildiz L. The relationship between breast milk leptin and neonatal weight gain. Acta Paediatr 2009;98:643-7. [Crossref] [PubMed]
- Oliver P, Pico C, Palou A. Ontogenesis of leptin expression in different adipose tissue depots in the rat. Pflugers Arch 2001;442:383-90. [Crossref] [PubMed]
- Bjørbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res 2004;59:305-31. [Crossref] [PubMed]
Cite this article as: Fang X, Li G, Zhang A, Lin H, Wang M, Chen Y, Li Y. Leptin in breast milk prevents hyperlipidemia in adult female rats born small for gestational age. Dig Med Res 2019;2:21.


