Nutritional intervention and efficacy analysis of children with biliary atresia after Kasai portoenterostomy
Introduction
Biliary atresia (BA) is a disease involving partial or total obstruction of the intrahepatic and extrahepatic bile ducts, which leads to cholestatic cirrhosis and ultimately liver failure (1). The etiology remains unclear but may be related to genes, viral infection, toxins, chronic inflammation or immune-mediated bile duct injury (2). Without surgery, most children die within a year, and liver transplantation (LT) is the only treatment for the disease. Early diagnosis and timely Kasai portoenterostomy (KPE) are the preferred treatment for BA (3). However, even when KPE is performed immediately after birth, children with end-stage liver disease will eventually require LT (4). The liver is the major organ involved in nutrient metabolism. Although bile flow is established after surgery in BA children, malnutrition still occurs easily. Children with a high risk of malnutrition are more prone to cholangitis after KPE, and the jaundice clearance rate is lower than that of children with a low nutritional risk; thus, these factors affect the 2-year survival rate of children with autologous livers after KPE (5). A recent study showed that severe or persistent malnutrition after KPE was one indication of a need for LT. Ideal pre-surgery management should actively optimize nutrition, growth and development of these children (6).
To study the nutritional status and strengthen the nutritional management of children with BA after KPE, this study conducted regular follow-ups of children with BA after KPE in the Department of Neonatal Surgery of our hospital over the last 7 years. We studied their post-KPE jaundice clearance status, physical development status and plasma levels of fat-soluble vitamins to better develop strategies for follow-up visits and nutritional intervention and to improve the long-term survival and quality of life of children with BA.
Methods
Research subjects
The study subjects are children with BA admitted to the Department of Neonatal Surgery of our hospital who survived with their autologous livers after KPE. Their physical status and fat-soluble vitamin levels in the peripheral blood were dynamically monitored to evaluate the post-KPE liver function, bilirubin level and nutritional status.
Research methods
Patients with BA after KPE were followed up in the multidisciplinary postoperative follow-up clinic (MDT) and evaluated by neonatal surgeons, gastroenterologists and clinical nutritionists. The follow-up time usually was 1, 2, 3, 4, 5, 6, 9 and 12 months after KPE, and a re-examination was conducted annually after the age of 1 year. This study was reviewed and approved by the Guangzhou Women and Children Medical Research Ethics Committee, and the approval number was 26001.
Nutritional intervention: a simple dietary survey was conducted to assess the nutritional intake, and individualized nutritional guidance was given to the children according to their nutritional statuses. Because children with BA are at high risk of vitamin D deficiency, all children were required to take a physiological dose of a vitamin D supplement (400–800 IU) (7,8). In addition to feeding guidance, children with malnutrition were given a medium-chain triglyceride (MCT)-enriched nutritional formulation (9).
Physical examination: the height/length, weight and head circumference were measured by referring to the physical measurement method of Zhu Futang’s Practical Pediatrics (the 8th edition). The weight of the infants and young children aged 1 to 3 years was measured with the electronic infant scale (seca376), whereas the weight of children aged 3 to 17 years was measured using the electronic standing scale (seca704s), which provided an accurate reading to 0.01 kg. The length of children under 3 years old was measured in the recumbent position (recumbent length) using the infant measuring bed (seca416). The height of children over 3 years old was measured using an electronic standing scale (seca704s), and the reading was recorded to within 0.1 cm. For children under 3 years old, the head circumference was measured using a seca201 measuring ruler, and the reading was recorded to within 0.1 cm. The above Seca series instruments were purchased from Seca Company, Germany. At each clinic visit, the growth curves were plotted to assess growth and development trends.
Lab tests: regular checks of liver function, prealbumin and plasma levels of fat-soluble vitamins A, D and E were performed. Liver function was measured using a turbidimetric immunoassay. The fat-soluble vitamins were detected by chemiluminescence. Jaundice clearance 3 months after KPE is defined as a total bilirubin (TBIL) level <20 µmol.
Statistical method: The Z-score of physique and the percentiles were calculated using the anthropometric calculator of the WHO Anthro software, and descriptive statistics were evaluated. An independent-samples t-test was used to compare pre- and post-KPE indicators and vitamins, with α<0.05 set as the statistical significance level.
Results
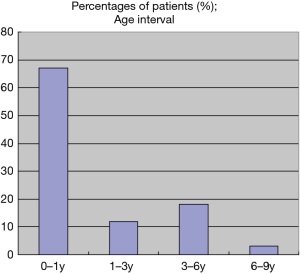
A total of 40 children (male: 24; female: 16) were included in the study. Among them, 25 children had good compliance and were followed up at least twice. The patients’ ages at the last follow-up were 13.38±21.70 months. The age distribution is shown in Figure 1. A total of 27 children were 0–1 years old, 5 children were 1–3 years old, 7 children were 3–6 years old, and 1 child was 6–9 years old. Nineteen children were followed up only once; the main reasons for the loss to follow-up were decreased treatment intention and waiting for a liver source in another city.
Pre- and post-KPE liver function
The pre- and post-KPE regular monitoring of liver function were compared (Table 1). The aspartate aminotransferase (AST), total protein (TP), globulin (GLO), albumin to globulin ratio (A/G), TBIL and direct bilirubin (DBIL) were all significantly improved after KPE.
Table 1
| Liver function index | N | Pre-KPE | N | 6 months post-KPE | T value | P value |
|---|---|---|---|---|---|---|
| ALT (U/L) | 16 | 189.00±107.51 | 25 | 131.36±91.94 | 1.8330 | 0.0744 |
| AST (U/L) | 16 | 235.38±125.42 | 25 | 128.76±87.80 | 3.2054 | 0.0027 |
| ALP (U/L) | 15 | 451.13±215.55 | 23 | 389.78±154.06 | 1.0243 | 0.3125 |
| Gamma-GT (U/L) | 16 | 944.50±551.23 | 24 | 989.25±2,031.62 | −0.0857 | 0.9322 |
| TP (g/L) | 16 | 57.93±5.00 | 25 | 64.36±6.37 | −3.4166 | 0.0015 |
| ALB (g/L) | 16 | 39.06±3.52 | 25 | 40.67±4.76 | −1.1672 | 0.2502 |
| GLO (g/L) | 16 | 18.87±4.84 | 25 | 23.68±4.38 | −3.2981 | 0.0021 |
| A/G | 16 | 2.18±0.49 | 25 | 1.78±0.41 | 2.8141 | 0.0076 |
| TBIL (μmol/L) | 16 | 123.60±30.72 | 25 | 62.54±86.15 | 2.7159 | 0.0098 |
| DBIL (μmol/L) | 16 | 102.28±24.84 | 25 | 48.86±70.73 | 2.8979 | 0.0061 |
| IBIL (μmol/L) | 16 | 21.32±8.35 | 25 | 13.69±15.80 | 1.7740 | 0.0839 |
| TBA (μmol/L) | 15 | 168.19±92.17 | 25 | 128.47±134.85 | 1.0058 | 0.3209 |
| PA (mg/L) | 9 | 130.83±70.56 | 20 | 150.82±47.48 | −0.6437 | 0.5268 |
Jaundice clearance rate within three months after KPE
The post-KPE TBIL level is shown in Figure 2. Twenty-five children were followed up with blood sampling, and the TBIL of 14 children (14/25, 56%) decreased to less than 20 µmol/L.
The pre- and post-KPE physical statuses
The pre- and post-KPE physical statuses are shown in Table 2.
Table 2
| Physical index | Birth | Admission | Last follow-up |
|---|---|---|---|
| Gestational age/month | 38.65±2.00 | 1.91±1.04 | 13.38±21.7 |
| Measured value | |||
| Weight (kg) | 3.12±0.54 | 4.9±0.78 | 6.54±2.46 |
| Length (cm) | 49.8±2.21 | 67.01±10.63 | |
| HC (cm) | 41.24±4.12 | ||
| BMI | 15.65±2.11 | ||
| Z-score | |||
| Weight | −0.34±1.02 | −0.51±1.09 | −1.49±1.54 |
| Length | 0.14±1.18 | −1.39±1.16 | |
| WFL | −0.82±1.35 | −0.48±1.13 | |
| BMI | −0.65±1.29 | ||
| HC | −1.43±1.93 | ||
| Percentile | |||
| Weight | 40.57±28.9 | 33.17±27.48 | 26.53±25.91 |
| Length | 54.2±31.83 | 18.93±18.96 | |
| WFL | 35.32±30.45 | 37.95±30.69 | |
| BMI | 37.5±31.36 | ||
| HC | 22.72±26.52 |
WFL, weight for length; HC, head circumference; BMI, body mass index.
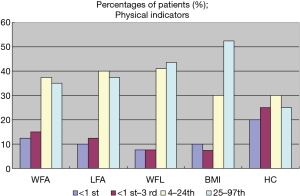
The percentile distributions of individual physical indicators at the last follow-up visit are shown in Figure 3. The incidence rate of malnutrition was as follows. In the weight for age (WFA) comparison, 11 children’s weights were less than the 3rd percentile (11/40, 27.5%), and 5 were less than the 1st percentile (5/40, 12.5%). In the length for age (LFA) comparison, 9 children were less than the 3rd percentile (9/40, 22.5%), and 4 were less than the 1st percentile (4/40, 10%). When the weight for length (WFL) was compared to the reference standards, 6 of the children’s WFL was less than the 3rd percentile (6/39, 15.4%), and 3 were less than the 1st percentile (3/39, 7.7%). In the comparison of their BMIs with those of their peers, 7 children’s BMIs were less than the 3rd percentile (7/40, 17.5%), and 4 were less than the 1st percentile (7/40, 17.5%). Compared with the head circumference (HC) of their peers, 18 children’s HCs were less than the 3rd percentile (18/40, 45%), and 8 were less than the 1st percentile (8/40, 20%).
Post-KPE fat-soluble vitamin levels
The fat-soluble vitamin levels in children with a jaundice clearance rate of 56% after KPE are shown in Table 3. BA patients are a high-risk group for vitamin deficiency. After supplementation with the physiological dose of vitamin D (400–800 IU), their fat-soluble vitamin levels were not significantly different from those of children of the same ages from the outpatient clinics.
Table 3
| Fat-soluble vitamin | Post-KPE group (n=22) | Control group (n=30) | T value | P value |
|---|---|---|---|---|
| Vitamin A (μmol/L) | 0.99±0.29 | 0.94±0.12 | 0.425 | 0.674 |
| Vitamin D (μmol/L) | 34.12±16.71 | 40.00±17.80 | −0.754 | 0.458 |
| Vitamin E (μg/mL) | 12.82±1.53 | 11.61±0.47 | 0.423 | 0.676 |
Discussion
For every 2,500 full-term infants, one child is born with cholestatic jaundice. The most common cause of neonatal cholestatic jaundice for newborn babies is BA (25–40%), which includes single-gene genetic disorders (25%) and other unknown or multifactorial causes (such as intravenous nutrition); each of these cases has its own time sensitivity and treatment plans (2). BA is an infantile disease with progressive fibrosis and obstruction of the intrahepatic and extrahepatic bile ducts. Its incidence rate varies slightly worldwide, with 1/19,000 among live born infants in Europe (10) and 1/18,000 among live born infants in North America (11). Children with this disease eventually develop end-stage liver disease requiring LT (6). The standard BA surgical procedure is KPE. Early diagnosis is extremely important, especially in infants with jaundice for up to 6 weeks. An earlier diagnosis leads to more successful KPE. Intraoperative cholangiogram evaluation and histological examination of the residual biliary duct are the gold standard for diagnosis of BA (2). Different from other cholestatic liver diseases, certain characteristics of the liver histopathology in BA patients are independent factors predicting high-risk LT, such as advanced-stage fibrosis, pancreatic duct plate structure, medium and advanced degree of bile duct damage, relatively old age when KPE is performed and elevation of the international normalized ratio (INR) (12).
Although nearly 50% of these children may need an LT before 2 years old, the other 50% may live with autologous livers for years without cirrhosis or chronic liver disease. However, many children suffer from a variety of chronic complications, such as recurrent cholangitis, portal hypertension, bleeding from varicose veins, growth problems, biochemical abnormalities and bone malnutrition. The incidence of these complications affects the quality of life of the children and their families (13).
Although nearly 50% of the children survive with autologous livers for more than 10 years after KPE (13,14), a 20-year study performed in Northern European countries showed that the treatment survival intention of BA children was lower than that of other children with chronic liver disease waiting for LT. Because these children have a younger age (<1 year), lower weight (<10 kg), higher bilirubin (>510 µmol/L), higher INR (>1.6), and higher pediatric end-stage liver disease (PELD) score (>20), they have the worst outcome (15). Children younger than 2 years of age with end-stage liver disease (YC2) are a uniquely vulnerable group awaiting LT, most of whom are children with BA. Multivariate analysis suggested that for YC2 waiting for LT, a PELD >21, body length <60.6 cm, weight >10 kg and initial creatinine >0.5 were independent risk factors of mortality (16). This finding suggests that the nutritional status after KPE and before LT is very important for the survival of BA patients.
KPE was performed on 106 children with type III BA who were divided into 2 groups according to the STRONGkids score: moderate nutritional risk (MNR) and high nutritional risk (HNR). The children in the HNR group were more prone to cholangitis, the post-KPE jaundice clearance rate for these children was lower than that in the MNR group, and the occurrence of the first postoperative cholangitis in the MNR group was later than that in the HNR group. Two-year survival was also significantly higher in the MNR group than in the HNR group (5). Therefore, nutritional support should be timely and effective. Although drugs such as steroid therapy can improve the short-term (≤1 year) jaundice clearance rate after KPE, no significant improvement has been noted in the long-term (≥2 years) jaundice clearance rate and the survival rate of children with autologous livers (17). The early nutritional status after KPE was found to be a predictive factor for the survival of children with autologous livers at the age of 2 years. The TBIL and albumin levels 3 months after KPE were independently correlated with the survival rate of children with autologous livers based on data from 217 BA children. Even in patients whose jaundice is completely cleared after KPE, an albumin level below 35 g/L indicates a poor prognosis (18). Therefore, improving the nutritional status after KPE is extremely important.
In this study, a nutritional follow-up visit was conducted with 40 children with a jaundice clearance rate up to 56% after KPE. We found that nutritional disorders appeared in the early stage. Regarding the incidence of malnutrition, 27.5% of the children had a weight lower than that of their peers, 15.4–17.5% were emaciated, 22.5% had a short stature, and 45% had a smaller-than-normal head circumference. These results differed from those of Mansi et al. (19), who investigated hospitalized children with chronic liver disease and found that 35.6% had a low weight, 49% had a short stature, and 5–10% were emaciated. In terms of the types of malnutrition, short stature is more concerning, because it reflects the long-term nutritional status of children, whereas weight reflects the short-term nutritional status. A total of 2/3 of these children are under the age of 1 year, nearly 80% of them are younger than 3 years old, and the percentage with a short stature is significantly higher than the percentage who are emaciated. Although KPE was completed as early as 2–3 months of age and obstructive jaundice was alleviated in these children, some of them still suffered from significant impairment of the linear growth rate.
In the comparisons of bilirubin, liver function and albumin before and after KPE, the efficacy of KPE for these children was significant, because TBIL, DBIL and AST decreased significantly, total protein increased significantly, and the jaundice clearance rate reached 56% within 3 months after KPE. If jaundice clearance is achieved within 3 months after KPE, significant improvement in the nutritional status of the patients can be expected. Studies have shown that children with BA are prone to deficiency of fat-soluble vitamins, especially vitamin D (7). Therefore, a vitamin D formulation should be routinely supplemented. In this study, nutritional interventions were conducted in a timely manner for children at high nutritional risk. At the follow-up visit, no significant differences in the fat-soluble vitamin levels were found compared with those of children of the same ages from the outpatient clinic. The liver is an important metabolic organ that plays a major role in integrating various biochemical pathways for metabolism. Therefore, the long-term nutritional and growth statuses still need to be followed up. In addition to supplementation with fat-soluble vitamins and the MCT-enriched nutritional formulation implemented in this study, we suggest that clinical dieticians be included in the multidisciplinary team (MDT) that will follow-up the patients after KPE to develop more comprehensive nutritional treatment and follow-up strategies, such as more sophisticated management of the relationship between drugs, foods and nutritional supplements and the sequence of administration.
In conclusion, long-term follow-up should be conducted for BA patients after KPE, and their growth and nutrient levels need to be monitored dynamically to improve the long-term survival rate of the children and particularly to strive for an optimal nutritional level before LT (10,20). Our study has some shortcomings: The follow-up time is relatively short, the rate of loss to follow-up is relatively high, and the number of cases is relatively few. In the future, we hope to standardize the follow-up system, increase the number of cases, group the children based on degrees of jaundice clearance after KPE, and perform a more accurate nutritional assessment to provide an objective basis for individualized nutrition management.
Conclusions
Among the children with BA who survived with autologous livers after KPE, 56% (14/25) achieved jaundice clearance 3 months after KPE. At the last follow-up at 13.38±21.70 months, 32.5% (13/40) of the children were in the ideal weight range of P25–P75, 37.5% (15/40) were in the ideal body length/height range, 28.2% (11/39) were in the ideal WFL range, and 42.5% (17/40) was in the ideal BMI range.
At the last follow-up, the emaciation rate was 17.5% (7/40), and the short stature rate was 22.5% (9/40), indicating that long-term malnutrition was prominent.
Long-term standardized follow-up and nutrition management for children after KPE should be carried out with multidisciplinary cooperation to achieve a more ideal nutritional level before LT.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Changhua Zhang and Liang Li) for the series “Nutritional Support for Digestive Surgery” published in Digestive Medicine Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2019.07.03). The series “Nutritional Support for Digestive Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of The Guangzhou Women and Children Medical Center Research Ethics Committee (No. 26001). Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet 2009;374:1704-13. [Crossref] [PubMed]
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017;64:154-68. [Crossref] [PubMed]
- Govindarajan KK. Biliary atresia: Where do we stand now? World J Hepatol 2016;8:1593-601. [Crossref] [PubMed]
- Lykavieris P, Chardot C, Sokhn M, et al. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005;41:366-71. [Crossref] [PubMed]
- Li D, Chen X, Fu K, et al. Preoperative nutritional status and its impact on cholangitis after Kasai portoenterostomy in biliary atresia patients. Pediatr Surg Int 2017;33:901-6. [Crossref] [PubMed]
- Sundaram SS, Mack CL, Feldman AG, et al. Biliary atresia: Indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl 2017;23:96-109. [Crossref] [PubMed]
- Ng J, Paul A, Wright N, et al. Vitamin D Levels in Infants With Biliary Atresia: Pre- and Post-Kasai Portoenterostomy. J Pediatr Gastroenterol Nutr 2016;62:746-50. [Crossref] [PubMed]
- Kelly DA, Bucuvalas JC, Alonso EM, et al. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013;19:798-825. [Crossref] [PubMed]
- Macías-Rosales R, Larrosa-Haro A, Ortíz-Gabriel G, et al. Effectiveness of Enteral Versus Oral Nutrition With a Medium-Chain Triglyceride Formula to Prevent Malnutrition and Growth Impairment in Infants With Biliary Atresia. J Pediatr Gastroenterol Nutr 2016;62:101-9. [Crossref] [PubMed]
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: Advances and future challenges. J Hepatol 2016;65:631-42. [Crossref] [PubMed]
- McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet 2000;355:25-9. [Crossref] [PubMed]
- Russo P, Magee JC, Anders RA, et al. Key Histopathologic Features of Liver Biopsies That Distinguish Biliary Atresia From Other Causes of Infantile Cholestasis and Their Correlation With Outcome: A Multicenter Study. Am J Surg Pathol 2016;40:1601-15. [Crossref] [PubMed]
- Wong KK, Wong CW. A review of long-term outcome and quality of life of patients after Kasai operation surviving with native livers. Pediatr Surg Int 2017;33:1283-7. [Crossref] [PubMed]
- Madadi-Sanjani O, Kuebler JF, Dippel S, et al. Long-term outcome and necessity of liver transplantation in infants with biliary atresia are independent of cytokine milieu in native liver and serum. Cytokine 2018;111:382-8. [Crossref] [PubMed]
- Malenicka S, Ericzon BG, Jørgensen MH, et al. Impaired intention-to-treat survival after listing for liver transplantation in children with biliary atresia compared to other chronic liver diseases: 20 years' experience from the Nordic countries. Pediatr Transplant 2017;21: [Crossref] [PubMed]
- Leung DH, Narang A, Minard CG, et al. A 10-Year united network for organ sharing review of mortality and risk factors in young children awaiting liver transplantation. Liver Transpl 2016;22:1584-92. [Crossref] [PubMed]
- Zhang MZ, Xun PC, He K, et al. Adjuvant steroid treatment following Kasai portoenterostomy and clinical outcomes of biliary atresia patients: an updated meta-analysis. World J Pediatr 2017;13:20-6. [Crossref] [PubMed]
- Nightingale S, Stormon MO, O'Loughlin EV, et al. Early Posthepatoportoenterostomy Predictors of Native Liver Survival in Biliary Atresia. J Pediatr Gastroenterol Nutr 2017;64:203-9. [Crossref] [PubMed]
- Mansi Y, Ghaffar SA, Sayed S, et al. The Effect of Nutritional Status on Outcome of Hospitalization in Paediatric Liver Disease Patients. J Clin Diagn Res 2016;10:SC01-5. [PubMed]
- Squires RH, Ng V, Romero R, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology 2014;60:362-98. [Crossref] [PubMed]
Cite this article as: Sun J, Wang Z, He Q, Zheng H, Zhang S, Wu J, Chen H, Liu X, Zhong W. Nutritional intervention and efficacy analysis of children with biliary atresia after Kasai portoenterostomy. Dig Med Res 2019;2:16.