Advanced gastric cancer with lymph node metastasis successfully treated using multimodal therapy: a case report
Introduction
Despite decreases in incidence worldwide, gastric cancer is the fourth most common cancer worldwide and accounts for 1.5% of all new diagnoses of cancer and 5.2% of all cancer deaths (1,2). The highest mortality rates are reported in East Asian countries such as China, Japan and Korea with 28.1 per 100,000 males and 13.0 per 100,000 females (3). Approximately 41% of all new gastric cancer diagnosis are in China (3). Gastric cancers are usually diagnosed late at an advanced stage where most patients are ineligible for gastric resection, since most patient with late stage gastric cancer who undergoes surgery as their primary treatment method have a high risk of recurrence, disease progression and mortality (4). This is particularly true for high-risk patients with locally advanced tumor stage, where the tumor is serosa positive and multiple lymph node metastases are present. Peri-operative chemotherapy or also known as neo-adjuvant chemotherapy appears to improve prognosis of such patients according to MAGIC, FNCLCC/FFCD trial and EORTC 40954 phase III clinical trials (5-7), but at the cost of increased surgical mortality. Our medical center has been pioneering in neo-adjuvant chemotherapy therapy in China, and here is a brief report of a patient of ours who likely benefitted from this form of treatment.
Case presentation
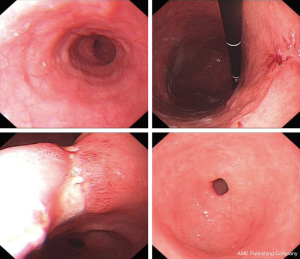
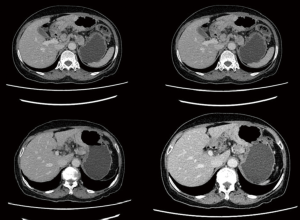
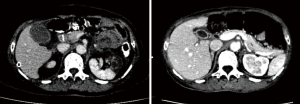
The patient was a 64 years old female patient who on a routine health check found elevated levels of CA19-9 at 6,744 U/L, a subsequent gastroscopy discovered a massive ulcerated mass along the small curvature of the stomach that covered up 1/3 of the surface area of the stomach (Figure 1), which was later histologically confirmed to be a gastric adenocarcinoma by lesion biopsy. Physical examination revealed no remarkable abnormalities other than a palpable mass around the epigastrium about 3 cm × 3 cm in size. The patient’s CT showed heterogenous enhancement of the gastric wall and antrum which combined with the previous test results confirmed the diagnosis of gastric cancer which had invaded across all the layers of the gastric wall into the adipose tissue surrounding the stomach. There were multiple enlarged lymph nodes detected by CT in the peritoneum, around the stomach and the spaces between the stomach and the liver, which were possible positive lymph node metastases (Figure 2). The patient’s positron emission tomography (PET)-CT further revealed thickened gastric walls and antral wall with remarked metabolic activity, multiple enlarged and highly metabolic lymph nodes in the peritoneum and the spaces surrounding the stomach. Endoscopic ultrasound showed an image that conformed to gastric cancer and detected small amount of ascites. All imaging tests did not detect any remarkable distant metastasis. Her case was then brought in front of our multi-disciplinary team (MDT) to set-up a treatment plan for her.
The patient was diagnosed with a cT4N2M0 gastric adenocarcinoma according to the TNM staging system of Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC), the most widely used staging system to evaluate the prognosis of gastric cancer patients, and the MDT suggested preoperative neoadjuvant chemotherapy using SOX chemotherapy regimen first.
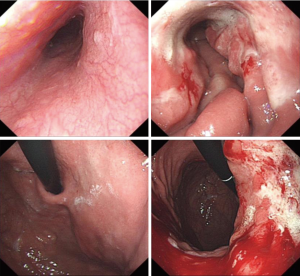
After 3 cycles of SOX regimen (oxaliplatin 200 mg + S-1 60 mg, BID 14 days) where the patient reported no severe adverse effect from the chemotherapy drugs, she underwent a second CT to observe the effect of neoadjuvant chemotherapy and the second CT showed that the gastric cancer still infiltrated the whole depth of the gastric wall but there were now slightly visible margins in the surrounding adipose tissue the previously enlarged peri-gastric lymph nodes were slightly smaller as seen in Figures 3 and 4. A repeat gastroscopy revealed a regression in size of the ulcerated mass in the stomach (Figure 5). After another round of MDT discussion, it was decided that the patient was responsive to chemotherapy and should continue 2 more rounds of neoadjuvant chemotherapy and then reassess her condition for surgery.
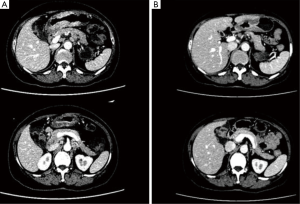
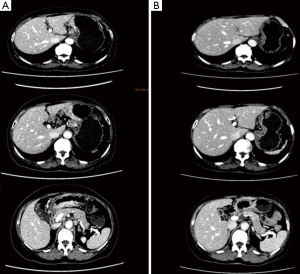
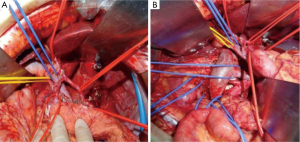
After 2 more cycles of SOX (oxaliplatin 200 mg + S-1 60 mg, BID 14 days), a repeat CT showed that the previously thickened gastric wall and antrum were slightly thinner, and the margins clearer, the lymph nodes were smaller compared to her previous CT. Blood tests showed a lowered level of CA19-9 of 62.75 U/mL. After another MDT discussion she was deemed surgery fit and was operated on the 26th August 2016, she underwent radical gastrectomy as seen in Figure 6 (D2 + PAND) (8,9), post-operative pathology confirmed that she had a poorly differentiated gastric adenocarcinoma which had invaded across all the thickness of the gastric wall and reached but not breached the serous layer, a total of 60 lymph nodes were positive for cancer cells out of 148, and the paraaortic lymph nodes were also positive for cancer cells. Immunohistochemical test showed that the tumor was CerbB-2 negative. According to the 8th version of AJCC cancer staging system, our patient was diagnosed with ypT4N3bM1, stage IV gastric adenocarcinoma.
Three days after surgery, the patient was put under hyperthermic intraperitoneal chemotherapy (HIPEC) with 1,000 mg of 5-FU once daily for 3 consecutive days (10). Afterwards she was given 6 cycles of adjuvant chemotherapy using SOX regimen (oxaliplatin 200 mg + S-1 60 mg, BID 14 days), where the first cycle of chemotherapy started a month after surgery. She has not complained of serious adverse reactions during her chemotherapy cycles, that is there were no grade 4 or 5 adverse events (according to Common Terminology Criteria for Adverse Events, CTCAE v4.0). She has had several follow-ups checks after surgery, and up to the last follow-up which was at the end of her adjuvant chemotherapy, she was disease-free without any sign of recurrence or disease progression as seen in Figure 7 which is a follow-up CT scan after surgery and adjuvant chemotherapy.
Discussion
Multimodal treatment including neoadjuvant chemotherapy, surgical resection with negative margins (R0-resection) followed by adjuvant chemotherapy is gaining more and more importance in the treatment of gastric cancer (11,12) after several previous trials have shown that perioperative chemotherapy to be superior to surgery alone in terms of disease-free survival and overall survival (5-7).
However, it seems that preoperative neo-adjuvant chemotherapy increases surgical morbidity and mortality (13,14). The most common complications in multimodal treatment using neo-adjuvant chemotherapy are surgical wound infections and anastomotic leakage (15,16), which ranges from 7–58.9% (17,18) and post-operative mortality which ranges from 0–13% (19,20). Hence careful measures should be undertaken to screen patients before the treatment and proper care taken during the course of the treatment to reduce operative morbidity and mortality.
In Asia, the chemotherapy regimen of choice is SOX, which is both recommended in NCCN, AJCC and has been shown to be more beneficial to Asian patients from the CLASSIC trial which has been carried out in Korea, Taiwan and China (21). Our patient has been responsive to this regimen and has shown next to no adverse reactions to it.
Usually the most challenging question to gastric cancer surgeons worldwide is when surgery should be done and which level of lymph node dissection should be done, for our patient the optimal window for surgery was decided by an MDT team which assessed her based on her clinical status and through thorough imaging modalities including endoscopic ultrasonography (EUS), CT, PET-CT, and endoscopy with lesion biopsy. In our medical center, a D2 lymph node resection is the norm for patients with T2 or higher, and PAN dissection is recommended in patients with confirmed second station enlarged lymph nodes confirmed pre-operatively (22,23), and since the patient CT and PET-CT both showed involvement of the PAN, the surgical team decided on a total gastrectomy with D2 and PAND resection.
Another important issue surrounding this patient treatment choices is the use of prophylactic HIPEC (10). In about 25% of all recurrent tumors of gastric origin, the recurring disease will be confined to the peritoneum, which is why surgeons focused on finding a way to stop the metastatic process of the tumor in the peritoneum. However, HIPEC in patients can also increase morbidity (24) and patients should be properly screened before treatment. This type of prophylactic HIPEC in patients at high risk of developing peritoneal metastatic tumors was first investigated by Japanese studies led by Fujimoto et al. in 1999 (25), where they submitted that patients with high grade serosa positive tumors had a higher 8 years overall survival rate when compared to radical surgery and adjuvant chemotherapy alone (62% vs. 49%) (25,26).
Our patient gastric cancer was a cT4N2M0 which in the recent past was indicative of a poor prognosis but with the development of treatment modalities and the advent of multidisciplinary team using and coordinating multiple treatment modalities, patients with more and more advanced stage of gastric cancer can gain from it. With a well-coordinated treatment plan consisting of perioperative chemotherapy, R0 resection and HIPEC our patient with a post-operative ypT4N3bM1, stage IV gastric adenocarcinoma is still alive and disease free till last follow-up.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (grant No. 81272643 and 81272637), the “3 & 3” project of The First Affiliated Hospital of Sun Yat-sen University, the National Natural Science Foundation of Guangdong Province (grant No. c15140600000016).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2019.01.01). YH serves as the Editor-in-Chief of Digestive Medicine Research. CZ serves as an Associate Editor-in-Chief of Digestive Medicine Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [Crossref] [PubMed]
- Van Cutsem E, Dicato M, Geva R, et al. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol 2011;22:v1-9. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Burke EC, Karpeh MS, Conlon KC, et al. Laparoscopy in the management of gastric adenocarcinoma. Ann Surg 1997;225:262-7. [Crossref] [PubMed]
- Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 2016;42:1123-31. [Crossref] [PubMed]
- Grundmann RT, Holscher AH, Bembenek A, et al. Diagnosis of and therapy for gastric cancer--work-flow. Zentralbl Chir 2009;134:362-74. [Crossref] [PubMed]
- Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer. Lancet 2009;374:477-90. [Crossref] [PubMed]
- Payne WG, Naidu DK, Wheeler CK, et al. Wound healing in patients with cancer. Eplasty 2008;8:e9. [PubMed]
- Dwight RW, Higgins GA, Keehn RJ. Factors influencing survival after resection in cancer of the colon and rectum. Am J Surg 1969;117:512-22. [Crossref] [PubMed]
- Grossmann EM, Longo WE, Virgo KS, et al. Morbidity and mortality of gastrectomy for cancer in Department of Veterans Affairs Medical Centers. Surgery 2002;131:484-90. [Crossref] [PubMed]
- Lang H, Piso P, Stukenborg C, et al. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol 2000;26:168-71. [Crossref] [PubMed]
- Hummel R, Bausch D. Anastomotic Leakage after Upper Gastrointestinal Surgery: Surgical Treatment. Visc Med 2017;33:207-11. [Crossref] [PubMed]
- Newman E, Marcus SG, Potmesil M, et al. Neoadjuvant chemotherapy with CPT-11 and cisplatin downstages locally advanced gastric cancer. J Gastrointest Surg 2002;6:212-23; discussion 223. [Crossref] [PubMed]
- Ciesielski M, Kruszewski WJ, Walczak J, et al. Analysis of postoperative morbidity and mortality following surgery for gastric cancer. Surgeon volume as the most significant prognostic factor. Prz Gastroenterol 2017;12:215-21. [Crossref] [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Zhang C, He Y, Schwarz RE, et al. Evaluation of para-aortic nodal dissection for locoregionally advanced gastric cancer with 1-3 involved para-aortic nodes. Chin Med J (Engl) 2014;127:435-41. [PubMed]
- Arikan AY, Senel FM, Akman RY, et al. Comparison of the effects of various anticancer agents on intestinal anastomosis after intraperitoneal administration. Surg Today 1999;29:741-6. [Crossref] [PubMed]
- Fujimoto S, Takahashi M, Mutou T, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999;85:529-34. [Crossref] [PubMed]
- Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol 2008;34:1246-52. [Crossref] [PubMed]
Cite this article as: Wei Z, Zheng J, Hao T, He Y, Zhang C. Advanced gastric cancer with lymph node metastasis successfully treated using multimodal therapy: a case report. Dig Med Res 2019;2:1.