
Appearance of Krukenberg tumor from gastric carcinoma, ultrasound and computed tomography evaluation
Introduction
Krukenberg tumor is a rare metastatic signet ring cell tumor of the ovary, accounting for 1–2% of all ovarian tumors. The stomach is the primary site in the majority (75%) of Krukenberg tumor cases, followed by carcinomas of the colon, appendix, and breast (1-4). Krukenberg tumor is considered as a late-stage disease with poor prognosis and may account for 5–30% of metastatic cancers to the ovaries. It is bilateral in 80% of the cases (5).
It is commonly metastatic tumors of the ovaries whose primary site is in the stomach to be called Krukenberg tumor. It was first described by Friedrich Krukenberg in 1896 who called it “sarcoma ovary mucocellulare (6). Finally, in 1960, Novak described this tumor as in filtered mucinocarcinoma with peripheral signet ring cells which sometimes will be observed with glandular structures (7).
Approximately 5% of all carcinomas metastatic to the ovaries are adenocarcinoma with pleomorphic mucin filled signet ring cells (8,9). Metastatic ovarian carcinoma grows rapidly and reaches the size of a fist up till the size of a child’s head, and, contrary to that, the primary tumor can be very small, and may not giving the slightest signs of its existence and revealing it even at autopsy (10).
Carcinoma of the stomach is a disease of the middle and old age group, with a tendency of shifting this border to a younger age, yet the occurrence of this disease in younger than 30 years is rare. It usually occurs between 40 and 60 years of age. Gender distribution of this disease varies from 2:1 to 2:1.5 among men and women depending on regional and racial affiliation, but it is never equalized.
The gastric carcinoma is on the rise in our population and it is characteristic that it is revealed in the already advanced stage when we have a large tumor mass, which affects almost all layers of the stomach wall and which penetrates through the serosa to the adjacent organs.
The macroscopic, as well as the radiological gastric cancer of the stomach, can be: protrusive (polyposis, encephaloid) which runs into the cavity of the organ; infiltrative (scirrhous, stenotic) which most often involves the deeper layers of the stomach wall and ulcerative.
About 10% of gastric carcinoma show locally or diffusely spread through the wall (linitis plastica) and 5% are superficially spreading into the normal mucosa of the stomach. The extensive spread of the mucosa of the stomach produces wide-plate lesions that level the gastric mucosa and unite the folds. The mucosa of the stomach loses its velvety appearance and becomes muddy, rigid and tied to deep layers. Even more frequent is the diffuse spreading through the wall where the entire thickness of the wall is infiltrated that is linitis plastica occurs. The wall is noticeably thickened up till 2–3 cm and becomes firm as cartilage. Without regard to the macroscopic appearance of the tumor, the histological picture usually shows a well-differentiated adenocarcinoma, but different degrees of differentiation can be found, up to a completely undifferentiated growth (11-13).
Radiological, in the infiltrative type of stomach cancer, there is always an expressed desmoplastic reaction of the stomach wall, so that the only symptom can be the rigidity of the wall. In the case of significant occupancy of the wall, the result is the narrowing of the lumen and the disappearance of the mucosa relief.
Before the involvement of adjacent organs, pathological deposits in the lymphatic, liver and distant organs are quite common. The route of metastasis to the ovaries is mainly due to retrograde lymphatic spread as the gastrointestinal tract mucosa, and submucosa have a rich lymphatic plexus and also close proximity of the retroperitoneal lymph nodes (14).
Case presentation
In this report, we describe a case of a 45-year-old woman with bilateral Krukenberg tumour. The patient was received to the Clinic of Surgery due to stomach pain, nausea, and vomiting, as well as anamnesis data that of weight loose approximately 20 kg in the last 6–8 weeks. The patient is at the age of 45, does not drink and does not smoke, denies diseases of interest, and gives the impression of a severely ill person. The diagnosis that occurs at the clinic after performing radiological, endoscopic and pathohistological tests is gastritis chronica.
The radiological examination of the stomach was performed and a diagnosis of chronic gastritis was made. A gastroscopy of the stomach was made, showing a thickened wall in the antrum area, and disturbed peristalsis. Mucosal folds are thickened, coarse and rigid, after air insufflation does not spread. A biopsy was made and 11 samples were taken for pathohistological analysis. The acquired pathopsychological findings are in favor of chronic gastritis.
Due to the severe general condition of a patient who is disproportionate to the above-described finding, she is again hospitalized at the Clinic of Surgery. Clinical investigations were made, and due to the severity of the clinical picture, repeated radiological investigations, ultrasound (US), and computed tomography (CT) of the stomach and abdomen were indicated. Sedimentation is drastically elevated and in the first hour, it is SE =160, while we also have a decrease in the value of hemoglobin, erythrocytes, hematocrit, and serum iron.
Serum tumor markers are made: CEA 1.9 (0.0–3.4) ng/mL, CA19-9 >400.0 (0.0–20.24) U/mL, CA125 >540.0 (0.0–35.0) U/mL, CA72-4 20.8 (0.0–9.6) U/mL. We have an extreme increase in CA19-9 and CA125, a moderate increase in CA72-4, while CEA is in the range of normal values. The obtained values from serum tumor markers, as well as high sedimentation, are in favor of neo-infiltrating stomach and ovarian disease.
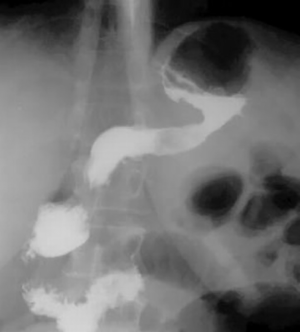
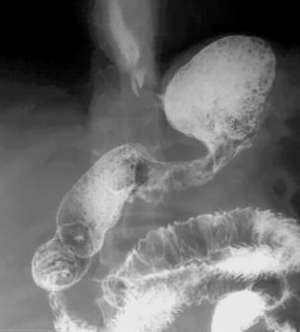
The repeated radiological examination of the stomach was made after the standard preparation of the patient with a monocontrast and double-contrast technique of examination. The stomach is with pronounced wall infiltration, with reduced volume, and with a strong diffuse rigidity. The process involves the corpus and the antrum by both curves, circularly. The esophagus, the fornix, duodenal bulbus and the duodenum are without changes (Figure 1). In the double-contrast examination of the stomach, after the administration of effervescent agent, no distension of the stomach was achieved, the reduction of volume and diffuse rigidity persisted further (Figure 2). The finding is in favor of the neoinfiltrative process of the stomach-linitis plastica.
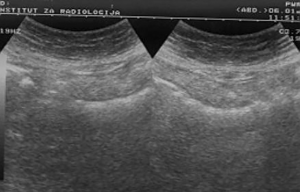
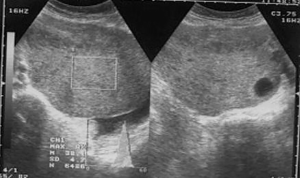
After standard preparation, an US examination of the abdomen and pelvis was made using a conventional examination technique. At the US examinations, infiltration of the stomach wall is seen, it is bold and rigid (Figure 3). There is free fluid in the peritoneal cavity, perihepatic and perilienal, paracolic gutter, between the intestinal loops, as well as in the pelvis. Focal pathological lesions in the parenchymal abdominal organs that accompany MS changes are not seen. US revealed, well defined hyper echoic predominantly solid pelvic tumor mass with cystic elements in right ovary measuring approximately 10 cm in diameter with visible cystic elements. Hypoechoic areas in the tumor formation in favor of necrotic zones (Figure 4). A similar change can be seen in the left ovary, but with significantly smaller dimensions. Intestinal loops are strongly distended, with stagnant fluid and contents like ileus. Ascites perihepatic, perisplenic, paracolic gutter on the right and left, between intestinal loops and in pelvis in a quantity greater than 1,500 mL. The right kidney calyx system is deformed, dilated and distended with no visible calculus, in favor of hydronephrosis of II degree. The proximal part of the right ureter is also dilated. This occurs as a consequence of compression of the right ureter by the tumor mass of the right ovary.
On the basis of previously performed radiological and US examination, a diagnosis of gastric carcinoma of scirrhous-type linitis plastica with MS changes in the ovaries-Krukenberg tumor has been established. To confirm the diagnosis, a CT examination of the stomach and abdomen was performed, including the pelvis, using a modified technique.
After water is given, as a contrast medium of 700 mL, and intravenous application of non-ionic contrast medium (Ultravist 300) of 90 mL, in the hypotonia achieved by intravenous application of glucagon, CT scans of the abdomen in position of supination with scan width and distance between the scans of 10 mm. The stomach is with a bold and rigid wall greater than 20–30 mm, diffusely infiltrated, with no signs of the breakthrough of the serosa and spreading to the adjacent organs. The volume of the stomach is markedly reduced, and the changes of the stomach wall are particularly pronounced in the area of the corpus and antrum (Figure 5). Parenchymal abdominal organs do not show signs of secondary pathological deposits, they are with neat structure and morphology. A collection of ascites fluid is perihepatic and perisplenic, between the intestinal loops, paracolic gutter as well as in the pelvis, which is in favor of MS changes in the peritoneum. There are no enlarged mesenteric and paraaortally lymph nodes, but there is no exclusion of secondary pathological deposits in them. The right kidney calyx system is deformed, dilated and distended in favor of hydronephrosis of II degree, and the left kidney is with no pathologic changes. The proximal part of the right ureter is dilated due to compression from the tumor mass in the pelvis. The intestinal loops in the pelvis are stagnant, and pushed laterally by soft-tissue tumor formations, which are non-homogeneous, predominantly solid, but with apparent zones of destructions that belong to the ovaries. The larger tumor mass belongs to the right ovary (Figure 6).
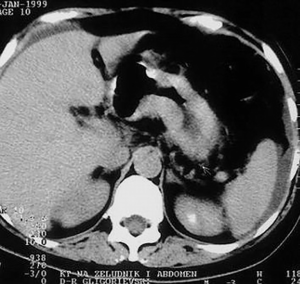
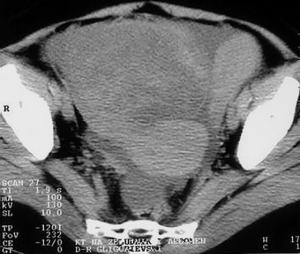
Based on the CT finding, a diagnosis of malignant tumor of the stomach of scirrhous-type linitis plastica with MS changes in the peritoneum and ovaries of the Krukenberg tumor was made (6).
An aspiration biopsy is made, the ascites is aspirated and given for cytological analysis. Millipore filter products made of 20 mL of yellowish liquid were found rare grouped cells with pronounced malignancy, around which there are numerous lymphocytes and rare macrophages. The result of the cytological finding is: classification group V.
By analyzing the obtained results from the clinical, laboratory, radiological and imaging methods, the final diagnosis was made, that is an inoperable stomach cancer of the scirrhous type—linitis plastica, with MS changes in the peritoneum and ovaries—Krukenberg tumor. In accordance with TNM classification, the tumor is in stage IV—T3N1M1. The case ends with a fatal outcome 2-month after the diagnosis has been established.
Discussion
Krukenberg tumour is a metastatic signet ring cell adenocarcinoma accounting for 1% to 2% of all ovarian tumours (15) usually presented in younger female with average age of 45 years (5,9). Krukenberg tumor is secondary to a neoplastic process in the gastrointestinal tract. Krukenberg tumors were more common in premenopausal women (75%) rather than in postmenopausal women (16). It was reported that patients with Krukenberg tumor were younger compared with those who had primary ovarian cancer, whereas the functioning ovary was prone to metastatic disease due to the rich ovarian blood supply predisposing to hematogenous metastasis (17). Most of the cases originate from gastric adenocarcinoma, followed by the colon (1,9). However, recent observations have reported a higher incidence of colorectal rather than gastric origin and, in particular, more frequently from the colon rather than the rectum (18)
Only 2% of all women with gastric cancer develop ovarian metastasis. Krukenberg tumor typically present as large, bilateral, solid ovarian masses, with clear well-defined margins and ascites. Krukenberg tumors have well defined histologic characteristics carcinoma with signet-ring cells and stroma with sarcomatoid reaction. Patients usually present with symptoms related to ovarian involvement such as abdominal pain and distension. Ascites is usually present in 50% of cases (9).
At US Krukenberg tumor typically present as irregular hyperechoic solid pattern and moth-eaten like cyst formation are also characteristic, allowing with some confidence to distinguish these lesions from primary ovarian neoplasms. CT shows solid masses and frequently intratumoral cysts, with strong contrast enhancement, a pattern that allows a differential diagnosis with primary ovarian cancer, where such a marked enhancement of the cyst walls is absent.
Majority of cases they are synchronous, but 20% to 30% occur as metachronous lesion after removal of primary. As the tumor is usually well encapsulated and rarely shows any ovarian surface involvement, theory of peritoneal seeding from primary lesion is questioned. Rich lymphatics draining gastric mucosa and submucosa initiating retrograde lymphatic spread to ovary is mostly accepted theory.
Carcinoma of the stomach is not only common but also a severe disease that is most often detected in an advanced stage, with a low survival rate that depends on the differentiation of the malignant and stage of the disease.
Digestive linitis plastica is a special form of poorly differentiated adenocarcinoma, which can affect all segments of the digestive tube, the most common localization in the stomach, and the most common is the double involvement of the stomach and rectum (12).
Berrada et al. In their study indicate the possibility of linitis plastica ingesting the entire digestive tube, and it is possible, however, to have a small percentage of a dual localization at the same time on the stomach and rectum. In our practice, we have cases of single localization of the stomach process, and very rarely on the rectum (three cases).
The case we present is gastric adenocarcinoma with MS changes in ovaries, i.e., synchronous Krukenberg tumor.
CT allows TNM staging in gastric carcinoma, in assessing the extent of the tumor process, the condition of the lymph nodes and distant metastases, thus allowing estimation of tumor operability and planning of operative treatment.
The involvement of the ovary, which is most commonly bilateral and can reach huge proportions, is thought to be the consequence of selective retrograde lymphatic spread of the primary tumor along the stomach ovarian axis (19).
The prognosis of patients with Krukenberg tumour is extremely poor with a median survival time of 14 months (9). Only 10% of patients survive more than 2 years after diagnosis (14). Peritoneal dissemination was reported as an adverse factor affecting survival (20).
We can conclude that Krukenberg tumor of the ovary is a rare metastatic tumor in young women. The primary tumor is gastrointestinal, most commonly the stomach. The pathophysiology is unclear. The diagnosis is often delayed. Imaging methods, and in particular, CT has great diagnostic value in setting the exact diagnosis and staging of the lesion. This allows for adequate planning of further treatment. The prognosis is bad.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/dmr.2018.06.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kiyokawa T, Young RH, Scully RE. Krukenberg tumours of the ovary: a clinicopathologic analysis of 120cases with emphasis on their variable pathologic manifestation. Am J Surg Pathol 2006;30:277-99. [Crossref] [PubMed]
- Young RH, Scully RE. Scully Metastatic tumors of the ovary. In: Kurman RJ (ed). Blaustein’s Pathology of the Female Genital Tract. 5th ed. New York: Springer, 2002:1063-101.
- Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol 2005;18:S99-111. [Crossref] [PubMed]
- Mambrini P, Giovanini M, Seitz JF, et al. Uterine metastasis revealing gastric adenocarcinoma. Gastroenterol Clin Biol 1995;19:725-8. [PubMed]
- Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary: part I. Historical perspective, general principles, mucinous tumors including the Krukenberg tumor. Adv Anat Pathol 2006;13:205-27. [Crossref] [PubMed]
- Schlagenhaufer F. Über das Fibrosarcoma Ovarii Mucocellulare (Carcinomatodes). Arch Gynäkol 1896;50:287-321. [Crossref]
- Hale RW. Krukenberg tumor of the ovaries. A review of 81 records. Obstet Gynecol 1968;32:221-5. [PubMed]
- Nirmal AK, Nirmal K, Kumar S, et al. Krukenberg Tumour of the Ovary in young Female: A Case Report. Int J Curr Res Med Sci 2016;2:60-64. [Crossref]
- Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: An overview. Arch Pathol Lab Med 2006;130:1725-30. [PubMed]
- Kim SH, Kim WH, Park KJ, et al. CT and MR findings of Krukenberg tumors: comparison with primary ovarian tumors. J Comput Assist Tomogr 1996;20:393-8. [Crossref] [PubMed]
- Levine MS, Pantongrag-Brown L, Aguilera NS, et al. Non-Hodgkin lymphoma of the stomach: a cause of linitis plastica. Radiology 1996;201:375-8. [Crossref] [PubMed]
- Berrada S, Alami A, Diouri M, et al. Linitis plastica of the digestive tract. A case with involvement of the stomach and rectum. Ann Gastroenterol Hepatol (Paris) 1996;32:120-2. [PubMed]
- Shafiuddin M, Caminker M, Batra S. Hereditary linitis plastica of the stomach. Am J Gastroenterol 1995;90:2062-3. [PubMed]
- Das S, Sahu D, Wani M, et al. A curious discourse of Krukenberg tumor: a case report. J Gastrointest Oncol 2014;5:E117-20. [PubMed]
- Mateş IN, Iosif C, Bănceanu G, et al. Features of Krukenberg-type tumors--clinical study and review. Chirurgia (Bucur) 2008;103:23-38. [PubMed]
- Wu F, Zhao X, Mi B, et al. Clinical characteristics and prognostic analysis of Krukenberg tumor. Mol Clin Oncol 2015;3:1323-8. [Crossref] [PubMed]
- La Fianza A, Alberici E, Pistorio A, et al. Differential diagnosis of Krukenberg tumors using multivariate analysis. Tumori 2002;88:284-7. [Crossref] [PubMed]
- Moore RG, Chung M, Granai CO, et al. Incidence of metastasis to the ovaries from nongenital tract primary tumors. Gynecol Oncol 2004;93:87-91. [Crossref] [PubMed]
- Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part II. Adv Anat Pathol 2007;14:149-77. [Crossref] [PubMed]
- Yook JH, Oh ST, Kim BS, et al. Clinical prognostic factors for ovarian metastasis in women with gastric cancer. Hepatogastroenterology 2007;54:955-9. [PubMed]
Cite this article as: Gligorievski A. Appearance of Krukenberg tumor from gastric carcinoma, ultrasound and computed tomography evaluation. Dig Med Res 2018;1:3.


